J.Cent.South Univ.Technol.(2010) 17: 289-294
DOI:10.1007/s1771-010-0044-0 
Thermodynamic study on reaction path of Hg(Ⅱ) with S(Ⅱ) in solution
CHAI Li-yuan(柴立元), WANG Qing-wei(王庆伟), WANG Yun-yan(王云燕),
LI Qing-zhu(李青竹), YANG Zhi-hui(杨志辉), SHU Yu-de(舒余德)
School of Metallurgical Science and Engineering, Central South University, Changsha 410083, China
? Central South University Press and Springer-Verlag Berlin Heidelberg 2010
Abstract: The mercury sulfidation experiments were conducted in the pH range from 1 to 13. The results show that Hg(Ⅱ) reacted with equimolar S(Ⅱ) has the lowest remained Hg(Ⅱ) concentration (9.7 mg/L) at pH 1.0 and the highest remained concentration (940.8 mg/L) at pH 13.0. Meanwhile, the changes of pH values were monitored exactly, which reveal that solution pH values change when mixing the same pH value solutions of HgCl2 and Na2S. In order to explain the phenomena and determine the reaction paths of Hg(Ⅱ) reacting with S(Ⅱ) in the solution, the concerned thermodynamics was studied. Species of S(Ⅱ)-H2O system and Hg(Ⅱ)-H2O system at different pH values were calculated, and then the species distribution diagrams of S(Ⅱ)-H2O system, Hg(Ⅱ)-H2O system and Hg(Ⅱ)-Cl--OH--H2O system were drawn. Combining the experimental data and thermodynamic calculation, the mechanism of Hg(Ⅱ) reacting with S(Ⅱ) was deduced. The results indicate that different species of S(Ⅱ) and Hg(Ⅱ) have the diverse reaction paths to form HgS precipitate at different pH values and the standard Gibbs free energies change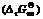 of those equations are also calculated, which can provide a guidance for mercury-containing wastewater treatment with Na2S.
of those equations are also calculated, which can provide a guidance for mercury-containing wastewater treatment with Na2S.
Key words: mercury species; Na2S; thermodynamics; reaction path; wastewater treatment;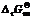
1 Introduction
Mercury pollution has been recognized as a global environmental problem because of its volatility and toxicity [1]. It can cause impairment of pulmonary function and kidney, chest pain and dyspnoea [2]. Mercury and its compounds have been widely employed in fungicides, dental preparations, electrical apparatus, industrial control instruments, dry batteries, paints, pharmaceuticals, explosives, metal plating, pesticides, pulp and paper industry [3-4].
Removal of mercury ions from wastewater is usually performed using varieties of traditional procedures viz. chemistry precipitation [5], electrolytic method [6], metal deoxidization [7], ion exchange [8], adsorption [9], reverse osmosis [10], solvent extraction [11], microorganism [12] and other procedures [13]. Among these methods, chemical precipitation with Na2S and NaHS is by far the most widely used process to remove mercury from wastewater. The main advantages of sulfidation treatment are high rate of mercury removal even at low pH, and low reactor detention time required due to the high reactivity of sulfides. So, the use of sulfides to precipitate mercury(Ⅱ) as HgS from industrial effluents is important [14-15]. Moreover, HgS is polymorphic and exists as the common red (α) form known as cinnabar and the black metastable (β) form, metacinnabar, and both forms are highly insoluble in aqueous solutions with a large Ksp value (4×10-53) [16-17].
In the past two decades, a great deal of attention has been paid to understanding the optimal parameters of S(Ⅱ) to treat mercury-containing wastewater. In these studies, the precipitation of metals was investigated to make them qualify the Integrated Wastewater Discharge Standard [14]. However, the mechanism of mercury treated by sulfidation agents was not further studied. The researchers usually use the equation Hg2++ S2-→HgS to describe this mechanism. However, this equation does not occur in the practical process because the species of Hg and S in the solution was not considered. Generally, pH value of solution is one of the important parameters governing the treatment of wastewater containing mercury using Na2S, Hg(Ⅱ) and S(Ⅱ) species were changed as pH value of solution varied. Various species of Hg(Ⅱ) and S(Ⅱ) have different reaction paths to form HgS precipitate, which directly affects the mercury removal efficiency. The objectives of this work were to investigate the reaction path of aqueous solution containing HgCl2 treated by Na2S at different pH values through thermodynamic calculation and experimental data.
