Effect of Friedel’s salt on strength enhancement of stabilized chloride saline soil
来源期刊:中南大学学报(英文版)2017年第4期
论文作者:黄新 程寅 李战国 白晓红
文章页码:937 - 946
Key words:chloride saline soil; stabilized soil; Friedel’s salt; strength; enhancement effect
Abstract: In the field of soil stabilization, only calcium silicate hydrate (CSH) and ettringite (AFt) as hydration products have been reported to directly contribute to the strength enhancement of the soil. A chloride dredger fill, an artificial chloride saline soil, and a non-saline soil were stabilized by Portland cement (PC) and PC with Ca(OH)2 (CH) with different contents. A series of unconfined compressive strength (UCS) tests of stabilized soil specimen after curing for 7 d and 28 d were carried out, and the hydration products and microstructure of the specimens were observed by X-ray diffractometry (XRD), scanning electronic microscopy (SEM), and energy-dispersive X-ray analysis (EDXA). The results showed that the strengths of PC+CH-stabilized chloride saline soils were much higher than those of PC-stabilized soils. A new hydration product of calcium aluminate chloride hydrate, also known as Friedel’s salt, appeared in the PC+CH-stabilized chloride saline soils. The solid-phase volume of Friedel’s salt expanded during the formation of the hydrate; this volume filled the pores in the stabilized soil. This pore-filling effect was the most important contribution to the significantly enhanced strength of the PC+CH-stabilized chloride saline soils. On the basis of this understanding, a new optimized stabilizer was designed according to the concept that the chloride in saline soil could be utilized as a component of the stabilizer. The strength of the chloride saline soils stabilized by the optimized stabilizer was even further increased compared with that of the PC+CH-stabilized soils.
Cite this article as: CHENG Yin, LI Zhan-guo, HUANG Xin, BAI Xiao-hong. Effect of Friedel’s salt on strength enhancement of stabilized chloride saline soil [J]. Journal of Central South University, 2017, 24(4): 937-946. DOI: 10.1007/s11771-017-3496-7.
J. Cent. South Univ. (2017) 24: 937-946
DOI: 10.1007/s11771-017-3496-7
CHENG Yin(程寅)1, LI Zhan-guo(李战国)1, HUANG Xin(黄新)1, BAI Xiao-hong(白晓红)2
1. School of Transportation Science and Engineering, Beihang University, Beijing 100191, China;
2. College of Architecture and Civil Engineering, Taiyuan University of Technology, Taiyuan 030024, China
 Central South University Press and Springer-Verlag Berlin Heidelberg 2017
Central South University Press and Springer-Verlag Berlin Heidelberg 2017
Abstract: In the field of soil stabilization, only calcium silicate hydrate (CSH) and ettringite (AFt) as hydration products have been reported to directly contribute to the strength enhancement of the soil. A chloride dredger fill, an artificial chloride saline soil, and a non-saline soil were stabilized by Portland cement (PC) and PC with Ca(OH)2 (CH) with different contents. A series of unconfined compressive strength (UCS) tests of stabilized soil specimen after curing for 7 d and 28 d were carried out, and the hydration products and microstructure of the specimens were observed by X-ray diffractometry (XRD), scanning electronic microscopy (SEM), and energy-dispersive X-ray analysis (EDXA). The results showed that the strengths of PC+CH-stabilized chloride saline soils were much higher than those of PC-stabilized soils. A new hydration product of calcium aluminate chloride hydrate, also known as Friedel’s salt, appeared in the PC+CH-stabilized chloride saline soils. The solid-phase volume of Friedel’s salt expanded during the formation of the hydrate; this volume filled the pores in the stabilized soil. This pore-filling effect was the most important contribution to the significantly enhanced strength of the PC+CH-stabilized chloride saline soils. On the basis of this understanding, a new optimized stabilizer was designed according to the concept that the chloride in saline soil could be utilized as a component of the stabilizer. The strength of the chloride saline soils stabilized by the optimized stabilizer was even further increased compared with that of the PC+CH-stabilized soils.
Key words: chloride saline soil; stabilized soil; Friedel’s salt; strength; enhancement effect
1 Introduction
In the coastal areas of China, more than 1×104 km2 of saline soil has formed by the long-term impregnation of sea water [1]. With large-scale reclamation projects underway in Chinese coastal provinces and cities, reclaimed areas have expanded by more than 100 km2 per year since 2005 [2]. Many reclaimed areas were formed by saline mud dredged from the seabed, which produced more large areas of new saline soil. For example, the dredged filled-in reclaimed land in the Tianjin Binhai New Area, China, contained salinity levels as high as 8%. The salinity of coastal saline soil can be between 1.5% and 10%, in which the chloride is dominant; the strength and deformation modulus of this variety of soil are poor [3, 4]. With the implementation of the east-coastal development strategy launched by the Chinese government, many large infrastructure projects, including traffic engineering, ports, nuclear power stations, and steel mills have begun in coastal areas. Some of these facilities are inevitably built on saline soil ground; thus, a solution to the treatment of soft saline soil ground is now urgently needed.
