
Thermal stability and curing kinetics of polycarbosilane fibers
ZHENG Chun-man (郑春满), LI Xiao-dong (李效东), WANG Hao (王 浩), ZHU Bin(朱 冰)
(State Key Laboratory of New Ceramic Fibers and composites, School of Aerospace and Materials Engineering, National University of Defense Technology, Changsha 410073, China)
Received 1 April 2005; accepted 5 Jun 2005
Abstract: Thermal stability and curing kinetics of polycarbosilane (PCS) fibers were studied by thermogravimetry (TG), Fourier transform infrared spectroscopy(FT-IR). Curing is an essential step in the preparation of SiC fibers and the properties of SiC fibers are affected greatly by curing conditions. TG measurement performed in air shows that mass gain starts at approximately 200℃ and PCS fibers are sensitive to oxygen. Curing with oxygen, which results in crosslinking on the surface, enabled PCS fibers to retain its shape during high-temperature pyrolysis. The curing of PCS fibers is oxidation of Si─H and Si─CH3, then Si─O─Si and Si─O─C bonds are formed. This is a first order reaction, with activation energy of 79.27 kJ/mol, and the pre-exponential factor is calculated as 3.07×106. The kinetics model was obtained and the experimental data of PCS fibers show good agreement with the kinetics model.
Key words: polycarbosilane; SiC fibers; thermal stability; curing; kinetics
1 Introduction
Silicon carbide(SiC) fibers, which have high tensile strength, high elastic modulus and good thermal stability, are one of the best candidates of the reinforcements available for continuous-fiber- reinforced ceramic-matrix composites (CMCs) [1, 2]. The methods of SiC fibers preparation include chemical vapor deposition (CVD), chemical vapor reaction(CVR) and preceramic polymer pyrolysis. SiC fibers derived from polymer pyrolysis have the advantages of the flexibility, fine-diameter form over those of SiC fibers from CVD. Since Yajima’s synthesis of polycarbosilane as precursor, polymer-derived SiC fibers have been extensively studied[3, 4].
The Yajima’s method includes four processes: synthesis, spinning, curing and pyrolysis[4]. The curing process in the SiC fibers preparation is oxidation of PCS fibers caused by heat treatment in air, which is to render the article infusible and retain its form, and also subsequently to obtain a high yield of ceramic[5]. It is an essential step because the fibers would lose much mass during the subsequent pyrolysis process. SiC fibers may not be obtained from uncured or poorly cured precursor fibers, not to mention their mechanical properties. The curing process can be carried out in air, oxygen, ozone and NO2, and it can also be carried out by irradiation with electron beam, UV-rays or γ-rays [6, 7]. The curing in air is widely used for the advantage of low cost and less pollution.
Therefore, it is necessary to make a thorough understanding of thermal stability and curing kinetics of PCS fibers so as to obtain excellent SiC fibers. In this paper, thermal stability of PCS fibers were carried out by employing a thermogravimetric analyzer, curing was carried out at various temperatures and curing kinetics was obtained based on the mass gain, and some useful information which was beneficial for curing techniques was gained.
2 Experimental
The PCS polymer was synthesized from polydimethylsilane(PDMS) at the normal pressure[8]. Its average molecular number was about 1 600 and the melting point was (210±3) ℃. The PCS was melted at about 300 ℃ in nitrogen and spun into fibers com- posed of 200 continuous filaments with a multi-holes spinning machine. The green fibers were then cured in air oven at a certain heating speed from the room temperature to 160-200 ℃ and the holding time was varied to obtain different curing degrees.
TG curves of PCS fibers were obtained with a high temperature NETZSCH STA 449C thermogravimetric analyses at a heating rate of 10 ℃/min. Approximately 8-10 mg sample was placed in an open alumina pan. A nitrogen flow of 40 mL/min was used prior and during pyrolysis. FT-IR spectra were measured by the KBr pellet method with a Nicolet-360 Fourier transform infrared spectro- photometer. The mass gain of the cured PCS fibers was measured at the room temperature by weighting PCS fibers before curing and after curing, and was calculated from Δm/m=(m2-m1)/m1, where Δm/m is the mass gain (%), m1 is the mass (g) of PCS fibers before curing, m2 is the mass (g) of PCS fibers after curing. The gel content is a usual index to characterize the solubility of macro-molecules. Here, a solvent of benzene and a 24 h reflux time were applied to get the gel content results.
3 Results and discussion
3.1 Thermal stability of PCS fibers
Fig.1 shows the TG results of PCS fibers. It indicated that weight increase started at approximately 200 ℃. From this temperature to 380 ℃, mass increase was observed. As shown in the Fig.1, the mass of the sample started to decrease at 400 ℃, and the mass loss could be ascribed to be the loss of hydrogen and low-molecular-weight hydrocarbons due to dehydrogenation and dehydrocarbonation condensation reaction and the thermal decomposition of fibers are very sensitive to oxygen during the side chains of the PCS fibers[9]. In a word, PCS fibers are very sensitive to oxygen during heat-treating process in air.