2 Thermodynamics
2.1 Distribution of S(Ⅱ) species in S(Ⅱ)-H2O system at different pH values
Total S(Ⅱ) concentration (ct(S2-)) in Na2S solution consists of c(S2-), c(HS-) and c(H2S).
ct(S2-)=c(S2-)+c(HS-)+c(H2S)
S2-+H+ HS- K1=10-14.15
HS- K1=10-14.15
HS-+H+ H2S K2=10-6.88
H2S K2=10-6.88
Herein:
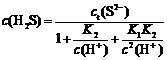
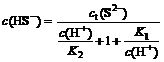
Those equations describe the measured concentrations of c(S2-), c(HS-) and c(H2S) as function of the total S(Ⅱ) concentration. Order α0=c(H2S)/ct(S2-), α1=c(HS-)/ct(S2-), α2=c(S2-)/ct(S2-). The proportion of H2S, HS- and S2- in Na2S solution at different pH values is shown in Fig.1.
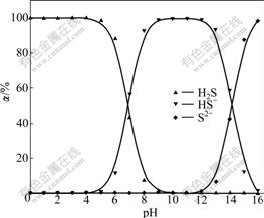
Fig.1 Proportions (α) of S2-, HS- and H2S in solution at different pH values
Fig.1 depicts that the dominant S(Ⅱ) species in the solution is S2- only at pH>13.0, HS- at pH 6-13 and H2S at pH<8, both HS- and H2S species exist between pH 6 and pH 8. At pH 12, the percentage of S2- is only 0.71%. H2S evaporation will occur as gaseous hydrogen sulfide from solution when H2S concentration in aqueous solution exceeds the solubility of 69.4 mmol/L at 40 ℃.
2.2 Distribution of Hg(Ⅱ) species in Hg(Ⅱ)-H2O system at different pH values
The main species of Hg(Ⅱ) that exist in solution are Hg2+, HgOH+, Hg(OH)2 and Hg(OH)3- at different pH values. The primary reaction of those species converting into each other and the equilibrium constants [17] can be described as follows:
Hg2++OH-  HgOH+ K3=1010.6
HgOH+ K3=1010.6
HgOH++OH- Hg(OH)2 K4=1011.2
Hg(OH)2 K4=1011.2
Hg(OH)2+OH- Hg(OH)3- K5=10-0.9
Hg(OH)3- K5=10-0.9
In dilute solution, mercury species distributing at different pH values are calculated according to the above equilibrium constants, let α′0, α′1, α′2 and α′3 represent the percentages of Hg2+, HgOH+, Hg(OH)2 and Hg(OH)3-, respectively. The calculated results are shown in Fig.2.
α′0= 1/[1+K3c(OH-)+K3K4c2(OH-)+K3K4K5c3(OH-)]
α′1=α′0K3c(OH-)
α′2=α′0K3K4c2(OH-)
α′3=α′0K3K4K5c3(OH-)
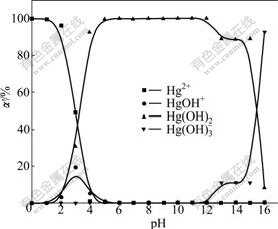
Fig.2 Proportions (α′) of Hg2+, HgOH+, Hg(OH)2 and Hg(OH)3- at different pH values
It can be seen from Fig.2 that the dominant mercury species in the solution is Hg2+ at pH<3.0, Hg(OH)2 at 5.0<pH<15.0 and both of these species between pH 3.0 and 5.0. In addition, small amount of HgOH+ is also present between pH 2 and 6. At pH 3.0, percentages of Hg2+, Hg(OH)- and Hg(OH)2 are 49.30%, 19.61% and 1.09%, respectively. When pH value is higher than 15.0, Hg(OH)3- is the predominant species in the solution.