As a cost-effective ground treatment technology, soft-soil stabilization is used in saline soil treatment [5-8], but most research on saline soil stabilization has focused on sulfate saline soil [9-11], with few studies focusing on chloride saline soil [12]. The Central Japan International Airport was built on an artificial reclamation island constructed with stabilized chloride saline soil, which was produced by mixing dredger fill and a stabilizer [13]. CHEW et al [14] and KAMRUZZAMAN et al [15] used cement to stabilize Singapore marine clay, and evaluated the microstructural behavior of the cement-treated clay through scanning electron microscopy (SEM) and X-ray diffraction (XRD) tests. The results indicated that the properties and behavior of cement-treated marine clay can be explained by the interaction of multiple microstructural mechanisms. ELAKNESWARAN et al [16] investigated the surface charge mechanism of cement hydrates and its relations to adsorption of chloride ions. The conclusion was that the chloride ions can react with cement to form some chloride-bearing hydrates and delay the development of calcium silicate hydrate (CSH). LUO [17] attempted the use of cement to stabilize chloride saline soil in the Tianjin Binhai New Area, but the results showed that the cement-stabilized chloride saline soil was unable to meet the engineering requirements of highway sub-grade areas. XING et al [18] reported that the mixing ratio of cement needed to stabilize seashore saline soil was much higher than that for stabilizing ordinary soil because of the adverse effects of chloride ions. ZHANG et al [19] evaluated the influence of salt concentrations on the cement-stabilized clay by electrical resistivity measurements; the result indicated that chloride had a detrimental effect on the strength of the cement-stabilized clay. Cement stabilization is still a major stabilization method for seashore saline soil, but the engineering properties and economic benefits of cement stabilization are not satisfactory because of the adverse effects of the salts [20, 21].
The performance of stabilized soil depends mainly on the composition of the soil and the structure formed by the hydration products of the stabilizer. Only two hydration products, CSH and ettringite (AFt), have been reported to have direct contributions to the strength of stabilized soil [22, 23]. When using Portland cement (PC) to stabilize soil, the major hydration product is CSH, which wraps around and binds together the soil particles to form a structure that contributes strength to the stabilized soil [24, 25]. By using stabilizer containing gypsum and alumina-bearing phases to stabilize some kinds of soil, AFt can be formed in the stabilized soil, and the volume expansion that occurs during the formation of AFt can fill the pores in the soil, and further improve the final strength [26, 27].
LEA [28] and TAYLOR [22] reported that NaCl could react with tricalcium aluminate (C3A, a cement clinker mineral) to form calcium aluminate chloride hydrate (Friedel’s salt; the chemical formula is 3CaO·Al2O3·CaCl2·10H2O), which is similar to volume expansion of AFt, the solid-phase volume of Friedel’s salt expands during the formation of the hydrate [29, 30]. Hence, a new idea for a stabilizer design is proposed. Using the chloride in saline soil as a component of the stabilizer, promoting the formation of Friedel’s salt in the stabilized soil with other aluminum-bearing components to increase the strength of the stabilized soil. In this work, the strength enhancement effect of Friedel’s salt in chloride saline soils is investigated.
2 Materials and experimental methods
2.1 Materials
2.1.1 Soil samples
Soil sample TT was composed of a chloride saline dredger fill obtained from the reclaimed land at 1.0-2.0 m depth below the ground surface in the Tianjin Binhai New Area, China. According to “Specifications for Design of Highway Subgrades” (JTG D30-2004), TT was a ultra-chloride saline soil. Soil sample BT was prepared by adjusting the moisture content of a soil collected from Beijing to match that of TT. The artificial chloride saline soil sample BT-N was prepared by mixing the soil of BT with 1% (mass fraction) NaCl, with the final Cl- content being approximately 50% of the TT. The physics and mechanics properties, major ions content and pH value of the soil samples are shown in Table 1 and Table 2, respectively.
2.1.2 Stabilizers
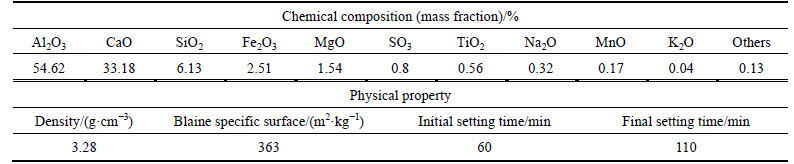
Stabilizer PC was P.C.32.5 Portland cement produced by the Beijing Qiangli Cement Plant, while stabilizer PC+CH was composed of PC and Ca(OH)2 (CH). CH (chemically pure) was supplied by the Beijing Yili Fine Chemicals Co., Ltd. The chemical composition and physical properties of PC are given in Table 3.
2.2 Experimental methods
Stabilized soil specimens were prepared by mixing the soil samples with the stabilizers PC and PC+CH at dosages of aw=10%, 15%, 20%, and 23.3% (aw: mass ratio of dry stabilizer to wet soil). The details of the stabilized soil specimens are given in Table 4. The water-stabilizer ratio (mass ratio of water to the dry stabilizer) was set to be 0.5. The unconfined compressive strength (UCS) of the specimens was measured, and the hydration products and microstructures of specimens TP-2 and TC-2 were analyzed by XRD, SEM, and energy-dispersive X-ray analysis (EDXA).