Fig.1 Thermogravimetry curve of PCS fibers in air
Fig.2 shows the TG results of PCS fibers under nitrogen atmosphere. From this curve, it can be seen that the process of PCS fibers turning into the inorganic state is divided into four states as[6]: the first stage from room temperature to 200 ℃, the second stage from 200 ℃ to about 400 ℃, the third stage from 400 ℃ to about 800 ℃ and the fourth stage above 800 ℃. In the first stage, there is little mass loss because of the evaporation of water. The mass loss is about 12%, which is caused by the evaporation of hydrogen and low-molecular-mass PCS during the second stage. It can be seen from the third stage that thermal decomposition of the side chains of the PCS leaded to about 20% mass loss. The fourth stage above 800 ℃, the conversion of the PCS into the inorganic state is almost terminated at about 800 ℃ and completes with further increase in temperature and the ceramic yield is about 50%[10].

Fig.2 Thermogravimetry curve of PCS fibers in nitrogen
3.2 Curing of PCS fibers
FT-IR of PCS fibers and cured PCS fibers heat-treated under different conditions are shown in Fig.3. The intensity of the absorption peak at 2 100 cm-1(Si─H, stretching vibration) of cured PCS fibers is evidently reduced compared with that of non-cured PCS fibers. The intensity of the peak at 1 250 cm-1 (Si─CH3, deformation vibration) is very similar. This reduction intensity at 2 100 cm-1 is considered to be attributed to the reaction of PCS fibers with oxygen, which supports the conclusion of Yajima et al[10] that the mechanism of curing involves the crosslinking upon oxidation of Si─H and Si─CH3 to produce Si─O─Si and Si─O─C bonds.
The Si─H bonds of PCS fibers decrease and the Si─O─Si bonds forms. Si─H reaction degree of PCS is measured through the characteristic peak’s ratio of Si─H to Si─CH3 in IR spectrum according to the formula[11] as

where A2100 and A1250 are the intensity of absorption at

Fig.3 FT-IR spectra of PCS fibers(a), cured PCS fibers heat-treated at 180 ℃ for 4 h(b) and cured PCS fibers heat-treated at 200 ℃ for 10 h(c)
2 100 cm-1 and 1 250 cm-1, respectively.
The relationship between PSi─H and mass gain in the cured PCS fibers is shown in Fig.4(a). PSi─H is Si—H reaction degree of PCS fibers. Fig.4(b) shows the relationship between gel content and mass gain in the cured PCS fibers. It shows that there is no gel content in cured PCS fibers when mass gain is smaller than 9%. The gel content increases with increasing mass gain and the gel content is 100% when mass gain is about 16%. It results in crosslinking on the surface[5], which enables the sample to retain its shape during high-temperature pyrolysis.
3.3 Theoretical derivation[12, 13]
Based on the experiment discussed above, curing process of PCS fibers in air can be described with a simple chemical equation as
≡Si—H+≡Si—CH3+O2(g)→≡Si—O—Si≡+
≡Si—O—C—Si≡+H2O+H2+CO2 (1)
A reaction rate may be generally defined as the reactant of conversion with respect to time. Assuming that curing of PCS fibers in air is a first-order chemical reaction, the kinetics equation can be written as
 (2)
(2)
where [O2] is oxygen concentration, which is constant because of the constant air flux during curing process. Y=H, CH3 and [Si─Y], [Si─Y]0, [Si─Y]c are the actual, initial, final concentration of Si─Y, respectively. Because the determination of the concentration of Si─Y is difficult, the mass gain in percentage is used in the kinetics equation. Then the first-order chemical reaction kinetics, Eqn.(3), is established, which is similar to the simple form of general conversion-dependence kinetics.