Hg(OH)2 precipitates as HgO in solution when Hg(OH)2 reaches the saturated concentration of 2.4×10-4 mol/L (K6=2.4×10-4) [18]. Fig.3 shows the calculated results of the solution containing 100.0 mg/L Hg(Ⅱ) by using the following equations and the equili- brium constants [17]:
Hg(OH)2(aq) Hg(OH)2(s) K6=10-3.76
Hg(OH)2(s) K6=10-3.76
Hg(OH)2 [HgOH+][OH-] K7=10-11.2
[HgOH+][OH-] K7=10-11.2
Hg(OH)2 [Hg2+][OH-]2 K8=10-21.8
[Hg2+][OH-]2 K8=10-21.8
Hg(OH)2+OH- Hg(OH)3- K5=10-0.9
Hg(OH)3- K5=10-0.9
As shown in Fig.3, Hg(Ⅱ) exists in water-soluble form in the solution at pH<3.2 and then transforms into HgO precipitation at pH 3.2. The content of Hg(OH)2 is saturated with 48.13 mg/L Hg(Ⅱ) in the solution in pH range of 3.2-13.0. If pH value is greater than 13, HgO precipitate is re-dissolved as Hg(OH)3-. At pH>15, HgO precipitate completely transforms into Hg(OH)3- and the concentration of Hg(Ⅱ) in solution reaches up to 100.0 mg/L. Accordingly, it is impossible to precipitate the mercury only by adjusting the pH value of the solution when the concentration of Hg(Ⅱ) is lower than 48.13 mg/L.
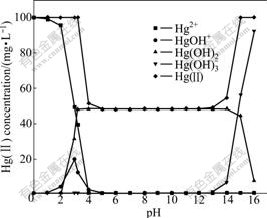
Fig.3 Concentrations of Hg(Ⅱ), Hg2+, HgOH+, Hg(OH)2 and Hg(OH)3- at different pH values (initial Hg(Ⅱ) concentration of 100 mg/L)
3 Experimental
3.1 Materials and analytical methods
HgCl2, Na2S·9H2O, KI, Na2S2O3, K2CrO4, Zn(CH3COO)2 were purchased from Sinopharm Chemical Reagent Co., Ltd. in Shanghai. All reagents used in this work were analytical reagent grade. Wastewater containing 0.24 mmol/L Hg(Ⅱ) was prepared by dissolving 0.065 0 g solid HgCl2. The Na2S solution was prepared by dissolving Na2S·9H2O crystals. Prior to use, Na2S·9H2O crystals were washed with distilled water to remove oxidized sulfur species from the surface of the crystals. S(Ⅱ) concentration in Na2S solution was standardized to 925.25 mg/L by a standard iodimetric method [19], 1 mol/L NaOH and 1 mol/L HCl solutions were used to adjust the solution pH value. All solutions were prepared in distilled water. Glassware was washed with detergent, rinsed with tap water, soaked with HNO3 (50%, volume fraction) for 24 h, and then rinsed with distilled water prior to drying. The pH value was measured with a digital ORION-420 pH meter calibrated with pH 4.01 and 6.86 standard buffers. Mercury was determined by a QM201 mercury analyzer (cold vapor atomic absorption spectrometer) using 25% SnCl2 reductant in 20% HCl.
3.2 Experimental method
Before mixing 150 mL synthetic wastewater containing Hg(Ⅱ) 0.24 mmol/L with 7.80 mL Na2S solution according to molar ratio of S(Ⅱ) to Hg(Ⅱ) of 1:1, the two solutions were adjusted to the same pH values of 1, 3, 5, 7, 9, 11 and 13 by HCl or NaOH. Thereafter, the reaction was carried out in water bath temperature was kept at 25 ℃. Remained mercury concentration and pH value in treated water were detected every 2 min in initial 20 min, every 5 min between 20-40 min and every 10 min between 40- 120 min.
4 Results
For the mercury sulfidation experiments, the remained mercury concentration with time at different pH values is shown in Fig.4.
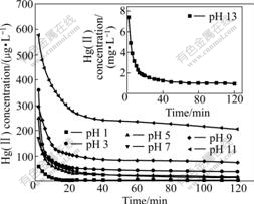
Fig.4 Dependence of remained mercury concentration on time at different pH values (initial Hg(Ⅱ) concentration of 48.13 mg/L)
Mercury solution of 48.13 mg/L cannot be removed by adjusting pH value via forming HgS precipitate. The results in Fig.4 clearly show that the reaction between Hg(Ⅱ) and S(Ⅱ) has the fastest reaction rate at pH 1.0 and the remained mercury concentration is 9.7 mg/L. The reaction rates are identical at pH 5.0 and 7.0, the remained mercury concentration are 16.68 and 3.78 mg/L, respectively, and this reaction rates were slightly higher than those at pH 3.0. The remained mercury concentration increased from 206.79 to 940.8 mg/L when the initial aqueous solution pH value increased from 9.0 to 13.0.