Table 1 Physical and mechanical properties of soil samples

Table 2 Major ions content and pH value of soil samples
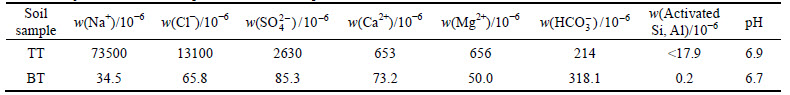
Table 3 Chemical composition and physical properties of PC
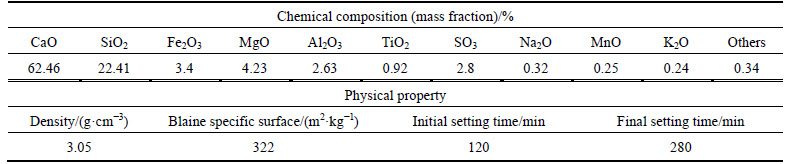
Table 4 Details and UCS values of stabilized soil specimens after 7 d and 28 d curing
The stabilized soil specimens were made according to the “Test Methods of Materials Stabilized with Inorganic Binders for Highway Engineering” (JTG E51-2009). The soil mixing apparatus was the SJ-160 mortar mixer, and the specimen preparation procedures were as follows:
1) According to Table 4, the soil sample and the stabilizer were weighed and mixed in a mixer pot.
2) The preparation for the mix included the following three steps: (1) mixing at low speed (60 r/min) for 30 s, (2) a high-speed (120 r/min) mixing for 60 s and a 30 s pause to scratch the samples from the mixing blade with a small shovel, and put the pot back into the mixer, and (3) another high-speed (120 r/min) mixing for 120 s.
3) The mixture was poured into a 50 mm×50 mm× 50 mm steel mold with 3 layers, then shaped completely, and covered with plastic film on the surface.
4) The specimen was carefully extracted after 24 h, and then stored in the curing room for the required curing time, as specified below, with a temperature of (20±2) °C and humidity ≥95%.
The UCS values of specimens after 7 d and 28 d of curing were tested based on the standard JTG E51-2009. The UCS of the stabilized soil was measured by the LQ-100S Pavement Material Strength Tester produced by the Beijing Tool Factory with a loading rate of 1 mm/min.
XRD analysis was performed on some of the specimens; a Rigaku diffractometer D/max-rc and a graphite monochromator were used with Cu Kα1 radiation of wavelength 1.5418  . SEM and EDXA tests were performed with an S-530 Hitachi Scanning Electron Microscope operated at 15 kV voltage. Specimens for XRD, SEM, and EDXA testing were prepared as follows: (1) The specimens were collected from central portion of the stabilized soil which had just undergone a UCS test; (2) all specimens were oven-dried under a temperature of 60 °C for 8 h; (3) the carbonized surfaces of the specimens were removed and the internal parts were made into 5 mm×5 mm×5 mm pieces, then the specimens were covered with powdered gold using a vacuum coating machine for SEM and EDXA tests, and pulverized and sieved using a No. 320 sieve for XRD testing.
. SEM and EDXA tests were performed with an S-530 Hitachi Scanning Electron Microscope operated at 15 kV voltage. Specimens for XRD, SEM, and EDXA testing were prepared as follows: (1) The specimens were collected from central portion of the stabilized soil which had just undergone a UCS test; (2) all specimens were oven-dried under a temperature of 60 °C for 8 h; (3) the carbonized surfaces of the specimens were removed and the internal parts were made into 5 mm×5 mm×5 mm pieces, then the specimens were covered with powdered gold using a vacuum coating machine for SEM and EDXA tests, and pulverized and sieved using a No. 320 sieve for XRD testing.
3 Results
3.1 UCS test results
The UCS values of the stabilized soil specimens after 7 d and 28 d curing are presented in Table 4. With the same aw and curing time, the strengths of PC+CH- stabilized TT samples are much higher than those of PC-stabilized TT samples. As for soil BT, the strengths of PC+CH-stabilized soils are almost the same as those of PC-stabilized soils. For the specimen stabilized with PC+CH, the strength of BT-N sample is much higher than those of BT.
3.2 XRD analysis
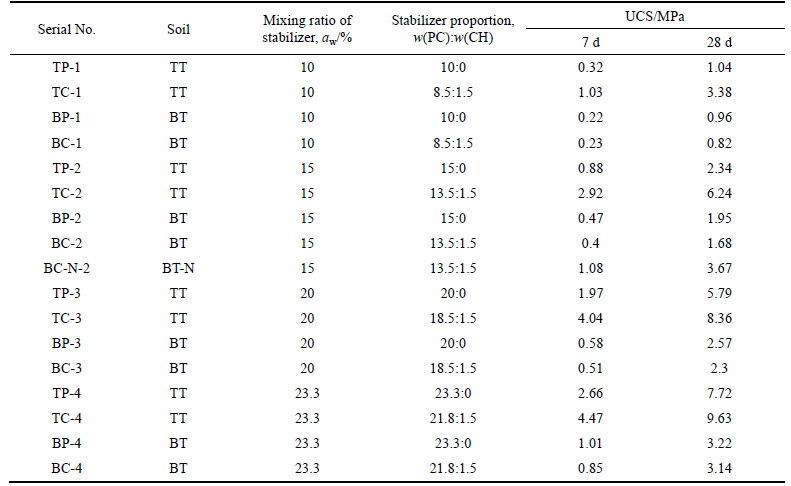
The XRD patterns of soil sample TT and specimens TP-2 and TC-2 after 1, 3, 7, and 28 d aging are presented in Fig. 1, and the XRD patterns of soil samples BT and BT-N and specimens BC-2 and BC-N-2 after 28 d aging are presented in Fig. 2. The main characteristic peaks corresponding to Friedel’s salt, NaCl, and CH are indicated in Fig. 1 and Fig. 2. In order to quantify the Friedel’s salt formed, the integral area of its most characteristic peak of the XRD pattern has been determined [31], and the semi-quantitative analysis results of Friedel’s salt in the stabilized soils after different curing times are presented in Fig. 3.
