Growth changes and tissues anatomical characteristics of giant reed (Arundo donax L.) in soil contaminated with arsenic, cadmium and lead
来源期刊:中南大学学报(英文版)2010年第4期
论文作者:郭朝晖 苗旭锋
文章页码:770 - 777
Key words:heavy metal; pollution; giant reed (Arundo donax L.); growth response; tissues anatomical characteristics; ecoremediation
Abstract: A greenhouse experiment was conducted to elucidate the growth changes and tissues anatomical characteristics of giant reed (Arundo donax L.), a perennial rhizomatous grass, which was cultivated for 70 d in soils contaminated with As, Cd and Pb. The results show that giant reed rapidly grows with big biomass of shoots in contaminated soil, possessing strong metal-tolerance with limited metal translocation from roots to shoots. When As, Cd and Pb concentrations in the soil are less than 254, 76.1 and 1 552 mg/kg, respectively, plant height and dried biomass are slightly reduced, the accumulation of As, Cd and Pb in shoots of giant reed is low while metal concentration in roots is high, and the anatomical characteristics of stem tissues are thick and homogeneous according to SEM images. However, plant height and dried biomass are significantly reduced and metal concentration in plant shoots and roots are significantly increased (P<0.05), the stems images become heterogeneous and the secretion in vascular bundles increases significantly when As, Cd and Pb concentrations in the soil exceed 334, 101 and 2 052 mg/kg, respectively. The giant reed is a promising, naturally occurring plant with strong metal-tolerance, which can be cultivated in soils contaminated with multiple metals for ecoremediation purposes.
基金信息:the National Natural Science Foundation of China
J. Cent. South Univ. Technol. (2010) 17: 770-777
DOI: 10.1007/s11771-010-0555-8![]()
GUO Zhao-hui(郭朝晖), MIAO Xu-feng(苗旭锋)
School of Metallurgical Science and Engineering, Central South University, Changsha 410083, China
? Central South University Press and Springer-Verlag Berlin Heidelberg 2010
Abstract: A greenhouse experiment was conducted to elucidate the growth changes and tissues anatomical characteristics of giant reed (Arundo donax L.), a perennial rhizomatous grass, which was cultivated for 70 d in soils contaminated with As, Cd and Pb. The results show that giant reed rapidly grows with big biomass of shoots in contaminated soil, possessing strong metal-tolerance with limited metal translocation from roots to shoots. When As, Cd and Pb concentrations in the soil are less than 254, 76.1 and 1 552 mg/kg, respectively, plant height and dried biomass are slightly reduced, the accumulation of As, Cd and Pb in shoots of giant reed is low while metal concentration in roots is high, and the anatomical characteristics of stem tissues are thick and homogeneous according to SEM images. However, plant height and dried biomass are significantly reduced and metal concentration in plant shoots and roots are significantly increased (P<0.05), the stems images become heterogeneous and the secretion in vascular bundles increases significantly when As, Cd and Pb concentrations in the soil exceed 334, 101 and 2 052 mg/kg, respectively. The giant reed is a promising, naturally occurring plant with strong metal-tolerance, which can be cultivated in soils contaminated with multiple metals for ecoremediation purposes.
Key words: heavy metal; pollution; giant reed (Arundo donax L.); growth response; tissues anatomical characteristics; ecoremediation
1 Introduction
Heavy metal pollution of the biosphere has accelerated rapidly since the onset of the industrial revolution and the toxicity of metal elements now poses a major environmental problem. Non-ferrous industrial activities, especially from the mining, mineral processing and smelting activities for non-ferrous metal ores, generate a large amount of waste materials that are deposited on the terrestrial surface, contributing to the enhanced soil concentrations of toxic elements, such as As, Cd and Pb. As and Cd are concomitant elements and Pb is an ore element in Pb/Zn ores. They can penetrate water and soil, reduce the crop quality through the adsorption by plant roots, directly enter the body by inhalation or ingestion of contaminated soil, plant or water [1-2], and result in a potential hazard for human health via the polluted food chain [3]. So, it is imperative to develop an environmentally friendly remediation technology for non-ferrous industrial contaminated soils in order to reduce the potential hazard of toxic metals for soil quality, environmental safety and human health. The immediate goals of soil remediation, however, are principally to restore a vegetative cover, minimize soil erosion and pollution spreading, and remove toxic metals from cultivated land by means of plants [4].