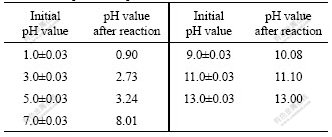
Accurate measurement of pH value is of primary importance for interpretation of aqueous chemical speciation. The changes of pH values before and after reaction are shown in Table 1. The results reveal that pH values of solution change after mixing the same pH value of HgCl2 and Na2S solutions. In acidic condition, the pH value of the mixed solution decreases, when the initial solutions pH value is 5.0, after reaction the pH value decreases to 3.24; at the initial pH 3, after reaction the mixed solution pH is 2.73; at the initial pH 1.0, the amount of H+ released from the reaction process is small relative to the strong acidic solution, so the decreased pH value is only 0.1. However, in neutral and alkaline condition, the pH value of the mixed solution increases, at the initial pH 7, the mixed solution pH value increase to 8.01; at initial pH 9, the pH of solution after reaction is 10.08, at pH 11 and 13, comparing to the OH- concentration in the solution the liberated OH- from the reaction process can be ignored, so the increased pH value is neglected.
5 Discussion
5.1 Distribution of Hg(Ⅱ) species in Hg(Ⅱ)-Cl-- OH--H2O system at different pH values
In the previous experiments, all the solution pH values were adjusted by HCl or NaOH. The accumulation of Cl- and OH- may affect the species of Hg(Ⅱ) in Hg(Ⅱ)-Cl--OH--H2O system. The species of Hg(Ⅱ) in 0.24 mmol/L HgCl2 solution were calculated using thermodynamic data shown as the following equations [16] considering the accumulation of Cl- and OH-, e.g., Cl- concentration in HgCl2 solution (0.24 mmol/L, pH=1) was nearly 0.1 mol/L. The results are depicted in Fig.5.
Hg2++Cl- HgCl+ K9=106.67
HgCl+ K9=106.67
HgCl++Cl- HgCl2 K10=106.44
HgCl2 K10=106.44
HgCl2+Cl- HgCl3- K11=100.87
HgCl3- K11=100.87
HgCl3-+Cl- HgCl42- K12=101.15
HgCl42- K12=101.15
Hg2++OH- HgOH+ K3=1010.6
HgOH+ K3=1010.6
HgOH++OH- Hg(OH)2 K4=1011.2
Hg(OH)2 K4=1011.2
Hg(OH)2+OH- Hg(OH)3- K5=10-0.9
Hg(OH)3- K5=10-0.9
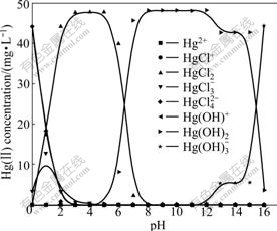
Fig.5 Thermodynamics of Hg(Ⅱ)-Cl--OH--H2O system (initial Hg(Ⅱ) concentration of 48.13 mg/L)
Comparing Fig.2 with Fig.5, it can be concluded that the species of Hg(Ⅱ) changes obviously, which is attributed to the accumulation of Cl- and OH-. In pH range of 0-3, the dominant mercury species are HgCl42-, HgCl3- and HgCl2 in the presence of Cl- and OH- (Fig.5) instead of Hg2+ and HgOH+ in the absence of Cl- (Fig.2). Correspondingly, the dominant species is HgCl2 at pH 3-5, HgCl2 and Hg(OH)2 at pH 5-8, Hg(OH)2 at pH 8-12, and Hg(OH)3- at pH>15. Moreover, both Hg(OH)2 and Hg(OH)3- exist at pH 12-15.
5.2 Reaction paths of Hg(Ⅱ) with S(Ⅱ)
In 0.24 mmol/L HgCl2 solution, mercury species are HgCl42-, HgCl3-, HgCl2, Hg(OH)2 and Hg(OH)3-; while in Na2S solution, S(Ⅱ) species are H2S, HS- and S2- at different pH values. The potential reactive routes of Hg(Ⅱ) reacted with S(Ⅱ) to form HgS precipitate are shown as the following equations and the 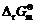 values of those reactions are calculated using the reported standard Gibbs energies [17].
values of those reactions are calculated using the reported standard Gibbs energies [17].