Figure 1 shows that the intensity of characteristic peak of Friedel’s salt is significant in the patterns of TC-2 at both 7 and 28 d of age, whereas it is rather weak in the pattern only from 28 d-aged TP-2, and the peak does not appear in the pattern from TP-2 after 7 d curing. In addition, the characteristic peaks of NaCl and CH in the XRD pattern of TC-2 are not present in the scans from the 7 d and 28 d aged specimens. In Fig. 2, the characteristic peak of Friedel’s salt appears in the XRD pattern of BC-N-2 but not in that of BC-2. The characteristic peaks of CH are retained in BC-2 but not in BC-N-2, whereas the characteristic peaks of NaCl do not appear in the XRD pattern of BC-N-2.
As shown in Fig. 3, the integral areas of most characteristic peak of Friedel’s salt in TC-2 and BC-N-2 after 28 d curing are much higher than those in TP-2 and BC-2; this suggests that Friedel’s salt is formed plentifully in TC-2 and BC-N-2, but only a small amount is formed in TP-2, whereas none forms in BC-2.
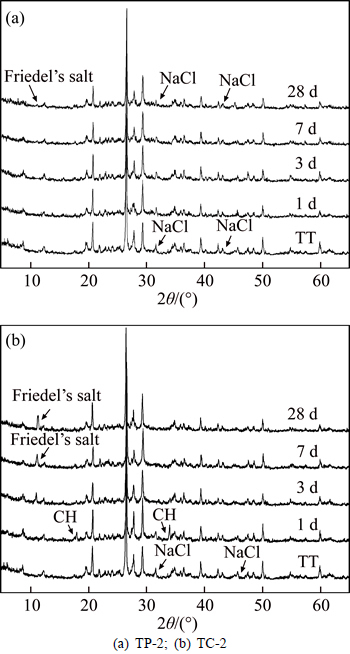
Fig. 1 XRD patterns of stabilized TT samples:
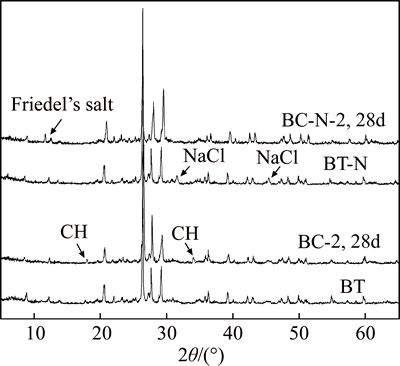
Fig. 2 XRD patterns of stabilized BT samples
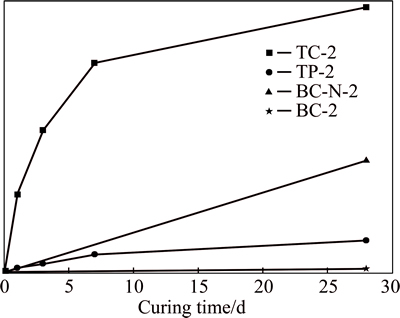
Fig. 3 Semi-quantitative analysis of Friedel’s salt in stabilized soil specimens
3.3 SEM analysis
The original SEM images of specimens TP-2 and TC-2 after 7 d curing are presented in Figs. 4(a)-(d). The images in Figs. 4(c) and (d) were processed by Image-Pro Plus (IPP) 6.0 software, and the binary images are shown in Figs. 4(c1) and (d1).
TC-2 is relatively compact while TP-2 is loose and porous, as seen in Figs. 4(a) and (b). The hydrates in TP-2 appear to be flocculated, which is related to the flocculated gel of CSH [32, 33], whereas in TC-2, many plate-like crystals appear in addition to the flocculated gel, as shown in Figs. 4(c) and (d). According to the reference [34], the morphology of Friedel’s salt is flat hexagonal or pseudohexagonal crystals; the plate-like crystals observed in specimen TC-2 may be Friedel’s salt.
As shown in Figs. 4(c1) and (d1), the basic situation of pore of images in Figs. 4(c) and (d) was determined by binarization analysis of IPP 6.0 software. The total pore count of TP-2 is more than that of TC-2, and the medium pores and macro pores in TP-2 are obviously more than those of TC-2 [8].
3.4 EDXA results
The EDXA images and results of TP -2 and TC-2 after 7 d curing are presented in Fig. 5, Tables 5 and 6, respectively. As shown in Fig.5, the morphologies of the microstructure at Points 1 and 2 are flocculated gel, and the morphologies of the microstructure at Points 3 and 4 are plate-like crystals. Tables 5 and 6 show that the level of Cl in the two locations of TC-2 is much greater than that in TP-2. The mass ratios of Cl, Al to Ca at different locations of TP-2 and TC-2 are listed in Table 7.