Phytoremediation is reported to be a quite efficient and environmentally friendly means for cleaning up metals and metalloids contaminated soils. The use of low cost, fast growing plants with efficient biomass producing plant species has been highly desired for metal-contaminated soils [5-6]. In particular, plants with multiple tolerances to heavy metals such as cattail (Typha latifolia) and common reed (Phragmites australis) are used successfully for Pb-Zn mine tailings stabilization [7-8], fibre crops such as flax (Linum usitatissimum L.) and hemp (Cannabis sativa L.) are suitable for growing in industrially polluted regions for the removal of heavy metals from soil [9], and giant reed (Arundo donax L.) can thrive in soils heavily contaminated with Cd and Ni [10-11] and in wastewater contaminated with As [12]. All of these plants show the strong metal tolerance and are potential of economic value after harvesting [13].
The giant reed, belonging to the Graminae family, is a tall perennial rhizomatous grass and is among the fastest growing terrestrial plants in the world. It is native to the freshwater regions of Eastern Asia and widely planted in other temperate and subtropical regions of the world, such as Southern Europe, Northern Africa, Australia, America, and can reproduce by spreading outwards or by clumps broken off from the adult plant. The giant reed has a variety of uses, such as for music tools with stem, for medicine with roots and for soil erosion control as revegetation. When being cultivated this fast-growing introduced crop attains a potentially high yielding non-food crop [10] that can meet requirements for energy, paper pulp production, biofuels and construction of building materials [14-16]. Giant reed may also act as chemically activated carbon [17] or biofiltering systems to treat wastewater [17-18], thus recycling nutrients and water, and produce value added products [18]. The high annual growth and cellulose content make the giant reed a potential crop for converting solar energy to industrial fibre or biofuels [14]. Although the giant reed is interesting for many scientists to use all kinds of objectives, there is limited information on the metal-tolerant capacity [10-11], the stress-related growth changes and tissues anatomical characteristics of the giant reed used for the ecological remediation of multiple-metal contaminated soils, especially for metal-contaminated soils from the mining and smelting areas [19].
In this work, the giant reed was found, which grew on multiple-metals contaminated soils from the non- ferrous mining and smelting areas of Hunan Province, China, in late 2002. It accumulated low concentrations of metals in the plant and had strong metal-tolerant capacity according to the field investigation and preliminary gravel hydroponic cultures utilizing half-strength Hoagland’s nutrient solution containing As, Cd or Pb. Based on the preliminary results, the objectives of this work were to: (1) study the metal-tolerant capacity and accumulation levels of the giant reed for As, Cd and Pb in spiked soils, (2) identify stress response of the giant reed in controlled environments by determining plant height and biomass yields, and stem anatomical characteristics using scanning electron microscopy (SEM) with energy dispersive X-ray (EDX), and (3) assess the potential of the giant reed to perform ecological remediation for metal-contaminated soils from industrially polluted areas, especially from the vicinity of non-ferrous metals mining and smelting areas.
