
Leaching of complex sulphide concentrate in acidic cupric chloride solutions
M. TCHOUMOU1, M. ROYNETTE2
1. Faculty of Science, Marien Ngouabi University, Brazzaville, Congo;
2. Laboratory of Applied Electrochemistry, EHICS, Louis Pasteur University, Strasbourg, France
Received 24 April 2006; accepted 4 December 2006
Abstract: The chemical analysis of a complex sulphide concentrate by emission spectrometry and X-ray diffraction shows that it contains essentially copper, lead, zinc and iron in the form of chalcopyrite, sphalerite and galena. A small amount of pyrite is also present in the ore but does not be detected with X-ray diffraction. The cupric chloride leaching of the sulphide concentrate at various durations and solid/liquid ratios at 100 ℃ shows that the rate of dissolution of the ore is the fastest in the first several hours, and after 12 h it does not evolve significantly. If oxygen is excluded from the aqueous cupric chloride solution during the leaching experiment at 100 ℃, the pyrite in the ore will not be leached. The determination of principal dissolved metals in the leaching liquor by flame atomic absorption spectrometry, and the chemical analysis of solid residues by emission spectrometry and X-ray diffraction allow to conclude that the rate of dissolution of the minerals contained in the complex sulphide concentrate are in the order of galena>sphalerite>chalcopyrite.
Key words: sulphide concentrate; rate of dissolution; dissolved metals; solid residue
1 Introduction
The leaching of the ore is a hydrometallurgical process that is to dissolve the minerals before extraction of principal metals by appropriate methods[1-2].
The leaching of the sulphide ore was generally carried out in sulphuric media[3-4], and in the chloride media by ferric solutions[5] or by the cupric chloride solutions[6-8]. It was carried out with ammoniacal solutions[9], or with hydrogen peroxide solutions[10].
If the leaching solution is decently chosen, the undesirable elements remain unreacted. During the leaching experiment, it is desirable to dissolve the maximum of the sulphide ore in the minimum of time and it is important to control the principal parameters which can affect the rate of dissolution as temperature, oxidant concentration, acidity of the solution, pulp density, redox potential, stirring speed and particle size[11]. The leaching of a sulphide ore by cupric chloride solution is by an electrochemical mechanism similar to the corrosion phenomenon; the dissolution is an oxidation—reduction reaction whose thermodynamic prevision can be easily understood by mean of the potential—pH diagram[12-13].
The leaching of a sulphide ore by an oxidant gives a solution containing the principal metallic ions to be extract, and the elemental sulphur that can be easily recovered. To avoid the precipitation of metallic hydroxides, the leaching experiments were carried out in acid media.
When the sulphide ore containing chalcopyrite, galena and sphalerite is leached by cupric chloride solutions, the reactions occurred can be written as
CuFeS2+3CuCl2→4CuCl+FeCl2+2S (1)
PbS+2CuCl2→2CuCl+PbCl2+S (2)
ZnS+2CuCl2→2CuCl+ZnCl2+S (3)
The chalcopyrite leaching by cupric chloride solutions was largely studied by the same authors that gave the results sometimes contradictory to the reactions occurred and the nature of the solid residue. However, GEORGE et al[14] agreed that the dissolution of chalcopyrite is not checked by the reaction (1), but by the reaction (4):
CuS+CuCl2→2CuCl+S (4)
This affirmation means that the chalcopyrite firstly decayed to give CuS and FeS according to the reaction (5):
CuFeS2→CuS+FeS (5)
In many studies carried out on the sulphide leaching, the researches are generally focused on the determination and the recuperation of principal dissolved elements in the resulting solution. Now, the chemical analysis of the solid residues after the leaching experiment allows to explain the behaviour of the minors elements during the leaching because the presence of the impurities in the leaching liquor is embarrassing for the electrolytic recovery of same metals. The objective of this experiment was to study in durations of the leaching experiment and solid/liquid ratios: 1) the variation of the concentration of principal dissolved metallic ions in the leaching liquor; 2) the variation of the quantity of minor elements in the solid residues.
2 Experimental
The complex sulphide ore used in the leaching experiment was a concentrate obtained from the Division of Mineralogy of BRGM (Orléans-la Source, France). The sulphide ore was finely crushed, then the leaching experiment was carried out with the fraction whose particle size was inferior to 100 ?m.