According to Ref. [35], the major elemental mass ratio of Friedel’s salt is w(Cl):w(Al):w(Ca)=1:0.76:2.25, and the original mass ratios of w(Cl):w(Al):w(Ca) in the two locations of the TP-2 specimen tested by EDXA are far from this specified ratio. Thus, it can be extrapolated to the conclusion that Friedel’s salt does not form sufficiently in TP-2, and the flocculated gel in TP-2 approaches the composition of CSH with a ratio of calcium to silicon falling in the range of 0.8-1.2. In contrast with TP-2, the original mass ratios of w(Cl):w(Al):w(Ca) in the two locations of the TC-2 specimen tested by EDXA are somewhat similar to those of Friedel’s salt, which could indicate that some Friedel’s salt may be formed in TC-2. Point 4 of TC-2 is chosen as an example for discussion. With the analysis scanning limit of the spectrum analyzer, the detected substances may include other hydrates, such as CSH, which may cause the test results to deviate. In order to exclude this possible deviation in analysis, according to the reference [36, 37], it was assumed that all Si is formed into a CSH gel with the w(Ca)/w(Si) ratio of 1.0. With the corresponding Ca mass deleted, the remaining elemental mass ratios can be calculated. A relatively accurate regulative mass ratio of w(Cl):w(Al):w(Ca)=1:0.72:2.15 is acquired, which is very similar to the elemental mass ratio of Friedel’s salt. By the same method, the regulative mass ratio of w(Cl):w(Al):w(Ca) at point 3 of TC-2 is about 1:0.88:2.4, which is also approximate to that of Friedel’s salt. Thus, it can be seen that the plate-like crystals in TC-2 are Friedel’s salt.

Fig. 4 Original SEM images (a)-(d) and binary images (c1)-(d1) of stabilized soil specimens:

Fig. 5 EDXA images:
Table 5 EDXA results of TP-2 (Mass fraction, %)

Table 6 EDXA results of TC-2 (Mass fraction, %)

Table 7 Mass ratios of Cl, Al, and Ca in different locations

4 Discussion
4.1 Relation between strength enhancement of stabilized soils and formation of Friedel’s salt
As shown in Table 4, the strengths of the TC specimens are much higher than those of TP with the same aw. As both specimens TP and TC use soil sample TT, the only difference between them is the stabilizer; thus, the strength difference between them must be caused by the different hydration products formed in the stabilized soils. The strengths of stabilized soil specimens BC and BP are similar, indicating that the hydration products produced by the two stabilizers in soil sample BT are not significantly different. With the same stabilizer, the stabilization effects observed in soil samples TT and BT are different. This phenomenon is mainly caused by the difference of salt ion contents in TT and BT, so it can be deduced that PC+CH may have reacted with some salt in soil TT to form some new hydration products. Some experimental evidence and discussion are provided to confirm this deduction.
Soil TT contains a high level of NaCl. NaCl can react with CH and C3A in the PC to form a new hydration product of 3CaO·Al2O3·CaCl2·10H2O (that is, Friedel’s salt). The chemical formula is as follows [38]:
3CaO·Al2O3+Ca(OH)2+2NaCl+10H2O→3CaO·Al2O3·CaCl2·10H2O+2NaOH (1)
The significant intensity of the characteristic peaks arising from Friedel’s salt, observed in Fig. 1(b), and the semi-quantitative analysis results in Fig. 3 prove the existence of Friedel’s salt in TC-2, with additional confirmation by the results of SEM and EDXA.
Based on the above analysis, the appearance of Friedel’s salt formed by the reaction of NaCl, CH, and C3A may be correlated to the strength enhancement observed in the TC series of specimens. This correlation is also supported by the fact that although the strengths of stabilized soil series BC and BP were similar, indicating that PC+CH is not better than PC to stabilize soil sample BT, the strength of specimen BC-N-2 is 1.2 times higher than that of BC-2. The only difference between the specimens is the 1% NaCl content in BC-N; however, Friedel’s salt is confirmed to have formed in BC-N-2 but not in BC-2, as shown in Fig. 2 and Fig. 3.
Figure 6 illustrates the UCS values of the specimens of the TC series, TP series, and BP series in Table 4 according to the amount of stabilizer added. A reasonable explanation for the phenomena demonstrated in Fig. 6, as follows, will provide further support to the above correlation.

Fig. 6 28 d UCS values of stabilized soil specimens at different aw values
The physics properties of unmodified BT are slightly better than those of TT. Thus, if the hydration products in the stabilized TP and BP soils are the same, the strengths of the two stabilized soils should be similar.
The hydration products of PC contain mainly CSH and CH. CSH is the main contributor to the strength of stabilized soil, and CH has little direct contribution to the strength [39]. However, the CH in the stabilized soil may be under-saturated, because the soil may consume much Ca2+ and OH– by ion exchange, physical adsorption, and coagulation reactions [40]. Using PC to stabilize TT, when aw=10%, the CH produced by PC might be consumed completely, meaning that no extra CH is available to react with NaCl and C3A to form Friedel’s salt. In this case, the hydration products that could affect strength in TP are similar to those in BP, indicating that the strengths of the two stabilized soils should be similar. When aw=15%, the CH produced by PC may have some surplus over what is consumed by the soil. This CH could react with NaCl and C3A to form a small amount of Friedel’s salt (as shown in the 28 d XRD patterns in Fig. 1(a)), which makes the strength of TP slightly higher than that of BP. When aw=20%-23.3%, the CH produced by PC is abundant, thus more Friedel’s salt is produced, which makes the strength of TP much greater than that of BP. However, the CH is still not sufficient to match C3A for the full formation of Friedel’s salt, thus the strength of TP is lower than that of TC.
