Trans. Nonferrous Met. Soc. China 29(2019) 1510-1515
Synthesis of Cu nanoparticles by chemical reduction method
M. S. AGUILAR1, R. ESPARZA2, G. ROSAS1
1. Instituto de Investigacion en Metalurgia y Materiales, UMSNH, Morelia Michoacan, 58000, Mexico;
2. Centro de Fisica Aplicada y Tecnologia Avanzada, UNAM, Santiago de Queretaro, 76230, Mexico
Received 29 September 2018; accepted 4 April 2019
Abstract: Cu nanoparticles (CuNPs) have been synthesized through an easy route by chemical reduction at room temperature. The Cu2+ ions were reduced and stabilized with sodium borohydride and polyvinylpyrrolidone, respectively. The effect of the variation of the reducing agent/precursor-salt (RA/PS) ratio on the size and morphology of the CuNPs was evaluated. The synthesized material was studied by ultraviolet-visible (UV-Vis) spectroscopy, X-ray diffraction (XRD), scanning electron microscopy (SEM), and transmission electron microscopy (TEM). The UV-Vis spectra showed a CuNPs plasmon peak at 569 nm and another peak belonging to Cu2O at 485 nm. XRD analysis showed the fcc-Cu phase with a small amount of fcc-Cu2O compound. SEM and TEM studies displayed that small semispherical CuNPs of approximately 7 nm were obtained at the RA/PS ratio of 2.6. The excess of polyvinylpyrrolidone stabilizer played an essential role in preventing CuNPs oxidation. On the other side, Cu2O polyhedral particles with larger sizes up to 150 nm were identified in the RA/PS ratio range of 2.0-1.84. In addition, Cu2O particles having star morphologies with quantum confinement at their tips were obtained at the RA/PS ratio of 1.66.
Key words: Cu nanoparticles; NaBH4; chemical reduction; polyvinylpyrrolidone stabilization; Cu2O
1 Introduction
In recent years, interest in the controlled synthesis of new nanomaterials has increased dramatically due to their wide range of applications in various areas of technology. However, the development of metal particles for nanoelectronic devices is affected by their instability and reactivity [1]. For example, synthesis of pure copper nanoparticles (CuNPs) is rare unless entire procedure is carried out in an inert atmosphere [2,3]. Although many synthesis processes of CuNPs report the use of a controlled atmosphere, they still obtain a mixture of Cu and Cu2O [4]. Therefore, the synthesis of stable Cu nanoparticles remains a challenge.
Several methods have been used to synthesize CuNPs, such as chemical reduction [5], electroreduction process [6], polyol method [7], laser ablation [8] and microwave irradiation [9]. Chemical reduction is one of the most feasible methods for the synthesis of nanoparticles and offers potential advantages, such as rapid and low-cost processing, and controlled particle size. For example, copper nanoparticles have been reported using L-ascorbic acid as reducing and capping agent in aqueous medium [10]. Regularly, surfactants such as polyvinylpyrrolidone (PVP) [11-13], cetyl- trimethyl-ammonium bromide (CTAB) [12] and sodium dodecyl sulfate (SDS) [11] are used to prevent growth and control oxidation. CuNPs have applications in catalysis [14,10], antibacterial agents [15], solar cells [16], nanodevices, nanoelectronics, and nano- sensors. In addition, copper is an important material because it has high electrical, optical and thermal properties.
In the present investigation, CuNPs were synthesized using a chemical reduction method. For this propose, sodium borohydride was used due to its high reducing capacity and polyvinylpyrrolidone for growth control, agglomeration, and avoiding oxidation. Although the most stable form of copper is copper oxide, it was possible to prevent the oxidation of CuNPs without the control of any atmosphere due to the excess of PVP used.
2 Experimental
Materials used for the synthesis of copper nanoparticles (CuNPs) are J. T. Baker-brand copper chloride (CuCl2·H2O), sodium borohydride (NaBH4), and polyvinylpyrrolidone (PVP) acquired from Sigma- Aldrich.