2 Materials and methods
2.1 Test soil
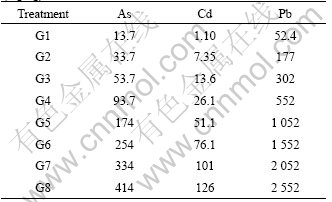
The tested soil belongs to allitic udic ferrisols, which developed from the typical quaternary red clay. Surface soil (0-20 cm) sample was collected from the vicinity of the Yuelu Campus of Central South University and the basic physiochemical properties were analyzed (Table 1). Tested soil (5 kg) was potted in each plastic tackle, of which the diameter was 32 cm and the height was 26 cm. The soil was homogeneously sprayed with aqueous solutions of test elements, which were prepared by dissolving salts Na3AsO4·12H2O, CdCl2·2?H2O and PbCl2·2H2O into deionized water, at seven contamination levels (hereafter termed treatments G2 to G8, see Table 2), respectively. The mole ratios of As, Cd and Pb in soils are based on the average ratio of As, Cd and Pb in contaminated soils, which are heavily contaminated by non-ferrous mining and smelting activities, from the vicinity of nonferrous industrial areas in Hunan Province of China. The spiked soils were stored in the dark for 15 d prior to planting. Treatment G1 was applied using deionized water without external As, Cd or Pb (Table 2). All treatments were applied to four replicates.
Table 1 Basic physiochemical properties of tested soil
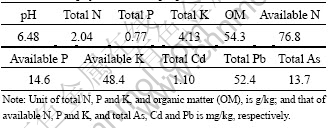
Table 2 Total concentrations of As, Cd and Pb in treated soils (mg/kg)
2.2 Culture and harvest for plant
Root systems (roots and rhizomes) of the giant reed, which had begun to sprout, were collected from the uncontaminated areas in the vicinity of mining and smelting areas of Hunan Province, China. The concentrations of total As, Cd and Pb in uniform size rhizome cuttings were 0.03, 0.01 and 0.07 mg/kg, respectively. Uniform size root systems cutting per pot was cultivated for the duration of the greenhouse experiment with a daily 14 h photoperiod (photosynthetic photon flux 260-350 mmol/(m2?s) provided by Phillips 400 W high-pressure sodium lamps) and a 10 h dark period, with temperature maintained at (22±3) ℃ during the photoperiod and (18±2) ℃ during the dark period.
During the growing period of giant reed, fertilizers containing 0.5 g CO(NH2)2, 1.5 g KH2PO4 and 1.6 g KNO3 were mixed to each pot before being transplanted and irrigating to each pot after being planted for 30 d, respectively. The plants were harvested after being cultivated for 70 d and carefully washed with tap water and deionized water. Shoots and roots were then separated and cut with stainless steel scissor, and dried at 60 ℃ for 48 h for elemental analysis.
Basic physiochemical soil properties were determined using the following procedures [20]. Specifically, pH was determined using a soil to water solution (1?2.5, g:mL). The soil organic matter was oxidized with K2Cr2O7. Available N was extracted with 1.0 mol/L NaOH by a micro-diffusion technique and titrated with 0.01 mol/L H2SO4; available P (Olsen-P) was extracted using 0.5 mol/L NaHCO3 with pH 8.5, while P in the extract was determined spectrophotometrically; and available K was extracted with 1.0 mol/L NH4OAc, pH 7.0 and determined with atomic absorption spectrometry (AA-6800, Shimadzu, Japan).
The soil and plant samples (including uniform size rhizome cuttings samples) were ground and passed through 0.25 mm sieve, then digested with HF-HNO3- HClO4 and HNO3-HClO4, respectively [20]. Dried soil sample about 0.20 g was accurately weighed into clean, dry digestion poly-tetrafluoro-ethylene crucible (50 mL) and firstly digested with a solution of HF (10 mL) and concentrated HNO3 (2 mL) to near dryness at 140 ℃. Subsequently, a second addition of HF (5 mL) was made and again the mixture was evaporated to near dryness. Finally, HClO4 (2 mL) alone was added and evaporated until the appearance of white fumes. The residue was dissolved in 1:1 HNO3 (2 mL) and diluted to 50 mL. The dried plant sample about 1.0 g was accurately weighed into clean, dry digestion poly-tetrafluoro-ethylene crucibles (50 mL). Concentrated HNO3 (5 mL) was added and allowed to stand overnight. On the following day, HClO4 (2 mL) was added and the crucibles were placed on a heating oven and the temperature was raised to 140 ℃ for digestion until the solution was nearly drying. After digestion the solutions were cooled, 2 mL 1:1 HNO3 was added and diluted to 50 mL with deionized water and filtered into acid-washed plastic bottles. The concentrations of metals in the solutions were determined using an inductively coupled plasma optical emission spectrometer (ICP-OES, IRIS Intrepid II XSP, USA). Quality assurance of metal analysis was assessed using duplicates, standard reference materials (soil of GBW 08303 and tea plant of GBW 08513) and standard reference solution in the process of analysis in order to verify the accuracy and precision of the digestion procedure and subsequent analysis. To determine the significance (P<0.05) of differences between treatments, the data were analyzed using software of SPSS 11.0.