When using PC+CH to stabilize soil sample TT, there is enough CH to match C3A to fully form Friedel’s salt, and the strength of TC is much higher than that of TP. As aw increases, the amount of C3A increases along with the amount of Friedel’s salt produced, so the strength increment between TC and BP correspondingly increases.
4.2 Strength enhancement mechanism of Friedel’s salt
The solid-phase volume increment of Friedel’s salt during formation can be calculated by the method used for calculating that of AFt [41]. The specific calculation is as follows:
3CaO·Al2O3+Ca(OH)2+2NaCl+10H2O→3CaO·Al2O3·CaCl2·10H2O+2NaOH (1)
The calculation values for solid-phase volume increments are given in Table 8.
Table 8 Calculation values for solid-phase volume increments
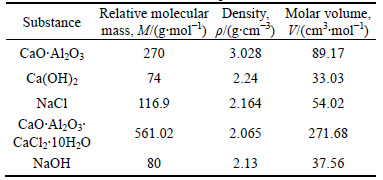
The calculation of solid-phase volume increments is as follows:
[(271.68+37.56-89.17-33.03-54.02)/(89.17+33.03+54.02)]×100%=75.5%
This indicates that the solid-phase volume of Friedel’s salt expands by 75.5% during the formation of the hydrate. The solid-phase volume expansion of AFt causes the pores in stabilized soil to be filled, thus densifying the structure of the stabilized soil and increasing the strength of the final product [42]. Similarly, the expansion of Friedel’s salt may also fill the pores in stabilized soil and thus increase the strength. This interpretation is supported by original SEM images (b) and (d) and binary image (d1) in Fig. 4, which indicate that the microstructure of specimen TC is more compact than that of TP. Based on these analyses, it is concluded that the expansion of Friedel’s salt filling pores in the stabilized soil is one of the major mechanisms underlying the strength enhancement of the soil.
4.3 Further discussion
The above analysis shows that the NaCl in the saline soil can react with the stabilizer to form Friedel’s salt, which can significantly increase the strength of stabilized soil. Hence, a new idea for optimizing the design of soil stabilizer is proposed, in which the chloride in saline soil is utilized as a component of the stabilizer.
For chloride saline soil stabilization, the PC+CH stabilizer may not be optimal. According to Eq. (1) and calculation of solid-phase volume increments, the amount of the alumina-bearing phases in PC+CH is a controlling factor over the final strength. The limited amount of alumina-bearing phases in PC+CH may hinder the increased production of Friedel’s salt, thus inherently limiting the increase in the strength of the stabilized soil. If the interpretation that Friedel’s salt enhances the strength of stabilized soil is correct, the strength of stabilized soil could be increased further by optimizing the stabilizer's composition. Moderately increasing the amount of alumina-bearing phases in the stabilizer would correlate to a moderate increase in the amount of Friedel’s salt produced. In order to confirm the above deduction, the following experiment was conducted.
The stabilizer was optimized by moderately increasing the amount of the alumina-bearing phase, as given in Table 9. The alumina-bearing phase was high- alumina cement (AC) produced by the Beijing Special Cement Plant, with chemical composition and physical properties of AC presented in Table 10. The total mixing ratio of the stabilizer was set to be aw=15%, and the water-stabilizer ratio was set to be 0.5. The other materials and the test procedures were the same as those listed above.
Table 10 shows that the AC contains high levels of Al2O3, and the results in Table 9 show that the strength of TA-2 is higher than that of TC-2, and the strength of BA-N-2 is also higher than that of BC-N-2. This proves that the strength of the stabilized soil can be enhanced further by optimizing the composition of the stabilizer, increasing the amount of alumina-bearing phase present to permit the increased production of Friedel’s salt. These results also confirm that Friedel’s salt, formed by the reaction of NaCl in saline soil with the stabilizer, can enhance the strength of stabilized saline soil.
Table 9 Test programs and results
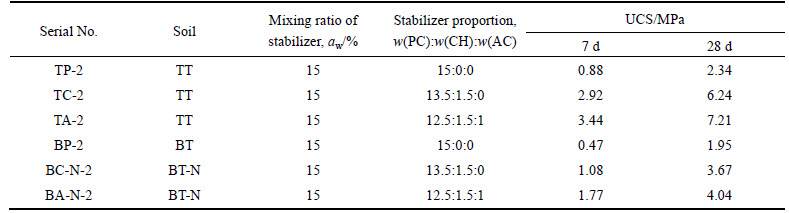
Table 10 Chemical composition and physical properties of AC
5 Conclusions
1) A chloride dredger fill, an artificial chloride saline soil, and a non-saline soil were stabilized by PC and PC+CH. The results showed that the strengths of PC+CH-stabilized chloride saline soils are higher than those of PC-stabilized chloride saline soils, whereas the performances of PC+CH and PC differ only slightly for stabilizing non-saline soil.