2.1 Synthesis of CuNPs
The synthesis of CuNPs was achieved through the reduction of CuCl2·H2O with NaBH4 in the presence of PVP. The reaction was carried out under magnetic stirring at room temperature. To synthesize the nanoparticles, initially, a dilution of 10 mL (5.6, 7.3, 7.9, 8.8 mol/L) of precursor salt, 7.7 mL (14.6 mol/L) of reducing agent, and 35 mL (0.9 mol/L) of surfactant were prepared separately. Subsequently, the reaction dilutions were made by adding the reducing agent and the surfactant agent to the precursor salt. From the above, a ratio of reducing agent to precursor salt (RA/PS) equal to 2.6, 2.0, 1.84 and 1.66 was deduced. When the reaction was carried out, the color of the solution changed to black and gradually to brown, characteristic of the formation of copper nanoparticles. After the synthesis, the solution obtained was stored at 10 °C for its subsequent characterization.
2.2 Structural characterization
The characterization of the solids synthesized was done by transmission electron microscopy (TEM) in a Phillips Tecnai F-20 microscope, whose filament is a field emission and works at a voltage of 200 keV. The analytical techniques used were a bright field and a high resolution. For the scanning electron microscopy (SEM), a JEOL JSM-7600F FEG-SEM microscope was used. The structural characterization was performed on a Bruker D8 ADVANCE DAVINCI diffractometer. The UV-Vis analysis was used to identify the presence of particles by absorption bands with a UV-Vis spectro- photometer Beckman Du-20.
3 Results and discussion
3.1 UV-Vis and XRD analyses
Figure 1(a) shows the results obtained after the chemical reduction of the sample with the higher RA/PS ratio (2.6) used in this work. A typical band of the surface plasmon resonance of CuNPs situated at 569 nm was observed. Also, a small peak of SPR, indicating the presence of Cu2O particles, was also found at 485 nm. The partial oxidation of the particles comes from their direct contact with the air, which is in agreement with previous works [17]. However, in this case, considering the differences in the intensity of both bands, the amount of CuNPs phase is higher compared to that of Cu2O.
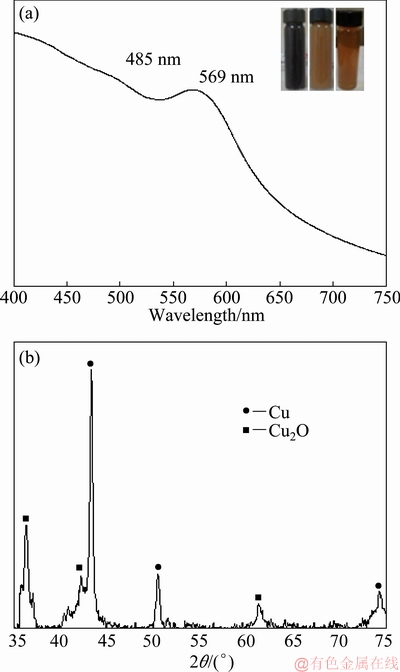
Fig. 1 UV-Vis spectrum of CuNPs synthesized for RA/PS ratio equal to 2.6 (a) and XRD pattern of obtained solids (b)
The X-ray diffraction technique was used to extend structural characterization. Figure 1(b) shows the XRD pattern of the as-synthesized powder, indicating a mixture of phases derived from the chemical reduction of Cu2+ ions with NaBH4 as a reducing agent and PVP as a surface stabilizer. A higher intensity of the (111) fcc-CuNPs diffraction peak in comparison to the (111) fcc-Cu2O particles indicates that more was obtained in the CuNPs crystalline phase. In order to quantify the percentage of Cu and Cu2O, the relative intensities of the Cu (111) peak and the Cu2O (111) peak were used, as outlined in Eq. (1) [18]:
 (1)
(1)
By using this technique, the CuNPs volume fraction of the as-synthesized sample was determined to be 72%. This result confirms that the present method has produced a large percentage of the CuNPs in comparison of the Cu2O phase. These results are in good agreement with the UV-Vis technique.
3.2 SEM studies
Figures 2(a) and (b) show a couple of scanning electron microscopy (SEM) images that illustrate the nano-solids formed after the chemical reduction of CuCl2. As seen in Fig. 2(a), a significant amount of NP was prepared to indicate a good yield for the synthesis of the reaction. Furthermore, judging from the backscattered image in Fig. 2(b), we can appreciate semispherical and polyhedral particles that have Z-contrast differences.