The chemical analysis of the ore was carried out by emission spectrometry according to a method, which is to melt the sulphide ore with Li2B4O7 at 1 000 ℃ in a carbon crucible and the pearl obtained was dissolved in a solution containing HCl, glycerine and bidistilled water, then transferred in a glass flask that was completed at the line before analysis[15]. The elements Fe, Al, Si and Ti were determined by Arc Emission Spectrometry with a quantometer ARL, type 14000, whereas Cu, Pb and Zn were determined by ICP Emission Spectrometry with a system ARL, type 35000C.
The sulphur contained in the sulphide concentrate was determined by a coulometric method that is to transform the sulphur ions to sulphur dioxide SO2. This gas was absorbed in the hydrogen peroxide solution contained in the cathodic compartment of electrolytic cell at a known pH, and the SO2 reacted with hydrogen peroxide according to the following reaction:
SO2+2H2O2→H2SO4 (6)
The formation of sulphuric acid involved the decreasing pH of the solution in the cathodic compartment until the end of the discharge of SO2; then the electrolysis of water begins and the electrochemical reaction occurred in the cathodic compartment is
2H2O+2e→H2+2OH- (7)
The pH of the solution in the cathodic compartment increases soon as the sulphuric acid was neutralised by the OH- produced by the electrolysis of water. The quantity of electricity is necessary to restore the pH at its initial value allowed to determine the amount of sulphur in the ore[16].
This coulometric determination of sulphur was carried out after the calibration with the standard whose mass fraction of sulphur were known. The identification of the different minerals contained in the sulphide ore was done by X-ray diffraction analysis with a diffractometer PHILIPS PW 1520. The composition of the solution used for the cupric chloride leaching of sulphide ore was CuCl2 0.5 mol/L, NaCl 3.5 mol/L and HCl 1 mol/L. The experiments were carried out at 100 ℃ in a vessel that was equipped with cooled condensers. A given quantity of finely crushed sulphide ore was introduced into a vessel containing 100 mL of cupric chloride solution, then heated in an oil bath to the desired temperature. To avoid the oxidation of cuprous to cupric ions, all experiments were carried out under nitrogen when the solution was stirred.
At the end of the leaching experiment, the resulting solution was separated from the solid residue by filtration. The solid residue constituted of the unreacted ore and the elemental sulphur was treated at 60 ℃ by 120 mL of carbon tetrachloride, then elemental sulphur was recovered by distillation of the organic solution under atmospheric pressure, whereas the mass of the final solid residue allowed to determine the percentage of the dissolved sulphide ore.
Table 1 lists the mass of sulphide concentrate introduced in the vessel containing 100 mL of cupric chloride solution for each solid/liquid ratio.
Table 1 Mass of sulphide ore introduced in vessel containing 100 mL of cupric chloride solution (g)

The dissolved metals as copper, zinc, lead and iron were determined in the leaching liquor by flame atomic absorption spectrometry respectively at 324.7, 217, 213.9 and 248.3 nm with a calibration method[17].
The solid residues after the leaching experiment were also analyzed by emission spectrometry and X-ray diffraction to explain the behaviour of minor elements during the oxidative leaching of the sulphide ore by the cupric chloride solutions.
3 Results and discussion
3.1 Chemical composition of sulphide concentrate
The chemical composition of the sulphide concentrate is listed in Table 2.
Table 2 Chemical composition of complex sulphide concentrate (%)

Except the principal elements whose mass fraction are given in Table 2, this sulphide concentrate contains equally same minor elements, such as Mn, Mg, Ti, K and Be.
It was supposed that all copper, iron, lead and zinc are present under divalent sulphides form, a calculation of theoretical mass fraction of sulphur gave 27.90%, whereas the value found by the coulometric determination was 29.15 %, that is to say a relative variation of 4.28%.
The chemical composition of the sulphide concentrate shows that the loss at 1 000 ℃ is very high, and the ratio of iron to copper is about 1.272, whereas its value is 0.878 in the stoichiometric composition of chalcopyrite CuFeS2, i.e. 44.8% of iron excess.
The high value of loss at 1 000 ℃ and the anomaly of the ratio of iron to copper in this sulphide concentrate can indicate that iron is also present in another form. Elsewhere, some studies already indicated the composition of chalcopyrite does not correspond to the stoichiometric composition[14].
