Ni-TiO2复合纳米晶的晶粒生长及热稳定性
来源期刊:中国有色金属学报(英文版)2017年第10期
论文作者:牛特 陈为为 程焕武 王鲁
文章页码:2300 - 2309
关键词:Ni;TiO2;纳米晶;晶粒生长;热稳定性;扩散机制;激活能
Key words:Ni; TiO2; nanocrystalline; grain growth; thermal stability; diffusion mechanism; activation energy
摘 要:系统研究Ni-TiO2复合纳米晶的晶粒生长及热稳定性。通过电沉积法制备Ni-TiO2复合纳米晶,并在电沉积过程中改变纳米TiO2纳米颗粒含量,制得不同TiO2含量的Ni-TiO2复合纳米晶。详细讨论TiO2含量对晶粒尺寸、相结构和显微硬度的影响;并考察不同TiO2含量Ni-TiO2复合纳米晶在加热过程中对应晶粒的生长和扩散机制。400 °C退火处理保温90 min之后,掺入20 g/L TiO2的复合纳米晶具有最佳的显微硬度,达到HV50 270。激活能的计算结果表明,高温时晶格扩散为复合纳米晶生长的主导机制;高温时增加TiO2纳米颗粒含量能提高晶粒生长的激活能,从而抑制晶粒生长。
Abstract: The grain growth and thermal stability of nanocrystalline Ni-TiO2 composites were systematically investigated. The nanocrystalline Ni-TiO2 composites with different contents of TiO2 were prepared via electroplating method with the variation of TiO2 nano-particles concentration. The effect of TiO2 content on the grain size, phase structure and microhardness was investigated in detail. The corresponding grain growth and diffusion mechanisms during the heating process were also discussed. The optimal microhardness of HV50 270 was achieved for the composite with addition of 20 g/L TiO2 nano-particles after annealing at 400 °C for 90 min. The calculation of the activation energy indicated that lattice diffusion dominated at high temperatures for the nanocrystalline Ni-TiO2 composites. It was indicated that the increase of TiO2 nano-particles content took effect on restricting the grain growth at high temperatures by increasing the grain growth activation energy.
Trans. Nonferrous Met. Soc. China 27(2017) 2300-2309
Te NIU, Wei-wei CHEN, Huan-wu CHENG, Lu WANG
School of Materials Science and Engineering, Beijing Institute of Technology, Beijing 100081, China
Received 30 December 2016; accepted 18 June 2017
Abstract: The grain growth and thermal stability of nanocrystalline Ni-TiO2 composites were systematically investigated. The nanocrystalline Ni-TiO2 composites with different contents of TiO2 were prepared via electroplating method with the variation of TiO2 nano-particles concentration. The effect of TiO2 content on the grain size, phase structure and microhardness was investigated in detail. The corresponding grain growth and diffusion mechanisms during the heating process were also discussed. The optimal microhardness of HV50 270 was achieved for the composite with addition of 20 g/L TiO2 nano-particles after annealing at 400 °C for 90 min. The calculation of the activation energy indicated that lattice diffusion dominated at high temperatures for the nanocrystalline Ni-TiO2 composites. It was indicated that the increase of TiO2 nano-particles content took effect on restricting the grain growth at high temperatures by increasing the grain growth activation energy.
Key words: Ni; TiO2; nanocrystalline; grain growth; thermal stability; diffusion mechanism; activation energy
1 Introduction
Nanocrystalline materials have extremely fine structure and large volume fraction of grain boundaries, exhibiting unique mechanical properties including high hardness and high strength. Some of them have been applied to practical production [1-4]. However, it has generally been confirmed that pure nanocrystalline materials are thermally unstable with rapid grain growth at relatively low temperatures [5-7]. For example, nanocrystalline nickel shows initiatory grain growth in the range of 150-300 °C [8]. The large free energy of nanocrystalline nickel provides a strong driving for grain growth, which significantly reduces the size-dependent properties of nanocrystalline nickel [9-11]. Therefore, potential technological applications of the materials are restricted by unsatisfactory thermal stability. It is necessary to find an effective way to improve the thermal properties of nanocrystalline materials.
Many researches have been conducted to find out key factors that affect the thermal stability and abnormal grain growth of nanocrystalline materials, such as type and concentration of solute atoms or second-phase particles [6], initial texture [12], initial grain size distribution [13], and structural defects [14,15]. Besides, KACHER et al [16] studied the thermal stability of Ni/NiO multilayers through in-situ TEM, and this multilayer structure was formed in a certain concentration of NiO film, indicating that with increasing NiO content, the thermal stability of nanocrystalline Ni was shown to increase although computational and theoretical studies have suggested that solute atoms can increase the likelihood of abnormal grain growth. Similarly, second-phase nano-particles are dispersed into nanocrystalline materials in a stable way, supposed to achieve an equal effect like multilayer structure. Related studies [17-21] indicated that the second-phase nano-particles effectively inhibited the grain growth of nanocrystalline materials. It is suggested that second-phase nano-particles significantly enhance the microhardness of nanocrystalline materials [22-24]. Theoretically, second-phase nano-particles hinder the lattice diffusion during the grain growth, which has been verified by related studies mentioned above. But the effect of the dispersed nano-particles on thermal stability has not been fully understood. Therefore, the grain growth and thermal stability of nanocrystalline composites are required to be systematically investigated in order to understand the reinforcement mechanism of the second-phase nano-particles.