Fig.4 Relationship between mass gain and PSi—H(a) and gel content of PCS fibers(b) under different curing conditions
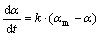 (3)
(3)
where  is mass gain in percentage of PCS fibers after curing time t,
is mass gain in percentage of PCS fibers after curing time t, 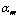 is the maximum ratio of mass gain after infinite curing time and k is the apparent reaction rate constant.
is the maximum ratio of mass gain after infinite curing time and k is the apparent reaction rate constant.
The temperature dependence of the rate constant, k, may be described by the Arrhenius expression:
 (4)
(4)
where A is the pre-exponential factor, E is the activation energy, R is the gas constant and T is the temperature. Substituting Eqn.(4) into Eqn.(3) and simplifying the result yields
 (5)
(5)
The integration of Eqn.(5) leads to the form as
 (6)
(6)
For the nonisothermal conditions, the practical curing process is generally carried out from room temperature to about 200 ℃ with a certain heating program. T is the function of t, thus a general form of the kinetic equation is obtained:
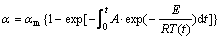 (7)
(7)
In fact, the right side of Eqn.(7) contains integral that generally cannot be integrable except for the isothermal process; numeric integration has to be adopted. In this process, the temperature zone is divided into much small zone and each zone is Ti-Ti+ΔT, the corresponding time zone is ti-ti+Δt. For each small zone, the temperature is considered to be a constant. Tav=(2Ti+ΔT)/2, which is the average temperature of Ti and Ti+ΔT. Thus, the mass gain ratio of PCS fibers can be expressed as
 (8)
(8)
From Eqn.(8), which describes the curing process of PCS fibers, we can obtain the mass gain of PCS fibers as a function of time and instruct the experiment with it.
In order to determine the activation energy in isothermal experiments, Eqn.(5) may be rearranged as
 (9)
(9)
The integration of Eqn.(9) leads to the integrated form as
 (10)
(10)
According to the above equation, the activation energy (E), can be obtained from the slope of  versus T-1 at a constant con- version level.
versus T-1 at a constant con- version level.
3.4 Results and discussions
In order to validate the theoretical derivation, curing of PCS fibers was performed under isothermal conditions. Table 1 lists the data of isothermal curing and related data processing, the holding time is 150 min.According to the discussion above, the activation energy (E), can be obtained from the slope of  as a function of T -1 (see Fig. 5).
as a function of T -1 (see Fig. 5).
Table 1 Data of isothermal curing and related data processing [14]

Fig.5 shows the relation between ln{ln[αm/(αm-α)]/t} as a function of T-1. It proves that the assuming of the curing of PCS fibers in air is a first-order chemical reaction is reasonable. The activation energy was obtained as 79.27 kJ/mol and the pre-exponential factor was calculated as 3.07×106.

Fig.5 Relationship of ln{ln[αm/(αm-α)]/150} and 1/T
Fig.6 shows the comparison of data between the experiment and the theory according to different heating schedules[15], HS means heating schedules. It shows good agreement and illuminates that the theoretical derivation is reasonable.