Semispherical particles are observed with a light- gray contrast and polyhedral particles with a medium- gray contrast. These results and their combination with XRD analyses show that the semispherical particles correspond to Cu, whereas the polyhedral morphologies correspond to Cu2O particles. The oxygen in the air reacts with Cu, oxidizing the material and modifying its morphology. The larger amount of copper nanoparticles observed compared to some previous works [5,13] may be related to the use of the excess of surface agent PVP, which achieved good stabilization of the Cu nanoparticles, retarding their oxidation.
An EDS chemical analysis (Fig. 2(c)) of the sample indicated the presence of Cu and O, confirming the chemical composition of the products. These findings are in good agreement with the previous characterization techniques.
3.3 TEM investigations
The CuNPs and Cu2O particles were also observed by transmission electron microscopy (TEM). Figures 3(a) and (b) display a couple of bright-field TEM images taken from the Cu and Cu2O particles, respectively. It can be seen that the Cu particles are semispherical in shape with narrow particle size distribution.

Fig. 2 SEM images of synthesized particles using RA/PS ratio of 2.6

Fig. 3 TEM images of semispherical CuNPs (a, b), TEM micrograph of Cu2O polyhedral particles (c) and HRTEM images showing inter-spacing distances that correspond to Cu cubic structure (d-f)
The average particle size, as determined by TEM micrographs, is 7 nm; whereas the polyhedral oxide particles have larger sizes up to 150 nm (Fig. 3(c)). Three typical HRTEM images of the CuNPs are shown in Figs. 3(d-f). The interlayer distances correspond to 0.203, 0.202, and 0.204 nm, respectively, that belong to the (111) crystallographic planes of the fcc-Cu.
Previous results showed that small particles were obtained at the ratio of reducing agent /precursor salt concentration (RA/PS) equaling 2.6. However, for low ratio of RA/PS, the average particle size of the products substantially increased. For example, Figures 4(a) and (b) show the morphology of the Cu2O particles for the RA/PS ratios of 2.0 and 1.84, respectively. It is found that particles have star morphology, and the average particle sizes are around 0.5 μm. As the RA/PS ratio decreased even more (1.66), the particles turn to semispherical morphologies. Under these conditions, the average particle size is near 2 μm (Fig. 4(c)). Figure 4(d) shows a chemical analysis of solids illustrated in Fig. 4(c), in which the copper with higher intensity is observed, followed by oxygen with a lower intensity; this indicates that the composition of the material obtained is Cu2O.
Figure 5 shows high-magnification TEM images of the Cu2O products. Figures 5(a) and (b) display bright- field and HAADF-TEM micrographs, of the star-shaped morphology. As can be observed, the tips of the star have sizes near the nanometric scale. Besides, some of them have sharp-tip type termination while others have a square shape.
This type of growth is due to the high amount of reduced copper ions that grow along with preferential directions. However, with the relative increases in the precursor salt (lower RA/PS ratio), the size of the products increased by the material saturation around the branch of the stars, giving spherical morphologies, as shown in Fig. 5(c).
HRTEM observations taken from one branch of the star-shape Cu2O morphology demonstrated their crystal structure, as shown in Fig. 5(d). The image indicates that the branch has an interlayer distance of 0.25 nm, which is in agreement with the (111) crystallographic planes of the cubic Cu2O phase. These results indicate that for high RA/PS ratios (2.6), the main product was CuNPs. As the RA/PS ratio decreased, the composition of the Cu2O particles changed, firstly with star-shaped morphology with RA/PS ratio of 2.0 and 1.84 and then to semispherical particles with RA/PS ratio of 1.66.