Nanocrystalline Ni-TiO2 composites were prepared in the manuscript in order to investigate the grain growth and thermal stability of the composites. For good understanding of the grain growth mechanism for the nanocrystalline pure metals typically Ni, we focused on the comparison of the grain growth and thermal stability of the Ni-TiO2 composites with different TiO2 contents. The effect of TiO2 content on the grain growth and thermal stability of the Ni-TiO2 composites was systematically investigated and the corresponding grain growth mechanism was discussed.
2 Experimental
TC4 plates with dimensions of 20 mm × 20 mm × 2 mm were used as the substrate, which were mechanically polished with SiC papers to a grit of #1200. Before electroplating, the specimens were pre-treated in a solution containing HCl 0.5 mL/L with HF 0.4 mL/L for 1 min. The concentrations of TiO2 nano-particles were set as 5, 10, 20 and 30 g/L (5, 10, 20 and 30 g/L samples are short for Ni-TiO2 composite samples with different TiO2 concentrations). After TiO2 nano-particles were added into the electrolyte and mechanically stirred at 200 r/min for 1 h, the electroplating was conducted immediately. At the same time, the solution was continuously magnetically stirred at 100 r/min. The electroplating was conducted at 50 mA/cm2 and room temperature for 30 min. The TiO2 nano-particles (China New Metal Co., Ltd.) have a crystalline phase of anatase type, with an average diameter of 50 nm.
In order to investigate the grain growth process, the samples were cut into dimensions of 5 mm × 5 mm × 2 mm by wire electrical discharge machining. Isochronal and isothermal annealing was conducted in air for the Ni-TiO2 nano-composites. During isochronal annealing, the composites were carried out at temperatures ranging from 100 to 400 °C for a constant time of 90 min, whereas for isothermal tests the composites were annealed at constant temperatures. 200, 250 and 350 °C were chosen respectively for different time ranging from 30 to 120 min. After annealing, the samples were quickly pulled out from the furnace and fast cooled in air.
The coating morphologies were analyzed using a field emission scanning electron microscope (FESEM) with an energy-dispersive spectroscopy (EDS) system. The phase structure of the coatings was determined using X-ray diffraction (XRD) with Cu Kα radiation (40 kV, 40 mA). Diffraction patterns were recorded in the 2θ range from 40o to 80o at a scanning rate of 0.02 (°)/s. Three FCC diffraction peaks, i.e., (111), (200) and (220), were used to calculate the average grain size of nanocrystalline Ni-TiO2 composites using the Scherrer equation. Moreover, the microhardness of the coatings was measured at a load of 50 g and a holding time of 15 s.
3 Results
3.1 Surface morphologies
Figure 1 shows surface morphologies of the Ni-TiO2 nano-composites with different concentrations of TiO2 nano-particles. Large spherical Ni nodules with sizes of 5-10 μm were clearly seen, on which there were many fine Ni nodules (~200 nm) as shown in the insets in Figs. 1(a) and (b). The size of the spherical Ni nodules in Fig. 1(c) is smaller than that in Figs. 1(a) and (b), which decreases to ~4 μm. The shape of the Ni nodules in Fig. 1(d) is different from that in Figs. 1(a)–(c). Besides a few spherical Ni nodules, there are more oval Ni nodules, which indicates a smoother surface for Ni-TiO2 composite with 30 g/L TiO2 compared with other composites.
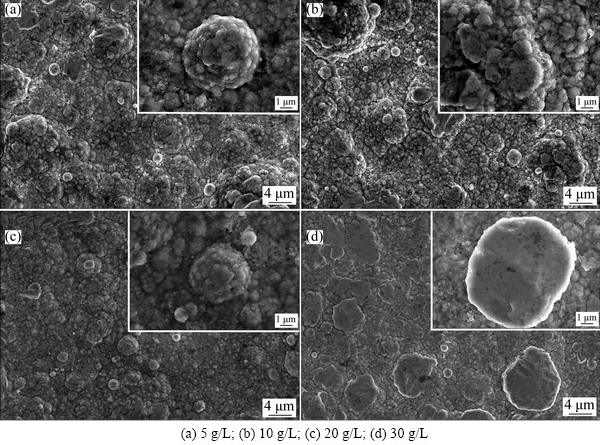
Fig. 1 SEM surface morphologies of Ni-TiO2 composites with different TiO2 concentrations
3.2 Content of TiO2
EDS spectrum was used to determine the mass fraction of TiO2 in the composites and to confirm a homogeneous TiO2 distribution throughout the thickness and along the faces of the electrodeposits. Repeated measurements were performed on the cross-sections of the composites to measure if any concentration gradients exist, owing to possible composition fluctuations during the electrodeposition process. Figure 2 shows the TiO2 content in the composites with different concentrations of TiO2 nano-particles. The TiO2 content was an average value calculated by three randomly chosen positions and was calculated based on element titanium content. The TiO2 content for 5 g/L samples amounts to 2.6% (mass fraction). For 10, 20 and 30 g/L samples, TiO2 contents are 3.3%, 3.9% and 5.6% (mass fraction), respectively.
