
Thermodynamic equilibrium of CaSO4-Ca(OH)2-H2O system
PENG Xiao-yu(彭小玉), WANG Yun-yan(王云燕), CHAI Li-yuan(柴立元), SHU Yu-de(舒余德)
School of Metallurgical Science and Engineering, Central South University, Changsha 410083, China
Received 18 January 2008; accepted 13 May 2008
Abstract: The pc—pH diagrams of the CaSO4-Ca(OH)2-H2O system and its two subsystems at 298.15 K were constructed according to the theory of thermodynamic equilibrium. The interaction characteristics between the solubility of CaSO4(s) and Ca(OH)2(s) can be found out from the diagrams. CaSO4(s), Ca(OH)2(s) and solution coexist when the pH value of solution is about 13.2. CaSO4(s) with the minimum solubility of 0.411 g/L is in equilibrium with solution when the pH value is lower than 13.2, and Ca(OH)2(s) with the minimum solubility of 2.749 g/L is in equilibrium with solution at the pH value over 13.2, which provides a theoretical basis for the treatment and reuse of industrial wastewater, especially for the wastewater containing sulfate which can be treated by lime-milk neutralization.
Key words: CaSO4-Ca(OH)2-H2O system; solubility; pc—pH diagram
1 Introduction
Large amounts of acidic sulfate wastewater containing heavy metals are discharged every year from the sulfate system in a number of industries such as electroplating, steel pickling, mining, nonferrous smelting and alkali making[1-4]. Now the lime-milk neutralization process is a conventional technique contributing to its advantages such as low investment, mature technique, simple and convenient operation[5-6]. However, the purified water treated by such method results in a series of problems which would influence the purification and reutilization of industrial wastewater for the high concentration of calcium ion and sulfate ion[7]. Some related research works have been done and it was pointed out that it is feasible to separate CaSO4 precipitates from Na2SO4-H2O system in addition of CaO[8-10], but the theoretical analysis concerned has not been reported in the literature up to now.
In this study, aiming at promoting and realizing the reuse and recycling of acidic sulfate wastewater treated by the lime-milk neutralization, the pc—pH diagram of the CaSO4-Ca(OH)2-H2O system at 298.15 K, as a theoretical guidance for the practical process, where the interaction characteristics between the solubility of CaSO4(s) and Ca(OH)2(s) could be found out, was constructed according to the thermodynamic equilibrium principle.
2 Drawing of thermodynamic equilibrium diagram
2.1 Thermodynamic relationship between Ca(OH)2(s) solubility and pH value
There are two kinds of calcium hydroxyl complex ions in Ca(OH)2-H2O system such as CaOH+ and Ca(OH)2. Correspondingly, their equilibrium equations and cumulative formation constants at 298.15 K are expressed as follows[11-14]:
Ca2++OH- CaOH+
CaOH+
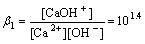 (1)
(1)
Ca2++2OH- Ca(OH)2
Ca(OH)2
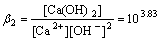 (2)
(2)
Knowing that:
Ca(OH)2(s) Ca2++2OH-
Ca2++2OH-
Ksp=[Ca2+][OH-]2=5.5×10-6 (3)
The following equation and constant can be obtained from Eq.(1) and Eq.(3)[15]:
Ca(OH)2(s) CaOH++OH-
CaOH++OH-
 (4)
(4)
Similarly, combination of Eq.(2) and Eq.(3) gives the following equation and constant:
Ca(OH)2(s) Ca(OH)2
Ca(OH)2
 (5)
(5)
Taking logarithm of both sides of Eqs.(3)-(5) gives
p[Ca2+]=2pH-22.74 (6)
p[CaOH+]=pH-10.14 (7)
p[Ca(OH)2]=1.43 (8)
The concentrations of [Ca2+], [CaOH+] and [Ca(OH)2] can be calculated by Eqs.(6)-(8) at given pH value, respectively. These values as functions of pH values are plotted as shown in Fig.1[16-18], which is the pc—pH diagram of the Ca(OH)2-H2O system at 298.15 K.
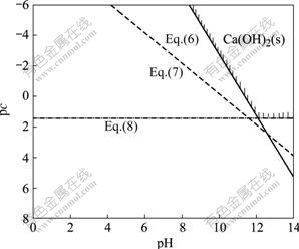
Fig.1 Diagram of pc—pH of Ca(OH)2-H2O system at 298.15 K
The lines in Fig.1 represent the concentrations of different calcium-containing species related to the pH values when the corresponding ions are in equilibrium with Ca(OH)2(s). The area surrounded by all lines is the stable zone of Ca(OH)2(s), in which Ca(OH)2(s) could exist stably. The others are the unsaturated zones of Ca(OH)2(s). Boundaries constituting this stable zone can approximately illustrate the total solubility of Ca(OH)2(s) at the different pH values in the system. From Fig.1 it can also be seen that the minimum solubility of Ca(OH)2(s) is 2.749 g/L as the pH value is located at 12.1-14.0, beyond this scope the solubility of Ca(OH)2(s) will increase gradually with the pH value declining.