2) The stabilized chloride saline soil specimens were analyzed by XRD, SEM, and EDXA; the results show that the difference between PC+CH-stabilized chloride saline soil specimen and PC-stabilized specimen is the formation of Friedel’s salt as a hydration product by the reaction between the stabilizer and NaCl in saline soil.
3) The solid-phase volume of Friedel’s salt expands by 75.5% during the formation of the hydrate; the Friedel’s salt fills the pores in the stabilized soil and thus densifies the structure of the stabilized soil. This behavior is the most important reason underlying the significantly enhanced strength of PC+CH-stabilized chloride saline soils.
4) A new idea for the design of a stabilizer was proposed to utilize the chloride in saline soil as a part of the composition of the stabilizer. An optimized stabilizer was made by moderately increasing the amount of alumina-bearing phase so as to moderately increase the amount of Friedel’s salt formed during stabilization. The strength of the optimized stabilizer-stabilized chloride saline soils exceeded that of the PC+CH-stabilized soils.
References
[1] WANG Zun-qin. China saline soil [M]. Beijing: Science Press, 1993: 250-256. (in Chinese)
[2] CHENG Yin, LI Zhan-guo, DENG Xiao-xuan, HUANG Xin. Experimental study on a new stabilizer for stabilizing coastal chlorine soil [J]. Chinese Journal of Geotechnical Engineering, 2011, 33(8): 1240-1245. (In Chinese)
[3] ZHU Jun-gao, YIN Jian-hua. Strain-rate-dependent stress-strain behavior of overconsolidated Hong Kong marine clay [J]. Canadian Geotechnical Journal, 2011, 37(6): 1272-1282.
[4] AHMED M A. Geotechnical characteristics of stabilized sabkha soils from the egyptian–libyan coast [J]. Geotechnical and Geological Engineering, 2015, 33(3): 1-6.
[5] MOAYED R Z, IZADI E, HEIDARI S. Stabilization of saline silty sand using lime and micro silica [J]. Journal of Central South University, 2012, 19(10): 3006-3011.
[6] WANG D, ABRIAK N E, ZENTAR R. Strength and deformation properties of Dunkirk marine sediments solidified with cement, lime and fly ash [J]. Engineering Geology, 2013, 166(9): 90-99.
[7] YU Hao, NING Jian-guo, ZHU Bao-lin. Effect of cation exchange capacity of soil on stabilized soil strength [J]. Soils and Foundations, 2014, 54(6): 1236-1240.
[8] HAN Peng-ju, WANG Shuai, FRANK C, BAI Xiao-hong. Mechanism of cement-stabilized soil polluted by magnesium sulfate [J]. Journal of Central South University, 2015, 22(5): 1869-1877.
[9] ALQASIMI A. Improving engineering properties of sabkha soils using Portland cement [D]. Columbia: University of South Carolina, 1993.
[10] AL-AMOUDI O S B. Characterization and chemical stabilization of Al-Qurayyah sabkha soil [J]. Journal of Materials in Civil Engineering, 2002, 14(6): 478-484.
[11] VASUDEV D. Performance studies on rigid pavement sections built on stabilized sulfate soils [D]. Arlington: The University of Texas, 2007.
[12] SALMAN F A, SABRE D K, AL-SAOUDI N S K. Compressibility characteristics of saline soils treated with cement [J]. International Journal of the Physical Sciences, 2011, 6(33): 7614-7628.
[13] KITAZUME M, SATOH T. Quality control in central Japan international airport construction [J]. Proceedings of the ICE-Ground Improvement, 2005, 9(2): 59-66.
[14] CHEW S H, KAMRUZZAMAN A H M, LEE F H. Physicochemical and engineering bahavior of cement treated clays [J]. Journal of Geotechnical and Geoenvironmental Engineering, 2004, 130(7): 696-706.
[15] KAMRUZZAMAN A H M, CHEW S H. Microstructure of cement-treated singapore marine clay [J]. Ground Improvement, 2006, 10(3): 113-123.
[16] ELAKNESWARAN Y, NAWA T, KURUMISAWA K. Electrokinetic potential of hydrated cement in relation to adsorption of chlorides [J]. Cement and Concrete Research, 2009, 39(4): 340-344.
[17] LUO Shu-hao. Study on comprehensive properties of curing chlorine saline soil in Tianjin Binhai new area [D]. Xi’an: Chang’an University, 2009. (in Chinese)
[18] XING Hao-feng, YANG Xiao-ming, XU Chao, YE Guan-bao. Strength characteristics and mechanisms of salt-rich soil–cement [J]. Engineering Geology, 2009, 103(3): 33-38.
[19] ZHANG Ding-wen, CAO Zhi-guo, FAN Li-bin, LIU Song-yu, LIU Wei-zheng. Evaluation of the influence of salt concentration on cement stabilized clay by electrical resistivity measurement method [J]. Engineering Geology, 2014, 170 (6): 80-88.
[20] LCPC-SETRA. Soil treatment with lime and/or hydraulic binders-application to the construction of fills and capping layers [M]. Paris: Laboratoire Central des Ponts et Chaussées, 2000: 22-56.
[21] YANG Xiao-ming. Microstructure and mechanism research on cement stabilized salt-rich clay [D]. Shanghai: Tongji University, 2006. (in Chinese)
[22] TAYLOR H F W. Cement chemistry [M]. London: Thomas Telford Ltd., 1997: 80-88.