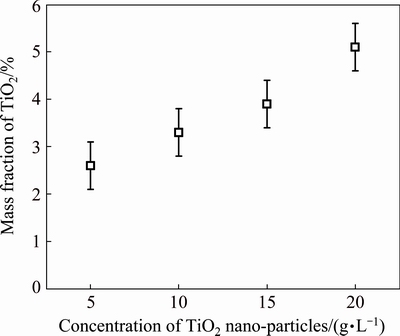
Fig. 2 Dependence of TiO2 content in composites on concentration of TiO2 nano-particles
According to related literature, GUGLIELMI [22] put forward a mechanism that the co-deposition of particles and metal was carried out by two steps. The first step is that particles are loosely absorbed into the cathode. The first step is physical absorption and reversible. In the second step, the charged particles are strongly absorbed onto the cathode under the action of electric field. The second step is nonreversible and the rate controlling step for co-deposition process. The mathematical model of co-deposition based on the above theory was experimentally validated in the Ni-TiO2 system. In general, the mass fraction of TiO2 is in parabolic curve relationship with concentration of TiO2 nano-particles. In Fig. 2, parabolic curve relationship was not completely presented. The mass fraction of TiO2 in the composite increased with the increase of the concentration of TiO2 nano-particles. It can be explained that the concentration of TiO2 nano-particles (30 g/L) in our experiment is not a saturated value. More TiO2 nano-particles are supposed to be absorbed onto cathode, and the mass fraction of TiO2 will continue to rise until TiO2 nano-particles saturate in the solution.
3.3 Phase structure
The effect of annealing on the phase structure of composites was analyzed from the XRD patterns in Fig. 3. Summarily, for the Ni-TiO2 composite with different concentrations of TiO2, electrodeposited nanocrystalline presents obviously preferential growth of Ni grains along (111) evidenced by the relative intensities for (111) as seen in Fig. 3. Annealing from 100 to 400 °C did not change preferential growth of Ni grains along (111) evidenced by the similar relative intensities of three peaks (111), (200) and (220). With the concentration of TiO2 nano-particles increasing, preferential growth of Ni grains along (111) is unchanged. For 5 and 10 g/L samples, annealing above 200 °C resulted in the slight growth of Ni grains along (200) evidenced by relative intensities of three peaks. For 20 and 30 g/L samples, annealing above 250 °C resulted in the slight growth of Ni grains along (200) evidenced by relative intensities of three peaks. Similarly, for all the Ni-TiO2 nano-composite samples, relative intensity of peak. (220) was obviously low even after annealing above 350 °C.
Therefore, the driving force for grain growth was probably same for the Ni-TiO2 composite with different concentrations of TiO2 nano-particles at these temperatures, although the transition temperature of TiO2 nano-particles (200 °C) is higher than that of the 5 and 10 g/L samples (250 °C). The 5, 20 and 30 g/L samples showed similar grain growth and orientation at high temperature as that at low temperature (Figs. 3(a), (c) and (d)). However, there is an exception shown in Fig. 3(b), the relative intensity of peak (200) is almost equal to that of peak (111), corresponding to the calculation of activation energy below.
Figure 4 shows the evolution of grain size after annealing for the Ni-TiO2 composites with different concentrations of TiO2 nano-particles. The grain size of nano-composites was calculated as ~25 nm. The grain sizes of the Ni-TiO2 composite with different concentrations of TiO2 nano-particles were almost stable when the annealing temperatures were below 150 °C. Therefore, given that the melting point (Tm) of Ni metal is 1453 °C (1726 K), the transition temperature can be calculated as 0.25Tm for the Ni-TiO2 composite. For all the Ni-TiO2 nano-composite samples, Ni grains significantly grew up when annealing was conducted above the transition temperature. And it can be concluded that the grain size continued to increase steadily at high temperatures (above 400 °C). In this work, TiO2 nano-particles were expected to enhance the thermal stability of the composites. With the content of TiO2 nano-particles increasing, the average grain size of 30 g/L samples was slightly smaller than that of other samples, and at high temperatures, the difference between 30 g/L samples and 5 g/L samples was considerable. It can be concluded that TiO2 nano- particles restrict the grain size during grain growth.