Fig.6 Comparison of data acquired from experiment (dot) and theory (line) according to different heating schedules
4 Conclusions
1) TG performed in air shows that there exists mass gain which starts approximately at 200 ℃ and PCS fibers are very sensitive to oxygen. In nitrogen, the PCS fibers would decompose with the increasing temperature in four stages and the ceramic yield is about 50%.
2) The curing of PCS fibers is oxidation of Si─H and Si─CH3, resulting in Si─O─Si and Si─O─C bonds, which results in crosslinking on the surface of the fibers and enables the sample to retain its shape during high-temperature pyrolysis.
3) The curing of PCS fibers is a first order chemical reaction. Its activation energy (E), is 79.27 kJ/mol, the pre-exponential factor (A), is calculated as 3.07×106. The related curing kinetics model of PCS fibers is  . Comparison of the theoretical date with experimental data of PCS fibers shows good agreement.
. Comparison of the theoretical date with experimental data of PCS fibers shows good agreement.
References
[1] JOHNSON D W, Evans A G, Goettler R W. Ceramic Fibers and Coatings: Advanced Materials for the Twenty-First Century [M]. Washington D C: National Academy Press, 1998. 1-49.
[2] LE P, Monthioux M, Oberlin A. Understanding Nicalon fiber [J]. J Euro Ceram Soc, 1993, 11(2): 95-103.
[3] Yajima S, Hayasgi J, Iimura M. Synthesis of continuous silicon carbide fiber with high tensile strength and high Young’s modulus [J]. Journal of Materials Science, 1978 (13): 2569-2576.
[4] Yajima S, Hayasgi J, Omori M, et al. Development of a SiC fibre with high tensile strength [J]. Nature, 1976, 261: 683-685.
[5] Ichikawa H, Teranishi H, Ishikawa T. Effect of curing conditions on the mechanical properties of SiC fiber (Nicalon) [J]. Journal of Materials Science Letters, 1987, 6(4): 420-422.
[6] Okamura K, Sato M. A study on the electron irradiation curing mechanism of polycarbosilane by solid-state 29Si high-resolution nuclear magnetic resonance spectroscopy [J]. Journal of Materials Science Letters, 1988 (7): 209-211.
[7] Sugimoto M, Shimoo T, Okamura K, et al. Reaction mechanisms of silicon carbide fiber synthesis by heat treatment of polycarbosilane fibers cured by radiation [J]. J of American Ceramic Society, 1995, 78(4): 1013-1016.
[8] WANG Y D, FENG C X, SONG Y C. Study on process of continuous SiC fibers [J]. Aerospace Materials and Technology, 1997(2): 21-25.(in Chinese)
[9] Hasegawa Y, Okamura K. Synthesis of continuous silicon carbide fibre(part 3): Pyrolysis of polycarbosilane and structure of the products [J]. Journal of Materials Science, 1983(18): 3633-3648.
[10] Hasegawa Y. Synthesis of continuous silicon carbide fibre(part 6): Pyrolysis process of cured polycarbosilane fibre and structure of SiC fibre [J]. Journal of Materials Science, 1989 (24): 1177-1190.
[11] ZHENG C M, LI X D, YU Y X. Preparation of high temperature resistance Si-Al-C-O fibers by polymer-derived method [J]. Journal of Materials Engineering, 2004, 12: 25-28.(in Chinese)
[12] LIN S T, HUANG S K. Study of curing kinetics of siloxane-modified DGEBA epoxy resins [J]. Journal of Applied Polymer Science, 1996, 62: 1641-1649.
[13] HE J, XUE D, XIN W Z. Study on the kinetics and mechanism of thermal degradation and crosslinking of PEEK by temperature programmed decomposition [J]. Acta Chimica Sinica, 1997, 55, 1152-1157.(in Chinese)
[14] WANG H. Study on the Curing Conditions and Inorganic Process of Polycarbosilane Fibers [M]. Changsha: National University of Defense Technology, 2000. 4. (in Chinese)
[15] ZHENG C M, ZHU B, LI X D. Study on thermal-curing of polycarbosilane fibers [J]. Acta Polymerica Sinica, 2004, 2: 246-250. (in Chinese)
Foundation item: Project (59972042) supported by the National Natural Science Foundation of China
Correspondence author: ZHENG Chun-man; Tel: +86-731-4576214; E-mail: zhengchunman@sohu.com
(Edited by LONG Huai-zhong)