Table 2 only lists the quantitative indications and does not allow knowing in which form each element is present in the complex sulphide concentrate.
The identification of different minerals by X-ray diffraction analysis indicates that the principal components are chalcopyrite, sphalerite and galena (Fig.1).
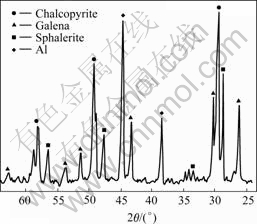
Fig.1 X-ray diffraction pattern of sulphide concentrate
As the X-ray diffraction analysis of sulphide concentrate did not indicate the presence of another mineral of iron, only the determination of the principal metals in the leaching liquor and the chemical analysis of the solid residues can allow to confirm or invalidate this hypothesis on the iron excess.
3.2 Effect of solid/liquid ratio and duration of leaching experiment on rate of dissolution of sulphide concentrate
Among the parameters that influence the rate of dissolution of the ore, only the duration of leaching experiment and solid/liquid ratio were studied. The different durations of leaching experiment were 3, 6, 12 and 24 h.
Since the volume of leaching solution was constant for each experiment, the mass of sulphide ore introduced in the vessel corresponds respectively to 35, 29.3, 24.1 and 17.5 g/L as value of solid/liquid ratio; the value 35 g/L of solid/liquid ratio corresponds to the stoichiometric quantity of oxidant required to dissolve all chalcopyrite, galena and sphalerite contained in the sulphide ore. It was found that the rate of dissolution of the sulphide ore was the function of solid/liquid ratio and the duration of leaching experiment. Fig.2 shows the variation of percentage of dissolved sulphide concentrate as function of duration of leaching experiment for each solid/liquid ratio.
These results indicate that the high dissolution of sulphide ore intervenes before 3 h, and after 12 h, the rate of dissolution does not evolve significantly. What ever the duration of the leaching experiment and solid/liquid ratio, the dissolution of sulphide ore is never complete. On the other hand, it is not necessary to use a very low solid/liquid ratio. In all cases, it remains near 5% of solid residue constituted only of unreacted minerals. The decrease of the leaching rate after 12 h can be explained by a possible passivation of the area of the mineral by elemental sulphur like certified by HACKEL et al[18].
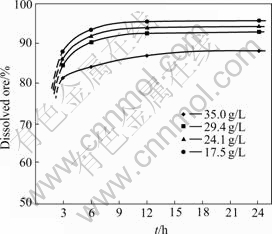
Fig.2 Percentage of dissolved ore as function of duration of leaching experiment (Temperature: 100 ℃; Concentration of CuCl2: 0.5 mol/L.)
3.3 Determination of dissolved metals
The determination of the concentration of principal metallic ions in the leaching liquor by flame atomic absorption spectrometry gives the reliable results for lead, zinc and iron.
However, since the sulphide concentrate is leached by cupric chloride solutions, the determination of copper is not accurate because its concentration is determined by a difference of two near numbers.
Fig.3 shows the variation of percentage of the dissolved metals as function of the duration of leaching experiment when the experiments were carried out with solid/liquid ratio of 29.3 g/L.
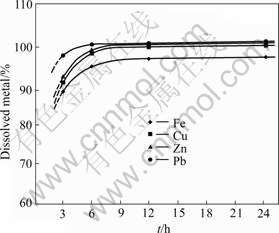
Fig.3 Percentage of dissolved metals as function of duration of attack (Solid/liquid ratio: 29.3 g/L, Temperaure: 100 ℃, Concentration of CuCl2: 0.5 mol/L)
This figure shows that lead, zinc and copper are completely dissolved after 6 h, while iron is not completely dissolved even after 24 h. Along the leaching experiment, the ratio iron/copper is not constant. This observation can indicate that either the chalcopyrite is not dissolved in a homogeneous way, or iron is equally present under another form that is not well leached under these conditions. The order of the dissolution of the metals is lead>zinc>copper>iron. This order allows to conclude that the principal minerals are dissolved in the order of galena>sphalerite>chalcopyrite.
3.4 Chemical analysis of solid residues
The chemical analysis of solid residues by emission spectrometry and X-ray diffraction, and the determination of principal dissolved metals by flame atomic absorption spectrometry allow to explain the behaviour of the sulphide concentrate during the leaching by cupric chloride solutions.