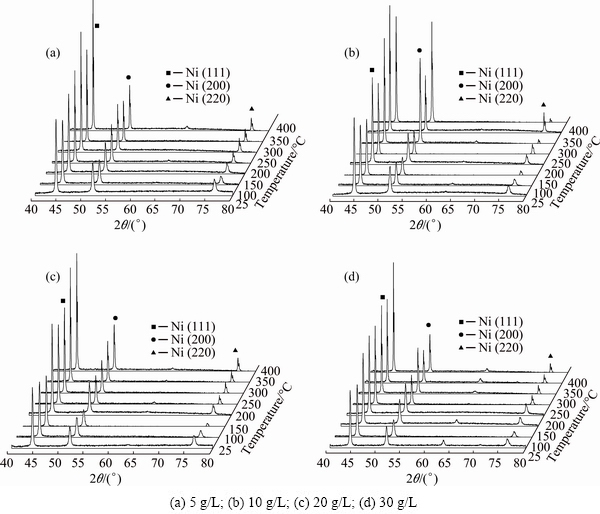
Fig. 3 XRD patterns of composites after annealing at different temperatures with different TiO2 concentrations
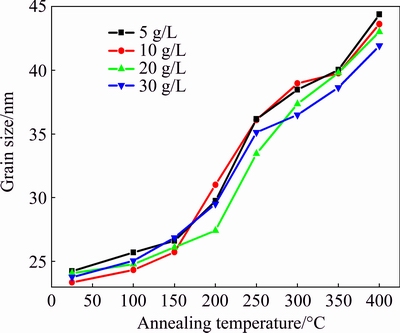
Fig. 4 Dependence of grain size on annealing temperature at different TiO2 concentrations
3.4 Microhardness
Figure 5 exhibits the effect of annealing on the microhardness of the Ni-TiO2 composites with different concentrations of TiO2 nano-particles. The 20 and 30 g/L samples possessed a higher microhardness of HV50 ~350 compared to HV50 ~300 of the 5 and 10 g/L samples. The microhardness of the 20 and 30 g/L samples was kept at HV50 ~350 when annealing below the transition temperature(150 °C, 0.25Tm), followed by a relatively steady decline, reaching HV50 ~270 when annealing at 400 °C for 90 min, which is consistent with the changes of grain size (Fig. 5). For the 5 and 10 g/L samples, the microhardness decreased from HV50 ~300 to HV50 ~210, which was similar to the 20 and 30 g/L samples.
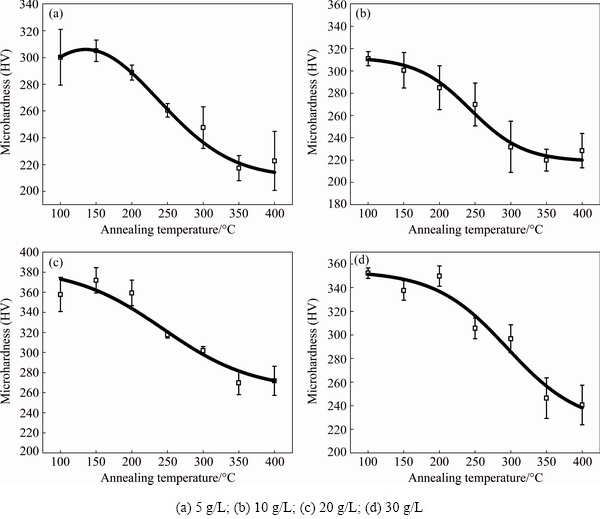
Fig. 5 Microhardness as function of annealing temperature for Ni-TiO2 composites with different TiO2 concentrations
4 Discussion
Based on the above experimental results, it is clear that the distribution of TiO2 nano-particles significantly influenced the grain growth behavior. The detailed mechanism for grain growth was discussed below.
4.1 Grain growth kinetics
The grain growth is a thermally activated process. In order to quantitatively understand the grain growth process of the traditional and sol-enhanced nano-composites, an Arrhenius equation is used below:
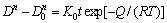 (1)
(1)
where D is the average grain size, D0 is the initial (pre-growth) average grain size, n is the grain growth exponent, K0 is the frequency term, t is the time, Q is the activation energy, R is the mole gas constant, and T is the absolute temperature. At a determined temperature, the grain growth exponent n can be calculated from a plot of the logarithm of grain growth rate, dD/dt, versus the logarithm of grain size D according to a mathematic conversion of Eq. (1) below:
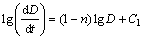 (2)
(2)
where C1 is a constant.
Figure 6 shows the isothermal annealing process for the grain size evolution as a function of annealing time. Using the grain growth data shown in Fig. 6, the logarithm of grain growth rate, dD/dt, was plotted against the logarithm of grain size dD as shown in Fig. 7. The uncertainty in the plots in Fig. 7 exists due to the uncertainty inherent to the computation of a derivative from the data in Fig. 7. The grain growth exponent n values for 5 g/L samples at 200, 250 and 350 °C were calculated to be 38.3, 44.3 and 53.5; for 10 g/L samples, the corresponding values were 15.3, 24.2, and 52.8; for 20 g/L samples, the corresponding values were 13.5, 27.7 and 57.3; and for 30 g/L samples, the corresponding values were 10.8, 17.3 and 60.1.