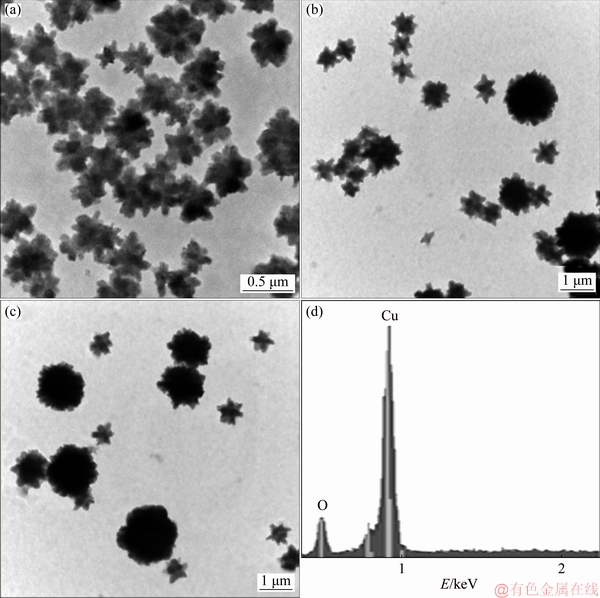
Fig. 4 SEM micrographs of Cu2O particles synthesized with RA/PS ratio equal to 2 (a), 1.84 (b) and 1.66 (c), respectively, and chemical analysis result of particles (d)

Fig. 5 Bright-field TEM (a) and HAADF-TEM (b) micrographs corresponding to Cu2O particles synthesized with RA/PS ratio equal to 2, bright-field TEM image of Cu2O particles synthesized with RA/PS ratio of 1.66 (c), and high-resolution images corresponding to (111) crystallographic planes of Cu2O cubic phase (d)
4 Conclusions
(1) CuNPs with small amounts of the copper oxide (Cu2O) were synthesized with NaBH4 as a reducing agent and PVP as a stabilizer.
(2) SEM micrographs illustrate that CuNPs have a semispherical morphology, while Cu2O particles have a polyhedral shape. EDS chemical analysis confirmed the presence of Cu and O in the reduction of products.
(3) The UV-Vis and XRD analyses confirmed the formation of CuNPs and small amounts of Cu2O particles with a concentration ratio for reducing agent/precursor salt of 2.6.
(4) TEM analysis determined that the average particle size of CuNPs was 7 nm; whereas the polyhedral oxide particles have larger sizes up to 150 nm. Besides, bigger Cu2O particles having star morphology and quantum confinement at the tips were obtained at lower RA/PS ratio (2.0).
(5) The excess of stabilizer used has a remarkable effect on the size of the copper nanoparticles as well as their oxidation.
Acknowledgements
M. S. AGUILAR would like to thank the National Council of Science and Technology (Conacyt) of Mexico for the financial support.
References
[1] BRUST M, FINK J, BETHELL D, SCHIFFRIN D J, KIELY C. Synthesis and reactions of functionalised gold nanoparticles [J]. Journal of the Chemical Society, Chemical Communications, 1995, 16: 1655-1656.
[2] DERRICK M, JEFFREY G, WANG Ling-yan, LUO Jin, ZHONG Chuan-jian. Synthesis of size controlled and shaped copper nanoparticles [J]. Langmuir, 2007, 23: 5740-5745.
[3] CHEN Shao-wei, SOMMERS J M. Alkanethiolate-protected copper nanoparticles: Spectroscopy, electrochemistry, and solid-state morphological evolution [J]. The Journal of Physical Chemistry B, 2001, 105: 8816-8820.
[4] LIU Qing-ming, ZHOU De-bi, YAMAMOTO Y, ICHINO R, MASAZUMI O. Preparation of Cu nanoparticles with NaBH4 by aqueous reduction method [J]. Transactions of Nonferrous Metals Society of China, 2012, 22: 117-123.
[5] AYESHA K, AUDIL R, RAFIA Y, REN Chong. A chemical reduction approach to the synthesis of copper nanoparticles [J]. International Nano Letters, 2016, 6: 21-26.
[6] RAJA M, SUBHA J, ALI FATHILAH B, RYU S H. Synthesis of copper nanoparticles by electroreduction process [J]. Materials and Manufacturing Processes, 2008, 23: 782-785.
[7] BLOSI M, ALBONETTI S, DONDI M, MARTELLI C, BALDI G. Microwave-assisted polyol synthesis of Cu nanoparticles [J]. Journal of Nanoparticle Research, 2011, 13: 127-138.
[8] TYURNINA A E, SHUR V Ya, KOZIN R V, KUZNETSOV D, PRYAKHINA V I, BURBAN G V. Synthesis and investigation of stable copper nanoparticle colloids [J]. Physics of the Solid State, 2014, 56: 1431-1437.
[9] ZHU Hai-tao, ZHANG Can-ying, YIN Yan-sheng. Novel synthesis of copper nanoparticles: Influence of the synthesis conditions on the particle size [J]. Nanotechnology, 2005, 16: 3079-3083.
[10] JAIN S, JAIN A, KACHHAWAH P, DEVRA V. Synthesis and size control of copper nanoparticles and their catalytic application [J]. Transactions of Nonferrous Metals Society of China, 2015, 25: 3995-4000.