2.4 Scanning electron microscopy
The anatomical structures of stems of plants in treatments G1 (control), G2 (slightly contaminated treatment), G4 (moderately contaminated treatment) and G7 (heavily contaminated treatment) was examined by means of scanning electron microscopy (SEM). The transversal section samples (about 5 mm×5 mm×3 mm) of the internodes of giant reed stems were fixed using a low-temperature freeze-drying method [21], which involved plunging specimens into 2.5% (volume fraction) glutaraldehyde for more than 2 h, then 1% osmium acid for 2 h, followed by three washes with 0.1 mol/L H3PO4 buffer solution. The samples were dehydrated in a tert-butanol dilution series of 50%, 70%, 80%, 90% and 95% (15 min in each solution). Finally, the stem samples were placed in a vacuum-freeze-drier and each specimen after Au coated was examined with a scanning electron microscope-energy dispersive X-ray (KYKY2800 SEM- EDX, Shimadzu, Japan).
3 Results
3.1 Giant reed’s height and biomass
After cultivation for 70 d, the shoot heights of plants in treatments G1 to G6 were similar and significantly (P<0.05) greater than those of plants growing in treatments G7 and G8 (Table 3). The shoot heights of plants in treatments G2 to G6 were slightly different by the presence of heavy metals in the soil, as compared with the control (treatment G1) (Table 3). The results showed that the shoot height was slightly affected under the light and moderate contamination of As, Cd and Pb in soils, where the metal contamination was less than 254, 76.1 and 1 552 mg/kg, respectively. However, the length of roots, which randomly changed from 0.23 to 0.29 m, was slightly different among different treatments as uniform size root systems were planted at the beginning of cultivation.
Table 3 Shoot height and dried biomass of giant reed plants after 70 d cultivation
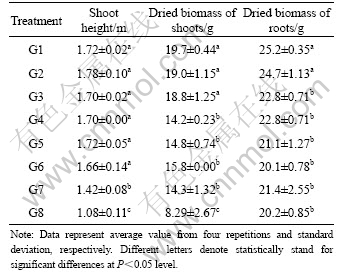
The dry weights of the shoots that included the stems and leaves and roots of the giant reed in the contaminated soils were obtained (Table 3). The dried biomass of shoots from treatments G1 to G3 was significantly higher (P<0.05) than that of plants growing in soils containing at least 93.7 mg/kg As, 26.1 mg/kg Cd and 552 mg/kg Pb. Although the concentrations of As, Cd and Pb in the soil increased sequentially from treatment G4 to G7, the dried biomass of plant shoots in these four treatments was still high and the plants were still able to grow with big biomass. However, the dried biomass of plant shoots in treatment G8 was significantly (P<0.05) reduced compared to that of plant shoots in treatments G1 to G7. Thus, extreme concentrations of As, Cd and Pb in contaminated soils reaching or exceeding 414, 126 and 2 552 mg/kg, respectively, were unsuitable for the growth of the giant reed. The dried biomass of plant roots in treatments G1 and G2 was also significantly (P<0.05) higher than that of plant roots in other treatments, respectively (Table 3). The dried biomass of plant roots in other treatments, however, was very similar and suitable for growing up with the increase of As, Cd and Pb concentrations in soils.