2.2 Thermodynamic relationship between CaSO4(s) solubility and pH value
There also exist two kinds of calcium hydroxyl complex ions in CaSO4-H2O system such as CaOH+ and Ca(OH)2. Correspondingly, their equilibrium equations and cumulative formation constants at 298.15 K are expressed as follows:
Ca2++H2O CaOH++H+
CaOH++H+
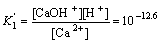 (9)
(9)
Ca2++2H2O Ca(OH)2+2H+
Ca(OH)2+2H+
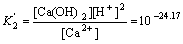 (10)
(10)
The mass balance for calcium in solution is[16]
[Ca]t=[Ca2+]+[CaOH+]+[Ca(OH)2] (11)
It should be noted that the [Ca]t, which represents the total soluble concentration of calcium, is also termed as the solubility of calcium.
From Eq.(9) and Eq.(10), the following equations can be deduced:
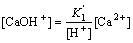 (12)
(12)
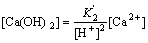 (13)
(13)
Inserting Eq.(12) and Eq.(13) into Eq.(11) gives
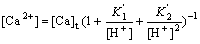 (14)
(14)
Sulphuric acid is a diprotic acid, which may yield two protons. Correspondingly, their ionization equations and equilibrium constants are expressed as follows[11]:
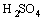

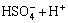
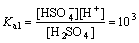 (15)
(15)
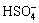

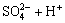
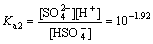 (16)
(16)
The mass balance for sulfate in solution is
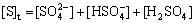 (17)
(17)
Similarly, [S]t represents the total concentration of sulfate. Inserting Eq.(15) and Eq.(16) into Eq.(17) gives
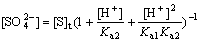 (18)
(18)
Calcium sulfate is considered to be insoluble commonly. However, it does dissolve slightly in water. The equilibrium equation and the solubility-product constant are expressed as follows:
CaSO4 Ca2++
Ca2++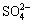
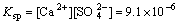 (19)
(19)
Inserting Eq.(14) and Eq.(18) into Eq.(19) gives
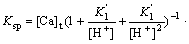
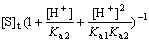 (20)
(20)
The total concentration of calcium is equal to that of sulfate in the system according to calcium sulfate ionization equation, and the pc—pH diagram of the CaSO4-H2O system can be obtained according to Eq.(20) as shown in Fig.2[16].
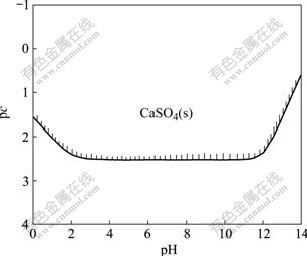
Fig.2 Diagram of pc—pH of CaSO4-H2O system at 298.15 K
The curve in Fig.2 represents the total concentration of calcium-containing species related to the pH value when the solution is in equilibrium with CaSO4(s). The area above the curve is the stable zone of CaSO4(s), where CaSO4(s) could exist stably, and below the curve is the unsaturated zone. Boundary constituting this stable zone can approximately illustrate the total solubility of CaSO4(s) at different pH values in the CaSO4-H2O system. The minimum solubility of CaSO4(s) is 0.411 g/L at the pH value of 3.5-11.0, beyond this scope the solubility of CaSO4(s) increases.
2.3 Thermodynamic equilibrium diagram of CaSO4- Ca(OH)2-H2O system
The thermodynamic equilibrium diagram of the CaSO4-Ca(OH)2-H2O system (Fig.3) is constructed by combination of Fig.2 and Fig.1[16-18]. From Fig.3 it can be concluded that the stable zones of Ca(OH)2(s) and CaSO4(s) overlap partially. The point a represents the triple equilibrium point where CaSO4(s) and Ca(OH)2(s) are in equilibrium with the solution simultaneously, and the dash line vertical to the pH axis from point a represents that both CaSO4(s) and Ca(OH)2(s) coexist at this pH value. The area on the left of the dash line is the stable zone of CaSO4(s), where the solubility of CaSO4(s) is less than that of Ca(OH)2(s), and Ca(OH)2(s) dissolves into the solution and CaSO4 precipitates. The area on the right of the dash line is the stable zone of Ca(OH)2(s), and CaSO4(s) dissolves and Ca(OH)2 precipitation occurs. The area above the solid line is the stable zone where the corresponding precipitates exist at different pH values, while the area below the solid line is the unsaturated zone where solution exists stably. And the solid line represents the total equilibrium concentration of calcium at different pH values. Fig.3 also shows that the minimum solubility of CaSO4(s) is 0.411 g/L at the pH values of 3.5-11.0, and the concentration of the sulfate retains its original concentration as the solubility of Ca(OH)2(s) keeps a constant of 2.749 g/L at the pH value over that of the triple point (13.2).