HgCl42-+H2S HgS+2H++4Cl-
HgS+2H++4Cl-
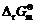 =-90.922 kJ/mol (1)
=-90.922 kJ/mol (1)
HgCl3-+H2S HgS+2H++3Cl-
HgS+2H++3Cl-
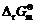 = -96.487 kJ/mol (2)
= -96.487 kJ/mol (2)
HgCl2+H2S HgS+2H++2Cl-
HgS+2H++2Cl-
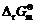 = -100.940 kJ/mol (3)
= -100.940 kJ/mol (3)
HgCl2+HS- HgS+H++2Cl-
HgS+H++2Cl-
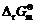 =-121.750 kJ/mol (4)
=-121.750 kJ/mol (4)
Hg(OH)2+H2S HgS+2H2O
HgS+2H2O
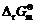 =-211.059 kJ/mol (5)
=-211.059 kJ/mol (5)
Hg(OH)2+HS- HgS+H2O+OH-
HgS+H2O+OH-
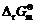 =-151.999 kJ/mol (6)
=-151.999 kJ/mol (6)
Hg(OH)2+S2- HgS+2OH-
HgS+2OH-
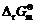 =7.750 kJ/mol (7)
=7.750 kJ/mol (7)
Hg(OH)3-+HS- HgS+H2O+2OH-
HgS+H2O+2OH-
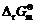 =-157.137 kJ/mol (8)
=-157.137 kJ/mol (8)
Hg(OH)3-+S2- HgS+3OH-
HgS+3OH-
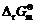 =2.613 kJ/mol (9)
=2.613 kJ/mol (9)
When solution pH value is lower than zero, species of Hg(Ⅱ) and S(Ⅱ) in solution are HgCl42- and H2S, respectively. The reaction path can be described using Eq.(1).
In pH range of 0-3, species of Hg(Ⅱ) in solution are HgCl42-, HgCl3- and HgCl2, and species of S(Ⅱ) is H2S. The potential reaction paths can be described using Eqs.(1)-(3). The  of the above three reactions are nearly equal, so the chemical reaction path depends on the species of Hg(Ⅱ). For instance, in the pH range of 0-1, the dominant species of Hg(Ⅱ) is HgCl42- and the chemical reaction path could be Eq.(1). At pH 1-2, the priority of the above three reactions is similar. At pH 2-3, the species of Hg(Ⅱ) is HgCl2 and the chemical reaction path could be Eq.(3).
of the above three reactions are nearly equal, so the chemical reaction path depends on the species of Hg(Ⅱ). For instance, in the pH range of 0-1, the dominant species of Hg(Ⅱ) is HgCl42- and the chemical reaction path could be Eq.(1). At pH 1-2, the priority of the above three reactions is similar. At pH 2-3, the species of Hg(Ⅱ) is HgCl2 and the chemical reaction path could be Eq.(3).
In pH range of 3-5, the dominant species of Hg(Ⅱ) and S(Ⅱ) are HgCl2 and H2S, respectively. The reaction path could be Eq.(3). H+ liberated from H2S results in the decreased pH value in the mixed solution, which is consistent with the experimental data (Table 1).
Table 1 Comparison of pH values before and after reaction
In pH range of 5-6, the dominant species of Hg(Ⅱ) is HgCl2 and the dominant species of S(Ⅱ) are H2S and HS-, the  values of Eqs.(3) and (4) are similar; the chemical reaction path should be determined by the concentration of H2S. Therefore, the chemical reaction path could be Eq.(3) according to Fig.1.
values of Eqs.(3) and (4) are similar; the chemical reaction path should be determined by the concentration of H2S. Therefore, the chemical reaction path could be Eq.(3) according to Fig.1.