3.2 Metals accumulation and stress symptoms of giant reed
The concentrations of As, Cd and Pb in giant reed shoots from treatment G1 were significantly (P<0.05) less than those from treatment G2, however, the concentrations of As and Cd in plant shoots from treatment G3 were slightly higher than those from treatment G2 while the concentrations of Pb in plant shoots from treatment G3 were significantly (P<0.05) higher than those from treatment G2. According to Table 4, the concentrations of As, Cd and Pb in plant shoots from treatments G1, G2 and G3 were very low. With the increase of concentrations of As, Cd and Pb in soils from treatments G4 to G7, the concentrations of As, Cd and Pb in plant shoots were significantly (P<0.05) increased compared to those from treatments G1 to G3, but, it was slight difference among treatments G5, G6 and G7, the concentrations of As, Cd and Pb in plant shoots from treatments G4 to G7 were still low. In treatments G4 to G7, the concentrations of As, Cd and Pb in plant shoots were increased from 1.51, 2.80 and 1.33 mg/kg to 1.76, 3.82 and 1.44 mg/kg, respectively. The accumulation of As, Cd and Pb in giant reed shoots was low in treatments G1 to G7 (Table 4). When the concentrations of As, Cd and Pb in soils arrived at those in treatment G8, the concentrations of As, Cd and Pb in plant shoots were extensively (P<0.05) increased compared to those from treatments G1 to G7, and arrived to 5.71, 4.96 and 1.65 mg/kg, respectively. The concentrations of As, Cd and Pb in plant roots, however, were obviously higher than those in plant shoot. The results showed that the accumulations of As, Cd and Pb in giant reed were mainly accumulated in the roots. The concentrations of As, Cd and Pb in giant reed roots in treatments G1 to G3 were only from 2.34, 1.46, 1.56 mg/kg to 8.14, 4.52, 34.45 mg/kg, respectively, but, the concentrations of As, Cd and Pb in plant roots from treatments G4 to G6 were very high and significantly increased from 24.96, 16.61 and 99.42 mg/kg in G4 plant roots to 30.61, 26.83 and 139.88 mg/kg in G6 plant roots (Table 4). The results showed that the giant reed accumulated high concentrations of As, Cd and Pb in plant roots with a limited ability to translocate metals from roots to shoots. The giant reed had strong metal tolerance in lightly or moderately contaminated soils, and could adjust to the high-stress environment when heavy metals were present, in which the metal concentrations of As, Cd and Pb in contaminated soil were less than 254, 76.1 and 1 552 mg/kg, respectively.
Table 4 Concentrations of As, Cd and Pb in dry giant reed plants after 70 d cultivation (mg/kg)

3.3 Stem anatomical characteristics of metal-stressed giant reed
Botanically distinct parts could have different responses to physiological mechanisms of metal tolerance [22]. Typical SEM images of the transversal section for the giant reed stem in different contamination conditions were illustrated and the anatomy characteristics differed significantly among treatments G1, G2, G4 and G7. The images of transversal sections of the internode revealed the prevalence of three tissue systems in Fig.1, including epidermal (or cortical parenchyma), fundamental (or ground parenchyma) and vascular (composed of fibro-vascular bundles). The images of epidermis and the cortical parenchyma of the transversal sections of the giant reed stem from treatments G1 and G2 were thick and the fibre rings between epidermis and inner cortex, and vascular tissues (ground parenchyma) were homogeneous (Figs.1(a)-(b)). The epidermis of the stem was circular with a large stoma, leaving a sharp margin between inner cortex and ground parenchyma in stem tissue. The cortical parenchyma was constituted by outer layers of thick-walled cells and thin-walled cells in the inner region arranged in a regular directional pattern. They comprised concentric rings of hard waxy epidermis and outer cortical cells, a thick sclerified fibre band and a thick inner cortex. The fibre band included small vascular bundles evenly distributed along its outer edge. The inner cortex comprised the bulk of the stem tissue and was made up of a mixture of vascular bundles and parenchyma cells. The reeds grown in treatments G1 and G2 had an obviously higher proportion of cortical vascular bundles with a continuous fibre and a lower proportion of xylem. All these tissue types