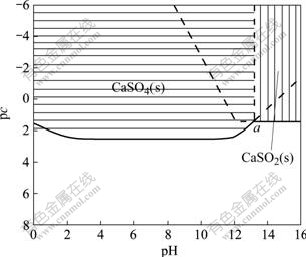
Fig.3 Diagram of pc—pH of CaSO4-Ca(OH)2-H2O system at 298.15 K
Fig.3 also indicates that Ca(OH)2(s) dissolves into the solution and CaSO4 precipitates in the ternary system located on the equilibrium line at the pH value below the triple equilibrium point. Reversely, Ca(OH)2 is preponderance at the pH value over the triple equilibrium point.
3 Conclusions
1) The pc—pH diagrams of CaSO4-Ca(OH)2-H2O system and its subsystem at 298.15 K are constructed according to the theory of thermodynamic equilibrium. The interaction characteristics between the solubility of CaSO4(s) and Ca(OH)2(s) can be found out from diagrams.
2) CaSO4(s), Ca(OH)2(s) and solution coexist at the pH value of about 13.2. In the solution equilibrated with CaSO4(s), the minimum solubility is 0.411 g/L at the pH value below 13.2, while the solution is in equilibrium with Ca(OH)2(s) and its minimum solubility is 2.749 g/L at the pH value over 13.2.
References
[1] WANG Guang-xing, GUO Lian-cai. Effective method of control SO42- content [J]. China Chlor-Alkali, 2006(1): 6-7. (in Chinese)
[2] GU Min, FU Bian-hong, YANG Ming-li. Progress in electroplating of copper from sulfate bath [J]. Journal of Materials Protection, 2006(1): 44-48. (in Chinese)
[3] WANG Hua, ZENG Zhen-ou, ZHAO Guo-peng, HU Yao-hong. Study on the process of thick chromium plating and coating performance from trivalent chromium sulfate electrolyte [J]. Electroplating & Finishing, 2007, 26(6): 13-17. (in Chinese)
[4] WU Hui-min, KANG Jian-qiang, ZUO Zheng-zhong, LIANG Guo-dong, ZHAO Guo-peng. Study of electroplating with trivalent chromium in sulfate system [J]. J Wuhan Univ (Nat Sci Ed), 2004, 50(2): 187-191. (in Chinese)
[5] WANG Xin-wen. Survey on treatment of acidic wastewater from large smelters in china [J]. Mining and Metallurgy, 2000, 9(2): 84-88. (in Chinese)
[6] LI Ying. Theory and practice of heavy metal industrial wastewater treatment recycle [J]. Hunan Nonferrous Metals, 2003, 19(2): 46-48.
[7] WANG Xiang-ping. Application of acidic waste water after treatment in non-ferrous hydrometallurgical enterprise [J]. Energy Saving of Non-ferrous Metallurgy, 2003, 20(4): 34-36. (in Chinese)
[8] MA Hong-wen, WANG Ying-bin, WANG Fang, SU Shuang-qing, LIU Hao, PENG Hui, YU Zi-jian. Chemical equilibrium in silicate system (PartⅡ): Reaction thermodynamics [J]. Geoscience, 2006, 20(3): 386-398. (in Chinese)
[9] LONG Cheng-ming, HU Zhong-ming. SO42- disposal process by calcium method [J]. China Chlor-Alkali, 2004(5): 18-20. (in Chinese)
[10] SUN Xiao-chun. Experiment of sulfate disposal by calcium chloride [J]. China Chlor-Alkali, 2000(5): 10-11. (in Chinese)
[11] YAO Yun-bin, XIE Tao, GAO Ying-min. Handbook of chemistry and physics [M]. Shanghai: Shanghai Scientific and Technical Publishers, 1985: 777-784, 812-817, 838-901. (in Chinese)
[12] JOHN A D. Lange’s chemistry handbook [M]. SHANG Jiu-fang, trans. Beijing: Science Press, 1991: 9-1-9-25. (in Chinese)
[13] IHSAN B. Thermochemical data of pure substances [M]. CHENG Nai-liang, trans. Beijing: Science Press, 2003: 623-765.
[14] WAGMAN, DONALD D. Selected values of chemical thermodynamic properties: Compounds of uranium, protactinium, thorium, actinium, and the alkali metals [M]. Washington: Department of Commerce, 1981: 152-156.
[15] SUN Fang, ZHAO Zhong-wei. Thermodynamic analysis on calcium phosphate system [J]. Rare Metals and Cemented Carbides, 2006, 34(2): 35-39. (in Chinese)
[16] CHEN Shao-yan. Aquatic chemistry [M]. Beijing: Irrigation and Electrics Press, 1989: 58-72, 84-97, 103-110. (in Chinese)
[17] FU Chong-shuo. Thermodynamic principles and calculation of metallurgical solution [M]. Beijing: Metallurgical Industry Press, 1989: 215-243. (in Chinese)
[18] ZHENG Qing-kou. Inorganic chemistry [M]. Beijing: China Industry Press, 1965: 121-165. (in Chinese)
Foundation item: Project(2007BAC25B01) supported by the National Key Project of Scientific and Technical Supporting Programs Funded by Ministry of Science and Technology of China during the 11th Five-year Plan
Corresponding author: WANG Yun-yan; Tel: +86-731-8830875; E-mail: wyy@mail.csu.edu.cn.
DOI: 10.1016/S1003-6326(08)60260-5
(Edited by LI Xiang-qun)