The chemical analysis of the solid residue indicates that: 1) The dissolution of lead is very fast because after 3 h, this metal is not found in the solid residue; 2) The dissolution of zinc is little slow, and becomes practically complete after 12 h; 3) The ratio of copper/iron decreases with the duration of leaching experiment. The decreasing ratio indicates that copper tends to be dissolved entirely whereas iron is dissolved with difficulty; 4) The mass fraction of the minors element as Si, Al, and Ti increases in the solid residue with the duration of leaching experiment. It is possible that those elements are present in a form that is not well leached by the cupric chloride solutions; 5) The loss at 1 000 ℃ increases with the increasing duration of the leaching experiment.
The X-ray diffraction spectra of solid residue reveal the peaks of pyrite and silica, whereas those of chalcopyrite, galena and sphalerite completely disappear (Fig.4).
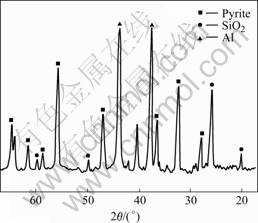
Fig.4 X-ray diffraction pattern of solid residue
The results indicate that if oxygen is excluded from the aqueous cupric chloride solution during the leaching experiment at 100 ℃, the pyrite in the ore will not be leached.
The presence of pyrite in the sulphide ore allows to explain the experimental results. The increase of the loss at 1 000 ℃ in the solid residue can be explained by the presence of the pyrite that was transformed into iron oxide according to the following reaction:
2FeS2+(11/2)O2→Fe2O3+4SO2 (8)
The presence of the pyrite in this sulphide ore also explains the decreasing ratio of copper/iron in the solid residue, and the anomaly of the ratio of iron/copper in the sulphide concentrate before the leaching experiment.
The results obtained confirm those of certain authors[19] who already indicated that the pyrite was not well leached during the oxidative leaching.
3.5 Recovery of elemental sulphur
The quantity of elemental sulphur was obtained, on one hand by difference between the mass of solid residue before and after treatment by carbon tetrachloride, on the other hand by weighing of the ball after distillation of organic solution.
The results are given in Table 3.
Table 3 Mass of recovered elemental sulphur
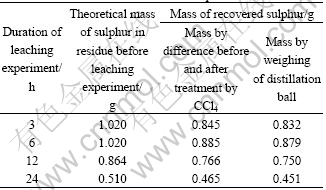
The concordance of the two quantities of elemental sulphur allowed to confirm that all sulphur was recovered. These results show that the quantity of recovered sulphur increases with the increasing duration of leaching experiment. The recovery of elemental sulphur is economically interesting since this sulphur can be used to the synthesis of another chemical compounds, like sulphuric acid.
4 Conclusions
1) The chemical analysis of a concentrate sulphide ore by emission spectrometry and X-ray diffraction indicates that the main components are chalcopyrite, sphalerite and galena. The leaching of the sulphide ore by cupric chloride solutions at 100 ℃ at various durations of leaching experiment and solid/liquid ratio shows that the percentage of dissolved ore fluctuates between 88% to 95%.
2) The dissolution of sulphide ore is the function of the duration of leaching experiment and solid/liquid ratio. It is not important to use a very low solid/liquid ratio because with 29.3 g/L as value of solid/liquid ratio, the percentage of dissolved ore is near 94% after 12 h.
3) The high dissolution of the sulphide ore intervenes in the beginning of leaching experiment, and after 12 h, the leaching rate does not evolve significantly. The determination of dissolved metals in the leaching liquor by flame atomic absorption spectrometry shows that the lead is dissolved with rapidity, followed by zinc, copper and iron.
4) The chemical analysis of solid residue by emission spectrometry and X-ray diffraction shows that the loss at 1 000 ℃ and the mass fraction of minor elements as Ti, Si and Al increase with the increasing duration of leaching experiment. On the other hand, the chemical analysis of the solid residue shows that the ratio of copper/iron decreases with the duration of leaching experiment.
5) The X-ray diffraction analysis of solid residue allows to confirm the presence of the pyrite in the ore. A small quantity of pyrite in the sulphide ore is difficult to detect with X-ray diffraction and this mineral is not well leached by the cupric chloride solutions.
6) The anomaly of the ratio of iron/copper in the sulphide ore before the leaching experiment can be explained by the presence of small quantity of pyrite together with the chalcopyrite.