[23] RAYMOND N Y, VAHID R O. Experimental study on instability of bases on natural and lime/cement-stabilized clayey soils [J]. Applied Clay Science, 2007, 35(20): 238-249.
[24] ONITSUKA K, MODMOLTIN C, KOUNO M. Investigation on microstructure and strength of lime and cement stabilized ariake clay [J]. Reports of the Faculty of Science and Engineering, Saga University, 2001, 30(1): 49-63.
[25] LIN Cheng, ZHU Wei, HAN Jie. Strength and leachability of solidified sewage sludge with different additives [J]. Journal of Materials in Civil Engineering, 2013, 25(11): 1594-1601.
[26] WILD S, KINUTHIA J M, JONES G I. Suppression of swelling associated with ettringite formation in lime stabilized sulphate bearing clay soils by partial substitution of lime with GGBS [J]. Engineering Geology, 1998, 51(4): 257-277.
[27] HUANG Xin, LI Zhan-guo, NING Jian-guo. Principle and method of optimization design for soft stabilizer [J]. Journal of Wuhan University of Technology. Materials Science Edition, 2009, 24(1): 155-161.
[28] LEA F M. The chemistry of cement and concrete [M]. London: Edward Arnold Ltd., 1956: 241-297.
[29] TRAETTEBERG A, GRATTAN-BELLEW P E. Hydration of 3CaO·Al2O3 and 3CaO·Al2O3+gypsum with and without CaCl2 [J]. Journal of the American Ceramic Society, 1975, 58(5): 221-227.
[30] BROWN P, BOTHE J. The system CaO-Al2O3-CaCl2-H2O at 23±2°C and the mechanisms of chloride binding in concrete [J]. Cement and Concrete Research, 2004, 34(9): 1549-1553.
[31] TALERO R, TRUSILEWICA L, DELGADO A. Comparative and semi-quantitative XRD analysis of Friedel’s salt originating from pozzolan and Portland cement [J]. Construction and Building Materials, 2011, 25(5): 2370-2380.
[32] RAO S N, RAJASEKARAN G. Reaction products formed in lime-stabilized marine clays [J]. Journal of Geotechnical Engineering, 1996, 122(5): 329-336.
[33] ZHA Fu-sheng, LIU Jing-jing, XU Long, CUI Ke-rui. Effect of cyclic drying and wetting on engineering properties of heavy metal contaminated soils solidified/stabilized with fly ash [J]. Journal of Central South University, 2013, 20(7): 1947-1952.
[34] ZHANG Dan-ni, JIA Yong-feng, MA Jia-yu, LI Zhi-bao. Removal of arsenic from water by Friedel’s salt (FS: 3CaO·Al2O3·CaCl2·10H2O) [J]. Journal of Hazardous Materials, 2011, 195(11): 398-404.
[35] MATSCHEI T, LOTHENBACH B, GLASSER F P. The AFm phase in Portland cement [J]. Cement and Concrete Research, 2007, 37(2): 118-130.
[36] WANG Fu-sheng, ZHU Yuan-na, MA Jin-long, SUN Rui-lian. Experimental research of sodium chloride on activation and binding mode of slag Portland blend cement [J]. Bulletin of the Chinese Ceramic Society, 2009, 28(4): 784-791. (in Chinese)
[37] ESCALANTE-GARCIA J I, MENDOZA G, SHARP J H. Indirect determination of the Ca/Si ratio of the C-S-H gel in Portland cements [J]. Cement and Concrete Research, 1999, 29(12): 1999-2003.
[38] SURYAVANSHI A, SCANTLEBURY J D, LYON S B. Mechanism of Friedel’s salt formation in cements rich in tri-calcium aluminate [J]. Cement and Concrete Research, 1996, 26(5): 717-727.
[39] NING Jian-guo. Hardening mechanism and structure forming model of stabilized soil and harden agent design [D]. Beijing: Beihang University, 2008. (in Chinese)
[40] SAITOH S, SUZUKI Y. Harding of soil improved by deep mixing method [C]// Proceedings of the 11th International Conference on Soil Mechanics and Foundation Engineering. Rotterdam: AA Balkema, 1985: 1613-1616.
[41] XUE Jun-gan, WU Zhong-wei. Expansive and self-stressing cement and its application [M]. Beijing: China Architecture & Building Press, 1985: 150-162. (in Chinese)
[42] OUHADI V R, YONG R N. Ettringite formation and behaviour in clayey soils [J]. Applied Clay Science, 2008, 42(1): 258-265.
(Edited by YANG Bing)
Cite this article as: CHENG Yin, LI Zhan-guo, HUANG Xin, BAI Xiao-hong. Effect of Friedel’s salt on strength enhancement of stabilized chloride saline soil [J]. Journal of Central South University, 2017, 24(4): 937-946. DOI: 10.1007/s11771-017-3496-7.
Foundation item: Project(51008007) supported by the National Natural Science Foundation of China; Project(2013318J01100) supported by the Science and Technology Project of Ministry of Communications, China
Received date: 2015-07-28; Accepted date: 2015-12-30
Corresponding author: HUANG Xin, Professor; Tel: +86-18910843036; E-mail: chengyin19840918@sina.com