were recognizable in a transversal section of the internode of the giant reed stem while all of these appearances in treatment G4 were similar (Fig.1(c)). By comparing the transversal section structures with those of treatments G4 and G7 (Figs.1(c)-(d)), the epidermis in treatments G4 and G7 was thin. The sizes of stoma between epidermis and inner cortex in treatment G4 became small and the margin between inner cortex and ground parenchyma in stem tissue became ambiguous, and in treatment G7, however, was dismissed. The images of the fibre rings and vascular tissues were multiplex, and the thickness of the cortical parenchyma was thin in treatment G7. The average content of the parenchyma in the giant reed was significantly higher while the proportion of vascular tissue and fibers was less compared to those in treatments G1 and G2. The images of epidermis and inner cortex became strongly sclerified and directly combined with the cortical parenchyma in treatment G7. The substantial changes in the transversal section of the internode appeared to depend on the conditions employed, such as the concentrations of As, Cd and Pb in soils, and in agreement with results inferred from transversal section properties.
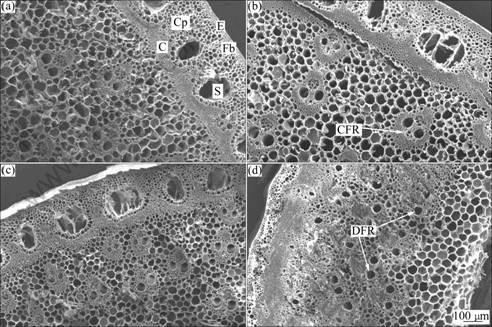
Fig.1 SEM images for transversal sections of internode of giant reed stems after 70 d cultivation (C, inner cortex; CFR, vascular bundles with continuous fibre rings; Cp, cortical parenchyma; DFR, vascular bundles with discontinuous fibre rings; E, epidermis and outer cortical cells; Fb, fibre band; S, stoma): (a) Treatment G1; (b) Treatment G2; (c) Treatment G4; (d) Treatment G7
The vascular bundle characteristics in the inner cortex of stem tissues of the giant reed in treatments G1, G2 and G4 were significantly different compared to those in treatment G7 (Fig.2). Under treatments G1 and G2, each vascular bundle consisted of three tissue types, including xylem, phloem and a larger fibre area, and a higher proportion of vascular bundles with a continuous fibre ring as reported by KOLESIK et al [23]. In treatments G1 and G2, the vascular bundles in the stem with continuous fibre rings regularly distributed and the cellular structure of the stems was homogeneous (Figs.2(a)-(b)), and the sizes of vascular bundles mostly were about 4.5 ?m. With the moderately contaminated treatment G4, the vascular bundles and cellular structure of the stems with fibre rings became discontinuous and distortion increased (Fig.2(c)). Vascular bundles became heterogeneous and disordered, with sizes ranging from about 4 to 2.5 ?m. The images of vascular tissues in treatment G4 still did not notably change compared to those in treatments G1 and G2, illustrating that the stress physiological response of giant reed stems was slight when grown in moderately contaminated soils. Vascular bundles in the stems from treatment G7, however, distributed more irregularly than those observed in treatment G4, and the sizes of vascular bundles were mostly from 2 to 3 ?m (Figs.2(c)-(d)). Vascular cells were even more distorted and contained an increased amount of secretion in vascular bundles compared to those in treatments G1, G2 and G4. The secretion in vascular bundles in treatment G7 was significantly increased, and resembled small balls whose sizes were from about 0.1 to 0.5 ?m and mainly comprised of carbon and oxygen (67% and 24%, respectively) according to the analysis of SEM with EDX.4 Discussion
The giant reed is an emergent-rooted wetland plant species with both the stems and the roots having the ability to propagate easily. In this work, there were no significant differences in the quantity of biomass produced in control and slightly contaminated treatments G1 to G3, in which the concentrations of As, Cd and Pb in soils were less than 53.7, 7.35 and 177 mg/kg, respectively and the giant reed was still suitable for growing in moderately contaminated treatments G4 to G6 with a big biomass yield and a low metal- accumulation, in which the concentrations of As, Cd and Pb in soils were less than 254, 76.1 and 1 552 mg/kg, respectively. The experimental results showed that the giant reed had high tolerance to As, Cd and Pb in soils, which could not be utilized for food production, and the ecoremediation using giant reed should be met the complex valuable use for both contaminated soils and plant biomass after harvesting.
