Effects of acidity,temperature and surfactants on electrochemical behavior of V5+ ion in sulfuric acid solutions
来源期刊:中国有色金属学报(英文版)2003年第6期
论文作者:易清风 刘云清 赵红钢 周秀林 刘小平 宋和付
文章页码:1430 - 1434
Key words:V5+; redox couple; voltammetry; electrochemistry;
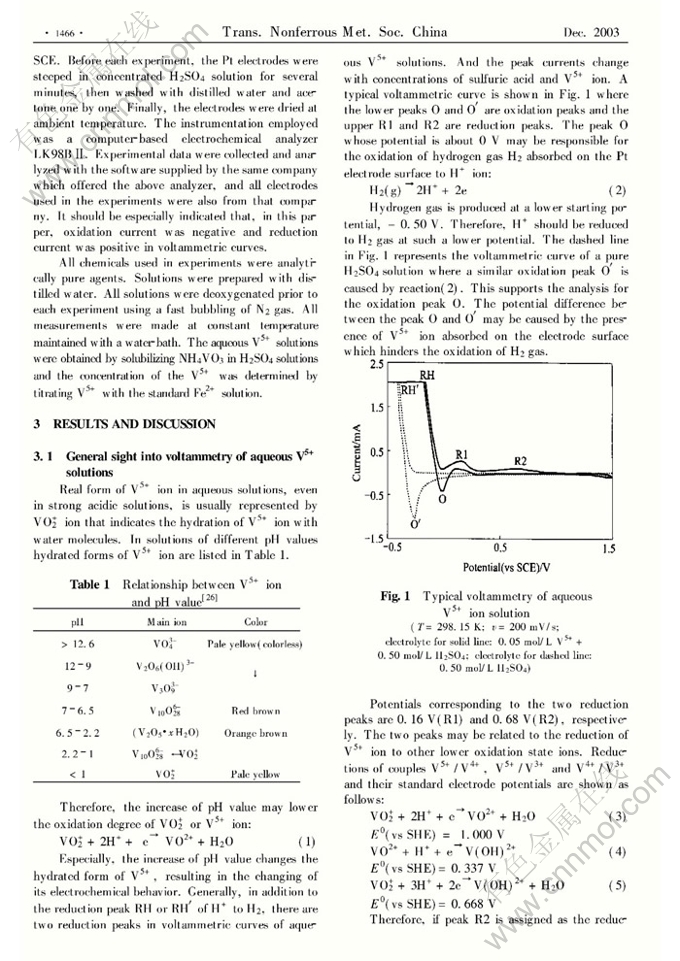
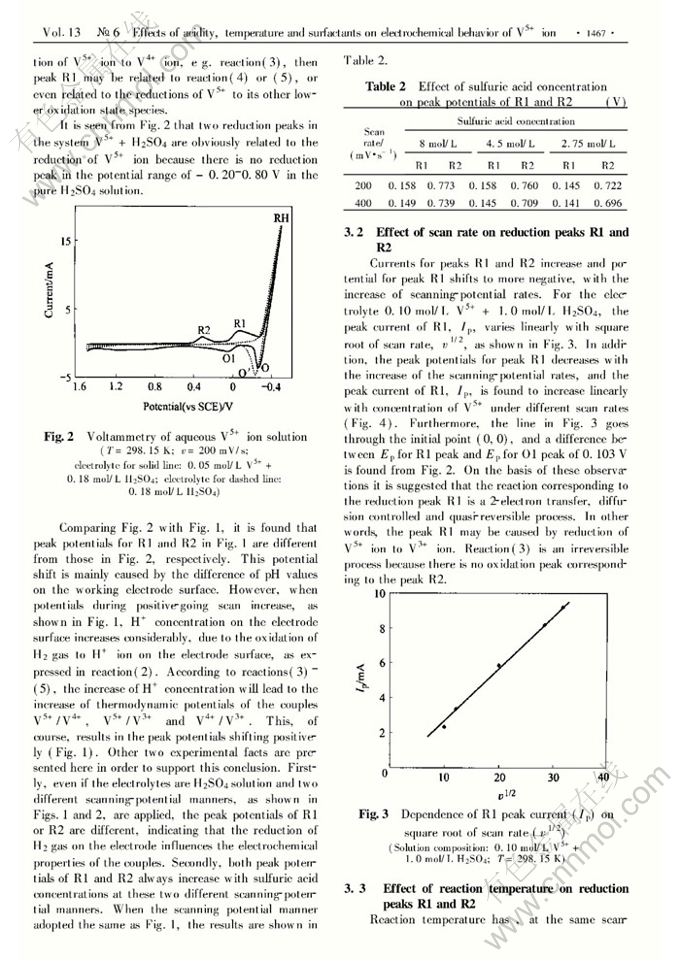
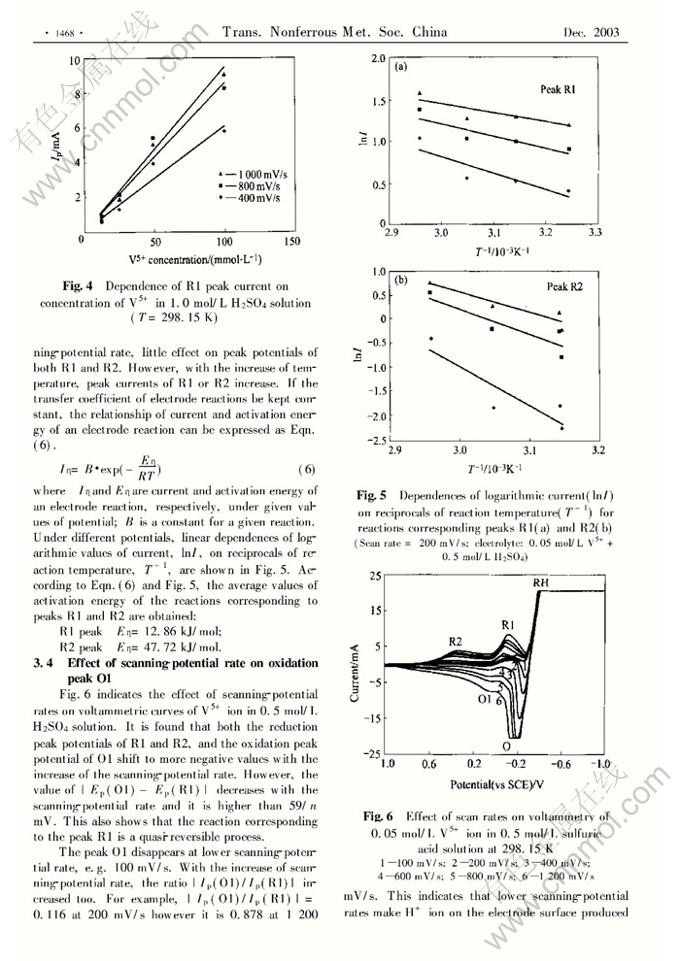
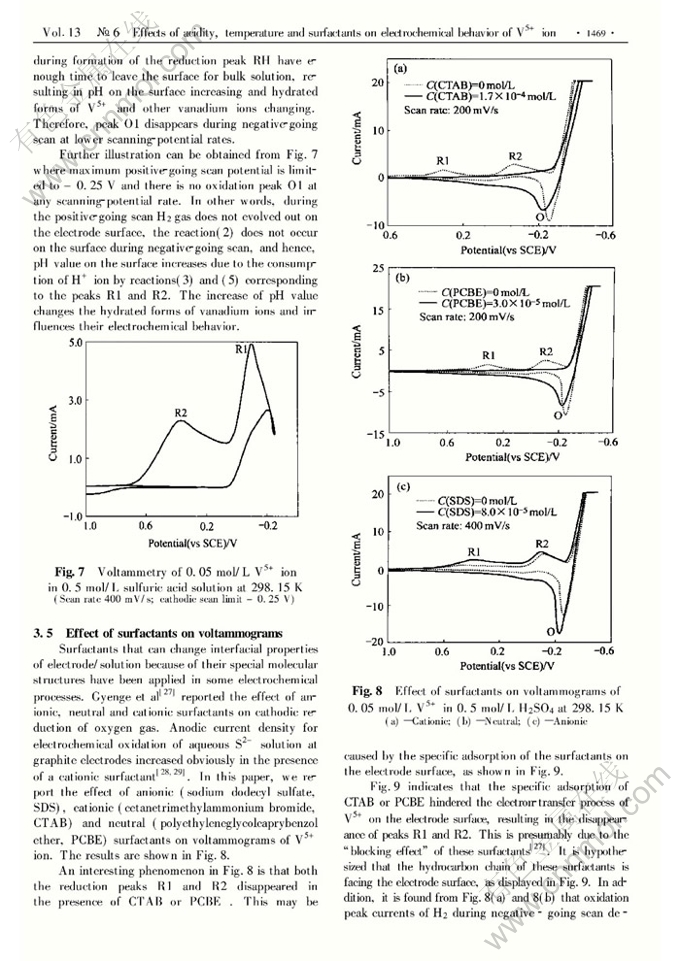
Abstract: The effects of sulfuric ac.id concentration, reaction temperature, potentiaFscanning rate and surfactants on electrochemical behavior of V5+ ion on platinum electrodes were investigated. In voltammetric curves of V5+ ion there are two reduction peaks corresponding to reductions of V5+ to V4+ ( R2) and V5+ to V3+ ( Rl) , which are irreversible and quasrreversible processes respectively. Oxidation peak of V3+ to V5+ is intensively affected by pH values on the electrode surface and scanning-potential rates. Only stronger ac,idity on the electrode surface and faster scanning- potential rates can lead to appearance of this oxidation peak. The neutral surfactant( PCBE) and cationic surfactant( CTAB) retard the V5+ electroreduction. The anionic surfactant( SDS) , even at a very low concentration, increases the currents of both the reduc- tion peaks Rl and R2, and the oxidation peak involves with the oxidation of H2 to H+ .