In pH range of 6-8, the species of Hg(Ⅱ) are Hg(OH)2 and HgCl2. The proportion of H2S and HS- in Na2S solution is similar. Eq.(5) is most likely to occur because the  of Eq.(5) is minimum among Eqs.(3)-(6).
of Eq.(5) is minimum among Eqs.(3)-(6). 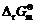 of reaction between Hg(OH)2 with H2S and HS- is less than that of reaction between HgCl2 with H2S and HS-. Thus, Hg(OH)2 is easier to react with H2S, HS- than HgCl2. In the initial solutions of pH 7, pH value in the mixed solution increases under no stirred condition. Accordingly, the reaction paths are Eqs.(5) and (6). The main species of S(Ⅱ) changes to HS- when pH value of Na2S solution approaches 8.0. As a result, the dominant reaction path is Eq.(6).
of reaction between Hg(OH)2 with H2S and HS- is less than that of reaction between HgCl2 with H2S and HS-. Thus, Hg(OH)2 is easier to react with H2S, HS- than HgCl2. In the initial solutions of pH 7, pH value in the mixed solution increases under no stirred condition. Accordingly, the reaction paths are Eqs.(5) and (6). The main species of S(Ⅱ) changes to HS- when pH value of Na2S solution approaches 8.0. As a result, the dominant reaction path is Eq.(6).
When the initial pH value ranges from 8 to 12, the dominant reaction path is Eq.(6). Because free OH- from Hg(OH)2 molecule enters the mixed solution, resulting in pH value increased. However, the reaction of Eq.(6) is more difficult to carry out because the OH- concentration increases along with the enhancement of system pH value, the higher remained mercury concentrations are determined in mixed solution, which is consistent with experimental results (Fig.4).
In pH range of 12-15, the species of Hg(Ⅱ) are Hg(OH)2 and Hg(OH)3-, and species of S(Ⅱ) are HS- and S2-. The potential reaction paths are Eqs.(6)-(9). Since the 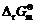 values of Eq.(7) and Eq.(9) are more than 0, both reactions cannot carry out spontaneously. Although the
values of Eq.(7) and Eq.(9) are more than 0, both reactions cannot carry out spontaneously. Although the 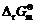 of Eq.(8) is less than 0, Hg(OH)3- and S2- are electronegative and mutually exclusive between the particles, so the reaction is also difficult to carry out. Correspondingly, the reaction process is Eq.(6) in pH range of 12-15. So in the strongly basic solution mercury is difficult to be removed by Na2S, which is confirmed by experimental results (Fig.4).
of Eq.(8) is less than 0, Hg(OH)3- and S2- are electronegative and mutually exclusive between the particles, so the reaction is also difficult to carry out. Correspondingly, the reaction process is Eq.(6) in pH range of 12-15. So in the strongly basic solution mercury is difficult to be removed by Na2S, which is confirmed by experimental results (Fig.4).
6 Conclusions
(1) The optimal pH value is 5-9 for wastewater containing mercury treated by Na2S because HgS and HgO precipitates can be formed simultaneously in this pH range.
(2) Thermodynamic calculation results indicate that S(Ⅱ) and Hg(Ⅱ) have different species in the solution of different pH values, so S(Ⅱ) and Hg(Ⅱ) have the various reaction routes to form HgS precipitate. The 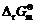 of Eq.(5) is minimum among Eqs.(1)-(9), which suggests that Eq.(5) is the most efficient reaction path for mercury-containing wastewater treated by Na2S.
of Eq.(5) is minimum among Eqs.(1)-(9), which suggests that Eq.(5) is the most efficient reaction path for mercury-containing wastewater treated by Na2S.
(3) The pH value of mixed solution changes after mixing HgCl2 and Na2S solution of the same pH value. The mixed solution pH decreases in acidic condition and increases in neutral and alkaline condition.
References
[1] JING Y D, HEA Z L, YANG X E. Effects of pH, organic acids, and competitive cations on mercury desorption in soils [J]. Chemosphere, 2007, 69(10): 1662-1669.
[2] SANDRA G, ISELA L, CARLOS B. Mercury removal from contaminated water by ultrasound-promoted reduction/vaporization in a microscale reactor [J]. Ultrasonics Sonochemistry, 2008, 15(3): 212-216.
[3] MEENA A K, MISHRA G K, RAI P K, RAJAGOPAL C, NAGAR P N. Removal of heavy metal ions from aqueous solutions using carbon aerogel as an adsorbent [J]. Journal of Hazardous Materials, 2005, 122(1/2): 161-170.
[4] RODRIGO S V, MARIAA M B. Dynamic and static adsorption and desorption of Hg(Ⅱ) ions on chitosan membranes and spheres [J]. Water Research, 2006, 40(8): 1726-1734.