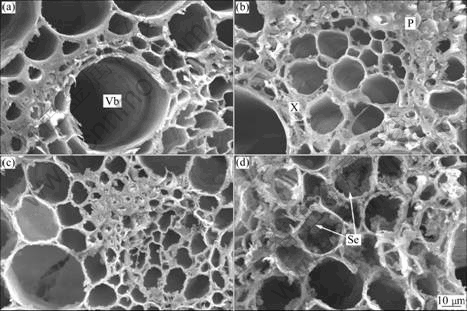
Fig.2 SEM images for vascular bundles of internode of giant reed stems after 70 d cultivation (P, phloem; Se, secretion; Vb, vascular bundle; X, xylem): (a) Treatment G1; (b) Treatment G2; (c) Treatment G4; (d) Treatment G7
The stem tissues anatomical characteristics of transversal sections of the giant reed also illustrated the low metal-accumulation and high tolerance to As, Cd and Pb in soil. The differences in the images for the transverse sections and anatomical characteristics of vascular bundles in the internode of giant reed stem tissues showed that the tissue constitution varied significantly between reeds of different quality, similar to the stress physiological mechanism of Viola principis H. de Boiss. to As and Pb [22]. Our finding demonstrated that vascular bundles in stem tissues with large, continuous fibre rings occurred as a response to slightly or moderately contaminated soil condition (treatments G1, G2 and G4) corresponding with small, semi- continuous fibre rings of stem tissues in the heavily contaminated soils (treatment G7), suggesting internal mechanisms inside plant cells limited As, Cd and Pb in soils to transfer from the plant roots to plant shoots. Although the results obtained with plants grown in pots could not be directly compared to those in field conditions, they were crucial to highlight the response of plants in controlled conditions. Nevertheless, more research regarding the giant reed would be performed under field conditions for multi-metal contaminated soils in the vicinity of non-ferrous metal mining and smelting areas in order to determine the utility of the giant reed for ecological remediation engineering.
5 Conclusions
(1) The height and biomass of giant reed shoots (including stems and leaves) are only slightly affected by slight or moderate contamination of As, Cd and Pb in the soil concentrations less than 254, 76.1 and 1 552 mg/kg, respectively. The accumulation of As, Cd and Pb in shoots of giant reed is low while metal concentration in roots is high, illustrating the limited capacity for metal translocation from roots to shoots. However, the height and biomass of giant reed shoots in contaminated soils are significantly (P<0.05) reduced while the concen- trations of As, Cd and Pb in the shoots and roots increase significantly, and stress symptoms are obvious when metal concentrations in the soil reach or exceed 334, 101 and 2 052 mg/kg, respectively.
(2) The anatomical characteristics of the transversal section of the stem tissues in treatments G1, G2 and G4 are thick and the images of the fibre rings and vascular tissues are homogeneous according to images from SEM analysis with EDX. But, the images of stem tissues become strongly sclerified and the vascular bundles become heterogeneous while the secretion in vascular bundles significantly increases when As, Cd and Pb concentrations in the soil approximately exceed 334, 101 and 2 052 mg/kg, respectively.
Acknowledgement
The authors thank Associate Professor Dr. JUHASZ A, from the Centre for Environmental Risk Assessment and Remediation, University of South Australia, for his assistance with the manuscript.