7) The treatment of solid residue by carbon tetrachloride allows recovering all elemental sulphur that can be used to the synthesis of chemical compounds like sulphuric acid.
Acknowledgement
The authors thank the government of the Republic of Congo to have agreed a grant in France, and Professor Jean Louis LEIBENGUTH for the direction of our research tasks to the Laboratory of Applied Electrochemistry.
References
[1] PHILIBERT J V, BRECHET Y A, COMBRADE P. Metallurgy from Ore to Materials [M]. Editions Dunod, 2ème Edition Paris, 2002.
[2] FOULETIER M, MATHIEU F J, NOUAL P. The Applications of Electrochemistry to Hydrometallurgy [M]. Editions Pluralis, 3ème Edition Paris, 1976.
[3] PADILLA R, ZAMBRANO P, RUIZ M C. Leaching of sulfized chalcopyrite with H2SO4-NaCl-O2 [M]. Met and Mater Trans B, 2003, 34B(2): 153-159.
[4] AKCIL A, CIFTCI H. A study of the selective leaching of complex sulphides from the Easter Black Sea Region Turkey [J]. Miner Eng, 2002, 15: 457-459.
[5] OLANIPEKUN E O. Kinetics of sphalerite leaching in acidic ferric chloride solutions [J]. Trans Ind Inst Met, 1999, 52(2/3): 73-79.
[6] GUY S, BROADBENT C P, LAWSON G J, JACKSON J D J. Cupric chloride leaching of a complex copper/zinc/lead ore [J]. Hydrometallurgy, 1983, 10: 243-255.
[7] BONAN M, DEMARTHE J M, RENON H, BARATIN F. Chalcopyrite leaching by CuCl2 in strong NaCl solutions [J]. Metal Trans B, 1981, 12: 269-280.
[8] DUTRIZAC J E. The leaching of sulphide minerals in chloride media [J]. Hydrometallurgy, 1992, 29: 1-45.
[9] FENG D, VAN DEVENTER J S J. Leaching behaviour of sulphides in ammoniacal thiosulphate systems [J]. Hydrometallurgy, 2002, 63: 189-200.
[10] ANTONIJEVIC M M, JANKOVIC Z D, DIMITRIJEVIC M D. Kinetics of chalcopyrite dissolution by hydrogen peroxide in sulphuric acid [J]. Hydrometallurgy, 2004, 71: 319-329.
[11] WILSON J P, FISCHER W W. Cupric chloride leaching of chalcopyrite [J]. J Metals, 1981, 33: 52-57.
[12] WARREN G W, HENEIN H, JIN Z. Reaction mechanism of the ferric chloride leaching of sphalerite [J]. Met Trans B, 1985, 16B: 715-724.
[13] ABRAITIS P K, PATTRICK R A D, KELSHALL G, VAUGHAN D J. Acid leaching and dissolution of majorsulphide ore minerals processes and galvanic effects in complex systems [J]. Miner Mag, 2004, 68(2): 343-351.
[14] GEORGE W, UDOVIC T J, DUMESIC J A, LANGER S H. Equilibria associated with cupric chloride leaching of chalcopyrite concentrate [J]. Hydrometallurgy, 1984, 13: 125-135.
[15] BESNUS Y, SAMUEL J, RAOULT R. Determination of metallic element in ore by inducted coupled plasma emission spectrometry [J]. Analusis, 1985, 13: 312-319.
[16] PFAADT M, STAUB R, BESNUS S J. Technic note of the Institute of geology, n°12, Louis Pasteur University. STRASBOURG, 1980.
[17] JORSHEM L, ENGMAN J. Determination of lead, cadmium, zinc, copper and iron in foods by atomic absorption spectrometry after microwave digestion [J]. J of AOAC Inter, 2000, 88(5): 1189-1203.
[18] HACKL R P, DREISINGER D B, PETERS E, KING J A. Passivation of chalcopyrite during oxidative leaching in sulfate media [J]. Hydrometallurgy, 1995, 39: 25-49.
[19] MIZOGUCHI T, HABASHI F. Oxidation of zinc sulphide, pyrite and their mixture in hydrochloric acid [J]. Trans Inst Mining Met, 1983, 92: C14-C19.
Corresponding author: M.TCHOUMOU; Tel: +242-668-64-91; E-mail: tchoumoutafel@yahoo.fr
(Edited by LI Xiang-qun)