[11] GIUFFRIDA S, COSTANZO L L, VENTIMIGLIA G, BONGIORNO C. Photochemical synthesis of copper nanoparticles incorporated in poly (vinyl pyrrolidone) [J]. Journal of Nanoparticle Research, 2008, 10: 1183-1192.
[12] GRANATA G, YAMAOKA T, PAGNANELLI F, FUWA A. Study of the synthesis of copper nanoparticles: The role of capping and kinetic towards control of particle size and stability [J]. Journal of Nanoparticle Research, 2016, 18: 133.
[13] YANG Ai-ling, LI Shun-pin, WANG Yu-jin, WANG Le-le, BAO Xi-chang, YANG Ren-qiang. Fabrication of Cu2O@Cu2O core-shell nanoparticles and conversion to Cu2O@Cu core-shell nanoparticles in solution [J]. Transactions of Nonferrous Metals Society of China, 2015 25: 3643-3650.
[14] SAMIM M, KAUSHIK N K, MAITRA A. Effect of size of copper nanoparticles on its catalytic behaviour in Ullman reaction [J]. Bulletin of Materials Science, 2007, 30: 535-540.
[15] ALZAHRANI E, AHMED R A. Synthesis of copper nanoparticles with various sizes and shapes: Application as a superior non-enzymatic sensor and antibacterial agent [J]. International Journal of Electrochemical Science, 2016, 11: 4712-4723.
[16] HUANG Chien-hua, WANG H P, LIAO Chang-yu. Nanosize copper encapsulated carbon thin films on a dye-sensitized solar cell cathode [J]. Journal of Nanoscience and Nanotechnology, 2010, 10: 1-4.
[17] JEONG S, WOO K, KIM D, LIM S, KIM J S, SHIN H, XIA Y, MOON J. Controlling the thickness of the surface oxide layer on Cu nanoparticles for the fabrication of conductive structures by ink-jet printing [J]. Advanced Functional Materials, 2008, 18: 679-686.
[18] KUMAR S, PARLETT C M A, ISAACS M A, JOWETT D V, DOUTHWAITE R E, COCKETT M C R, LEE A F. Facile synthesis of hierarchical Cu2O nanocubes as visible light photocatalysts [J]. Applied Catalysis B: Environmental, 2016, 189: 226-232.
化学还原法合成Cu纳米粒子
M. S. AGUILAR1, R. ESPARZA2, G. ROSAS1
1. Instituto de Investigacion en Metalurgia y Materiales, UMSNH, Morelia Michoacan, 58000, Mexico;
2. Centro de Fisica Aplicada y Tecnologia Avanzada, UNAM, Santiago de Queretaro, 76230, Mexico
摘 要:通过室温化学还原合成Cu纳米粒子(CuNPs)。采用硼氢化钠还原Cu2+离子,并用聚乙烯吡咯烷酮进行稳定化处理。考察还原剂/前驱体盐 (RA/PS) 比值的变化对CuNPs粒径和形貌的影响。利用紫外-可见光谱(UV-Vis)、X射线衍射(XRD)、扫描电镜(SEM)和透射电镜(TEM)对合成的材料进行表征。UV-Vis光谱显示,569 nm处存在一CuNPs的等离激元峰;另一峰位于485 nm处,为Cu2O的特征峰。XRD分析表明,合成的材料为fcc-Cu相,并含有少量的fcc-Cu2O化合物。SEM和TEM研究显示,当RA/PS比值为2.6时,得到粒径约7 nm的半球形CuNPs微粒。过量的聚乙烯吡咯烷酮稳定剂对于防止CuNPs的氧化至关重要。另一方面,当RA/PS比在2.0~1.84范围内时,获得较大粒径的多面体形Cu2O颗粒,最大粒径可达150 nm。此外,在RA/PS比为1.66时,得到在其尖端具有量子限域效应的星形Cu2O粒子。
关键词:铜纳米粒子;NaBH4;化学还原;聚乙烯吡咯烷酮稳定化;星形Cu2O
(Edited by Bing YANG)
Corresponding author: G. ROSAS; E-mail: grtrejo@umich.mx, grtrejo07@yahoo.com.mx
DOI: 10.1016/S1003-6326(19)65058-2