[5] FRIDER E S, SHELEFB G, KRESS N. Mercury speciation in sediments at a municipal sewage sludge marine disposal site [J]. Marine Environmental Research, 2007, 64 (5): 601-615.
[6] ZAMBRANO J B, ABORIE S L, VIERS P, RAKIB M, DUTAND G. Mercury removal and recovery from aqueous solutions by coupled complexation-ultrafiltration and electrolysis [J]. Journal of Membrane Science, 2004, 229(1/2): 179-186.
[7] GRAU J M, BISANG J M. Removal and recovery of mercury from chloride solutions by contact deposition on iron felt [J]. Journal of Chemical Technology and Biotechnology, 1995, 62(2): 153-158.
[8] MONTEAGYDO J M, ORTIZ M J. Removal of inorganic mercury from mine waste water by ion exchange [J]. Journal of Chemical Technology and Biotechnology, 2000, 75(9): 767-772.
[9] CHAI Li-yuan, LI Qing-zhu, WANG Qing-wei, YANG Zhi-hui, WANG Yun-yan. Removal of Hg(Ⅱ) from aqueous solutions using spent grain [C]// 2009 Annual TMS Meeting and Exhibition. San Francisco: Minerals, Metals and Materials Society, 2009: 1019- 1023.
[10] HUANG Y C, KOSEOGLU S S. Separation of heavy metals from industrial waste streams by membrane separation technology [J]. Waste Management, 1993, 13(5/7): 481-501.
[11] REDDY M L P, FRANCIS T. Recent advances in the solvent extraction of mercury(Ⅱ) with calixarenes and crown ethers [J]. Solvent Extraction and Ion Exchange, 2001, 19(5): 839-863.
[12] KINGJ K, HARMON S M, THERESA T F, GLADDEN J B. Mercury removal, methylmercury formation, and sulfate-reducing bacteria profiles in wetland mesocosms [J]. Chemosphere, 2002, 46(6): 859-870.
[13] HASANY S M, AHMAD R. The potential of cost-effective coconut husk for the removal of toxic metal ions for environmental protection [J]. Journal of Environmental Management, 2006, 81(3): 286-295.
[14] TOKUDA H, KUCHARA D, MIHARA N, KUBOTA M, MATSUDA H, FUKUTA T. Study on reaction kinetics and selective precipitation of Cu, Zn, Ni and Sn with H2S in single-metal and multi-metal systems [J]. Chemosphere, 2008, 73(19): 1448-1452.
[15] ZHENG Ya-jie, WANG Yong, XIAO Fa-xin, LUO Yuan. Recovery of copper sulfate after treating As-containing wastewater by precipitation method [J]. Journal of Central South University of Technology, 2009, 16(2): 42-46.
[16] BRANDON N P, FRANCIS P A, JEFFREY J, KELSALL G H, YIN Q. Thermodynamics and electrochemical behaviour of Hg-S-Cl-H2O systems [J]. Journal of Electroanalytical Chemistry, 2001, 497(1/2): 18-32.
[17] LI Meng-long. Concise handbook of chemical date [M]. Beijing: Chemical Industry Press, 2004. (in Chinese)
[18] JAMES N B. Ionic equilibrium: A mathematical approach [M]. Massachusetts: Addison-wesley Educational Publishers, 1964.
[19] CAO Jie-shan. Determination of sulfides in water and wastewater by iodonetric method [J]. Environmental Monitoring in China, 2001, 17(4): 31-33. (in Chinese)
Foundation item: Project(50925417) supported by China National Funds for Distinguished Young Scientists; Project(50830301) supported by the Key Project of the National Natural Science Foundation of China; Project(308019) supported by the Key Science and Technical Project of Ministry of Science and Technology of China; Project(2007BAC25B01) supported by the National Key Project of Science and Technical Supporting Programs Funded by Ministry of Science and Technology of China during the 11th Five-Year Plan; Project(08JJ3020) supported by the Natural Science Foundation of Hunan Province, China
Received date: 2009-08-24; Accepted date: 2009-12-19
Corresponding author: WANG Yun-yan, PhD; Tel: +86-731-88830875; E-mail: wyy@mail.csu.edu.cn
(Edited by YANG You-ping)