The elementary theories of grain growth, either based on the proportionality of the growth rate to the interfacial free energy per unit volume [24] or based on the inverse proportionality of the rate of boundary migration to the boundary curvature [25], predict a value of 2 for n. However, most nanocrystalline materials show n values in a range of 1.5-40 [6,23,24]. The calculation of 10, 20 and 30 g/L samples exhibited similarly low values of n as 15.3, 13.5 and 10.8 respectively, at the relatively low temperatures, implying similar kinetics for the grain growth. The significant differences of n between the relatively high and low temperature ranges were related to changes of grain growth mechanisms.

Fig. 6 Instantaneous grain size as function of annealing time for Ni-TiO2 composites with different TiO2 concentrations and annealed at different temperatures
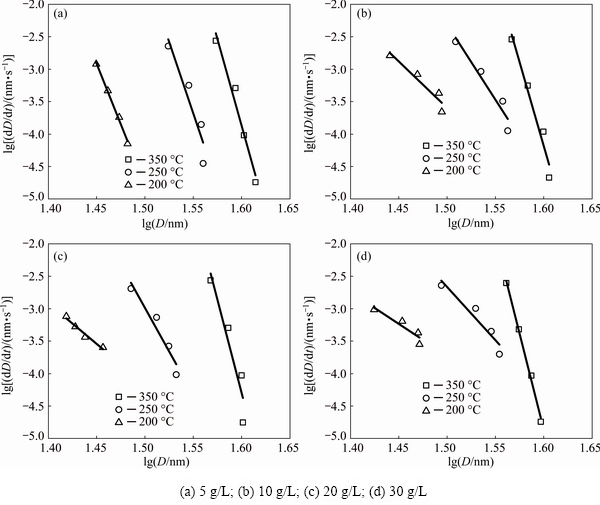
Fig. 7 Plots of lg(dD/dt) versus lg D for Ni-TiO2 composites with different TiO2 concentrations and annealed at different temperatures
4.2 Activation energy
The activation energy Q for the grain growth can be evaluated from a plot of ln(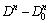 ) versus the reciprocal of absolute temperature, 1/T, according to a conversion form of Eq. (1) as follows:
) versus the reciprocal of absolute temperature, 1/T, according to a conversion form of Eq. (1) as follows:
 (3)
(3)
Due to the variation of n with temperature, the activation energy Q calculated with Eq. (3) will not be in such an accuracy. However, the values of n in the corresponding temperature sections are still rational in determining the ranges of activation energy Q [6]. In the present annealing process, given that the transition temperature was 0.25-0.3Tm for both composites, the values of n were regarded to be 38.3, 15.3, 13.5, 10.8 at temperatures below 200 °C for 5, 10, 20 and 30 g/L samples, respectively. Meanwhile, at high temperatures above (250 and 350) °C, n values were regarded as 44.2 and 53.8 for the 5 g/L samples, correspondingly, 24.2 and 52.8 for the 10 g/L samples. 27.7 and 57.3 for the 20 g/L samples, 17.3 and 60.1 for the 30 g/L samples.
Using the grain size data in Fig. 4, the plots of ln(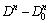 ) against 1/T are shown in Fig. 8 with two temperature ranges. Linear relationships were well observed. Accordingly, the values of Q were obtained from the slope of the straight lines (-Q/R).
) against 1/T are shown in Fig. 8 with two temperature ranges. Linear relationships were well observed. Accordingly, the values of Q were obtained from the slope of the straight lines (-Q/R).
At low temperatures below 0.25Tm, the grain growth activation energy Q for the 5 g/L samples was calculated to be 81 kJ/mol (Fig. 8(a1)), 63.8 kJ/mol (Fig. 8(b1)) for the 10 g/L samples, 33.7 kJ/mol (Fig. 8(c1)) for the 20 g/L samples and 36.5 kJ/mol (Fig. 8(d1)) for the 30 g/L samples. Whereas above 0.25Tm, Q was 196-242 kJ/mol for 5 g/L samples (Fig. 8(a2)). The corresponding values for the 10, 20 and 30 g/L samples were 101-220 kJ/mol (Fig. 8(b2)), 156-329 kJ/mol (Fig. 8(c2)) and 69.2-240 kJ/mol (Fig. 8(d2)), respectively. The activation energy for grain boundary diffusion for nanocrystalline Ni was calculated to be 100-140 kJ/mol in Refs. [26-31] and the corresponding value for lattice diffusion was reported to be 200- 290 kJ/mol [27,29,32]. Obviously, the present activation energy values at low temperatures, i.e., 63.8, 33.7 and 36.5 kJ/mol for the 10, 20 and 30 g/L samples respectively, are much lower than those required for grain boundary diffusion or lattice diffusion, reflecting that the grain growth at low temperatures may be controlled by the re-ordering of grain boundaries. CHAUHAN and MOHAMED [23] reported that the activation energy for the re-ordering of grain boundaries was 11 kJ/mol for electrodeposited nanocrystalline Ni, which is close to the value of the present Ni-TiO2 composites (Figs. 8(c1) and (d1)). Typically, nano- crystalline materials have high free energy associated with the large volume fraction of grain boundary component [32-34]. Correspondingly, a low energy is required to relax structure and rearrange atoms at grain boundaries.
By comparing with the literature values mentioned above, we can conclude that the grain growth for the Ni-TiO2 composite with different concentrations of TiO2 was controlled by the lattice diffusion at high temperatures. Different grain growth processes for all nano-composites were possibly caused by the dispersive distribution of second-phase nano-particles. It is known that the resistant force, P, of second-phase particles against the grain growth is expressed as
 (4)
(4)
where f is the volume fraction of second-phase particles, γ is the interfacial energy, and r is the particle size. It is implied that the composites mixed with the most second-phase particles should require the highest activation energy.
However, it can be noticed in Figs. 8(a2)-(d2) that the variation of activation energy is not expected as the formula above at high temperature. The addition TiO2 nano-particles was supposed to limit the grain growth and further influence the diffusion process radically, so increasing the TiO2 content is likely to enhance the resistant force. According to the results in Fig. 4, the average grain size at high temperatures proves that the resistant force of nano-particles exists and with the TiO2 content increasing, the resistant force has been enhanced to some extent. In the low temperature range, recovery is the dominant thermally activated phenomenon. In this process, dislocation movement is the fundamental assumption [35] and thus an increase in the activation energy for the grain growth of the composite material can be attributed to fact that dislocation movement has been decelerated by the nano-particles. At higher temperatures, pinning of grain boundaries is the possible mechanism for the increase of grain growth activation energy. It seems that nano-particles have great effect in this process, as the activation energy of 242.2 kJ/mol for the composite with 5 g/L nano-particles has increased to 329.3 kJ/mol for the composite with 20 g/L nano- particles.

Fig. 8 Arrhenius plots of ln( ) versus 1/T to estimate activation energy for grain growth of Ni-TiO2 composites with different TiO2 concentrations
) versus 1/T to estimate activation energy for grain growth of Ni-TiO2 composites with different TiO2 concentrations
Meanwhile, there is no similar corresponding relation in the composite with 10 g/L nano-particles. This can be attributed to the fact that the grain growth is also controlled by phase difference among grains. The phase difference of neighboring grains has significant influence on the migration of grain boundaries [36]. If there are high-angle grain boundaries among the grains, migration velocity of boundaries is accelerated due to the increase of grain boundary energy and diffusion coefficient. For nanocrystalline Ni prepared by electrodeposition, (111) (200) and (220) are common preferential growth of Ni grains. As shown in Fig. 3, the XRD pattern of the samples with 10 g/L TiO2 nano-particles is quite different from others. When the samples with 10 g/L TiO2 nano-particles were maintained at 400 °C, the preferred orientation growth changed from (111) into (200). It seems reasonable to conjecture that the variation of the preferred orientation growth affects the activation energy.
5 Conclusions
1) Ni-TiO2 composites with different contents of TiO2 nano-particles were prepared by electroplating. The microstructure of each Ni-TiO2 composite has certain degree of similarities. The variation of content of nano-particles changes the mass fraction of TiO2 in the Ni-TiO2 composites.
2) Ni-TiO2 composite with the highest content of TiO2 nano-particles did not show the highest microhardness and the most optimal thermal stability. Thermal and kinetics analysis indicated that with content of nano-particles changing, interaction between nano- particles and Ni nanocrystalline at low or high temperature was completely different. Calculation of the activation energy indicated that lattice diffusion dominated at high temperatures for the nanocrystalline Ni-TiO2 composites.
3) Nano-particles have been fairly effective at high temperature, as the activation energy of 242.2 kJ/mol for the composites with 5 g/L nano-particles has increased to 329.3 kJ/mol for the composites with 20 g/L nano- particles. However, there is no similar corresponding relation in the composites with 10 g/L nano-particles. The variation of the preferred orientation growth may well influence the activation energy.
4) The related experiment will proceed to describe the interaction between second phase and nanocrystalline at high temperature in a more scientific way. Our experiment provided a theoretical understanding for the development of thermal stability of electroplating composites, which will have potential applications in various fields.
References
[1] RAMASAMY P, LIM D H, KIM B, LEE S H, LEE M S, LEE J S.All-inorganic cesium lead halide perovskite nanocrystals for photo detector applications [J]. Chemical Communications, 2016, 52(10): 2067-2070.
[2] KUMAR V, SHARMA D K, SHARMA K, DWIVEDI D K. Structural, optical and electrical characterization of vacuum- evaporated nanocrystalline CdSe thin films for photo sensor applications [J]. Applied Physics A, 2016, 122(11): 960-966.
[3] LIU Guang-yin, WANG Hui-yuan, LIU Guo-qiang, YANG Zhi-zheng, JIN Bo, JIANG Qi-chuan. Facile synthesis of nanocrystalline Li4Ti5O12, by microemulsion and its application as anode material for Li-ion batteries[J]. Journal of Power Sources, 2012, 220(220): 84-88.
[4] KHAYATI G R, JANGHORBAN K. An investigation on the application of process control agents in the preparation and consolidation behavior of nanocrystalline silver by mechanochemical method [J]. Advanced Powder Technology, 2012, 23(6): 808-813.
[5] CHOOKAJORN T, MURDOCH H A, SCHUH C A. Design of stable nanocrystalline alloys [J]. Science, 2012, 337(6097): 951-955.
[6] LIN C S, LEE C Y, CHANG C F, CHANG C H. Annealing behavior of electrodeposited Ni-TiO2 composite coatings [J]. Surface and Coatings Technology, 2006, 200(12): 3690-3697.
[7] LIU Yi-chun, LIU Lei, SHEN Bin, HU Wen-bin. A study of thermal stability in electrodeposited nanocrystalline Fe-Ni invar alloy [J]. Materials Science and Engineering A, 2011, 528(18): 5701-5705.
[8] CHEN Wei-wei, GAO Wei. Thermal stability and tensile properties of sol-enhanced nanostructured Ni-TiO2 composites [J]. Composites (Part A): Applied Science and Manufacturing, 2011, 42(11): 1627-1634.
[9] GODON A, CREUS J, COHENDOZ S, CONFORTO E, FEAUGASX, GIRAULT P, SAVALL C. Effects of grain orientation on the Hall-Petch relationship in electrodeposited nickel with nanocrystalline grains [J]. Scripta Materialia, 2010, 62(2): 403-406.
[10] MARVEL C, YIN D, CANTWELL P. The influence of oxygen contamination on the thermal stability and hardness of nanocrystalline Ni-W alloys [J]. Materials Science and Engineering A, 2016, 664: 49-57.
[11] TIAN Zong-jun, WANG Dong-sheng WANG Gui-feng, SHEN Li-da, LIU Zhi-dong, HUANG Yin-hui. Microstructure and properties of nanocrystalline nickel coatings prepared by pulse jet electrodeposition [J]. Transactions of Nonferrous Metals Society of China, 2010, 20(6): 1037-1042.
[12] KACHER J, HATTAR K, ROBERTSON I M. Initial texture effects on the thermal stability and grain growth behavior of nanocrystalline Ni thin films [J]. Materials Science and Engineering A, 2016, 675: 110-119.
[13] LIU X C, ZHANG H W, LU K. Strain-induced ultrahard and ultrastable nanolaminated structure in nickel [J]. Science, 2013, 342(6156): 337-340.
[14] WASEKAR N P, HARIDOSS P, SESHADRI S K, SUNDARARAJAN G. Sliding wear behavior of nanocrystalline nickel coatings: Influence of grain size [J]. Wear, 2012, 296(1-2): 536-546.
[15] HIBBARD G D, RADMILOVIC V., AUST K T, ERB U. Grain boundary migration during abnormal grain growth in nanocrystalline Ni [J]. Materials Science and Engineering A, 2008, 494(1-2): 232-238.
[16] KACHER J, ELIZAGA P, HOUSE D S, HATTAR K, NOWELL M, ROBERTSON M I. Thermal stability of Ni/NiO multilayers [J] Materials Science and Engineering A, 2013, 568: 49-60.
[17] Lü Biao, HU Zhen-feng, WANG Xiao-he, XU Bin-shi. Thermal stability of electrodeposited nanocrystalline nickel assisted by flexible friction [J]. Transactions of Nonferrous Metals Society of China, 2015, 25(10): 3297-3304.
[18] CHAI Guang-yu, SUN Ying, SUN Jian-ren, CHENQuan-fang. Mechanical properties of carbon nanotube copper nanocomposites [J]. Journal of Micromechanics & Microengineering, 2008, 18(3): 237-262.
[19] ARAI S, SAITO T, ENDO M. Cu-MWCNT composite films fabricated by electrodeposition [J]. Journal of the Electrochemical Society, 2010, 157(3): 147-153.
[20] HAQ I U, AKHTAR K, KHAN T I, SHAH A A. Electrodeposition of Ni-Fe2O3, nanocomposite coating on steel [J]. Surface and Coatings Technology, 2013, 235: 691-698.
[21] LI Zhi-ming, YANG Xiao-ping, ZHANG Jun-bao, ZHENG Bao-long, ZHOU Yi-zhang, SHAN Ai-dang, ENRIQUE J L. Microstructure evolution and mechanical behavior of cold-sprayed, bulk nanostructured titanium [J]. Metallurgical and Materials Transactions A, 2014, 45(11): 5017-5028.
[22] GUGLIELMI N. Kinetics of the deposition of inert particles from electrolytic baths [J]. Journal of the Electrochemical Society, 1972, 119(8): 1009-1012.
[23] CHAUHAN M, MOHAMED F A. Investigation of low temperature thermal stability in bulk nanocrystalline Ni [J]. Materials Science and Engineering A, 2006, 427(S): 7-15.
[24] TJONG S C, CHEN H. Nanocrystalline materials and coatings [J]. Materials Science and Engineering R, 2004, 45(1): 1-88.
[25] MOHAMED F A, SHEN-ANN S, LANGDON T G. The activation energies associated with superplastic flow [J]. Acta Metallurgica, 1975, 23(12): 1443-1450.
[26] FARKAS D, MOHANTY S, MONK J. Linear grain growth kinetics and rotation in nanocrystalline Ni [J]. Physical Review Letters, 2007, 98(16):165502.
[27] BASU R, ESKANDARI M, UPADHAYAY L, MOHTADI-BONAB M A, SZPUNA J A. A systematic investigation on the role of microstructure on phase transformation behavior in Ni-Ti-Fe shape memory alloys [J]. Journal of Alloys and Compounds, 2015, 645(7): 213-222.
[28] GUPTA R K, RAMAN R K S, KOCH C C. Electrochemical characteristics of nano and microcrystalline Fe-Cr alloys [J]. Journal of Materials Science, 2012, 47(16): 1-7.
[29] MA Li, ZHANG Long, LI Xiao-bing , LI Zhi-you , ZHOU Ke-chao. Fabrication and characterization of electrodeposited nanocrystalline Ni-Fe alloys for NiFe2O4 spinel coatings [J]. Transactions of Nonferrous Metals Society of China, 2015, 25(1): 146-153.
[30] PROKOSHKINA D, ESIN V A, WILDE G, DIVINSKI S V. Grain boundary width, energy and self-diffusion in nickel: Effect of material purity [J]. Acta Materialia, 2013, 61(14): 5188-5197.
[31] HOSSEINI S N, ENAYATI M H, KARIMZADEH F. Nanoscale grain growth behavior of CoAl intermetallic synthesized by mechanical alloying [J]. Bulletin of Materials Science, 2014, 37(3): 383-387.
[32] NI H T, ZHANG X Y, CHEN X, YU L. Strain effects on annealing behaviors of moderately deformed nanocrystalline nickel [J]. Materials Science and Technology, 2012, 28(6): 754-759.
[33] PELLICER E, VAREA A, SIVARAMAN K M, PANE S, SURINACH S, BARO M D, NOGUES J, NELSON B J, SORT J. Grain boundary segregation and interdiffusion effects in nickel–copper alloys: an effective means to improve the thermal stability of nanocrystalline nickel [J]. ACS Applied Materials & Interfaces, 2011, 3(7): 2265-2274.
[34] SAWATZKI S,  C, ENER S. Grain boundary diffusion in nanocrystalline Nd-Fe-B permanent magnets with low-melting eutectics [J]. Acta Materialia, 2016, 115: 354-363.
C, ENER S. Grain boundary diffusion in nanocrystalline Nd-Fe-B permanent magnets with low-melting eutectics [J]. Acta Materialia, 2016, 115: 354-363.
[35] RADI Y, MAHMUDI R. Effect of Al2O3, nano-particles on the microstructural stability of AZ31 Mg alloy after equal channel angular pressing [J]. Materials Science and Engineering A, 2010, 527: 2764-2771.
[36] HU Geng-xiang, CAI Xu, RONG Yong-hua. Fundamentals of materials science [M]. 3rd ed. Shanghai: Shanghai Jiao Tong University Press, 2012. (in Chinese).
牛 特,陈为为,程焕武,王 鲁
北京理工大学 材料学院,北京 100081
摘 要:系统研究Ni-TiO2复合纳米晶的晶粒生长及热稳定性。通过电沉积法制备Ni-TiO2复合纳米晶,并在电沉积过程中改变纳米TiO2纳米颗粒含量,制得不同TiO2含量的Ni-TiO2复合纳米晶。详细讨论TiO2含量对晶粒尺寸、相结构和显微硬度的影响;并考察不同TiO2含量Ni-TiO2复合纳米晶在加热过程中对应晶粒的生长和扩散机制。400 °C退火处理保温90 min之后,掺入20 g/L TiO2的复合纳米晶具有最佳的显微硬度,达到HV50 270。激活能的计算结果表明,高温时晶格扩散为复合纳米晶生长的主导机制;高温时增加TiO2纳米颗粒含量能提高晶粒生长的激活能,从而抑制晶粒生长。
关键词:Ni;TiO2;纳米晶;晶粒生长;热稳定性;扩散机制;激活能
(Edited by Wei-ping CHEN)
Foundation item: Project (51401024) supported by the National Natural Science Foundation of China; Project (20150942006) supported by Basic Research Program of Beijing Institute of Technology, China
Corresponding author: Wei-wei CHEN; Tel: +86-10-68912709-109; E-mail: wwchen@bit.edu.cn
DOI: 10.1016/S1003-6326(17)60256-5