References
[1] MCGRATH S P, LOMBI E, GRAY C W, CAILLE N, DUNHAM S J, ZHAO F J. Field evaluation of Cd and Zn phytoextraction potential by the hyperaccumulators Thlaspi caerulescens and Arabidopsis halleri [J]. Environmental Pollution, 2006, 141(1): 115-125.
[2] CHARY N S, KAMALA C T, RAJ D S S. Assessing risk of heavy metals from consuming food grown on sewage irrigated soils and food chain transfer [J]. Ecotoxicology and Environmental Safety, 2008, 69(3): 513-524.
[3] CUI Y J, ZHU Y G, ZHAI R H, CHEN D Y, HUANG Y Z, YI Q, LIANG J Z. Transfer of metals from soil to vegetables in an area near a smelter in Nanning, China [J]. Environment International, 2004, 30(6): 785-791.
[4] WONG M H. Ecological restoration of mine degraded soils, with emphasis on metal contaminated soils [J]. Chemosphere, 2003, 50(6): 775-780.
[5] WEI C Y, CHEN T B. Arsenic accumulation by two brake ferns growing on an arsenic mine and their potential in phytoremediation [J]. Chemosphere, 2006, 63(6): 1048-1053
[6] TANHAN P, KRUATRACHUE M, POKETHITIYOOK P, CHAIYARAT R. Uptake and accumulation of cadmium, lead and zinc by Siam weed [Chromolaena odorata (L.) King & Robinson] [J]. Chemosphere, 2007, 68(2): 323-329.
[7] YE Z H, BAKER A J M, WONG M H, WILLIS A J. Zinc, lead and cadmium tolerance, uptake and accumulation by Typha latifolia [J]. New Phytologist, 1997, 136(3): 469-480.
[8] YE Z H, BAKER A J M, WONG M H, WILLIS A J. Zinc, lead and cadmium tolerance, uptake and accumulation by the common reed, Phragmites australis (Cav.) Trin. ex Steudel [J]. Annals of Botany, 1997, 80(3): 363-370.
[9] ANGELOVA V, IVANOVA R, DELIBALTOVA V. Bio-accumulation and distribution of heavy metals in fibre crops (flax, cotton and hemp) [J]. Industrial Crops and Products, 2004, 19(3): 197-205.
[10] PAPAZOGLOU E G, KARANTOUNIAS G A, VEMMOS S N, BOURANIS D L. Photosynthesis and growth responses of giant reed (Arundo donax L.) to the heavy metals Cd and Ni [J]. Environment International, 2005, 31(2): 243-249.
[11] PAPAZOGLOU E G. Arundo donax L. stress tolerance under irrigation with heavy metal aqueous solutions [J]. Desalination, 2007, 211(1/3): 304-313.
[12] MIRZA N, MAHMOOD Q, PERVEZ A, AHMAD R, FAROOQ R, SHAH M M, AZIM M R. Phytoremediation potential of Arundo donax in arsenic-contaminated synthetic wastewater [J]. Bioresource Technology, 2010, 101(15): 5815-5819.
[13] FISCHEROV? Z, TLUSTO? P, SZ?KOV? J, ?ICHOROV? K. A comparison of phytoremediation capability of selected plant species for given trace elements [J]. Environmental Pollution, 2006, 144(1): 93-100.
[14] NASSO N N O D, ANGELINI L G, BONARI E. Influence of fertilization and harvest time on fuel quality of giant reed (Arundo donax L.) in central Italy [J]. European Journal of Agronomy, 2010, 32(3): 219-227.
[15] VERVERIS C, GEORGHIOU K, CHRISTODOULAKIS N, SANTAS P, SANTAS R. Fiber dimensions, lignin and cellulose content of various plant materials and their suitability for paper production [J]. Industrial Crops and Products, 2004, 19(3): 245-254.
[16] ABRANTES S, EM?LIA M, COSTA A P, SHATALOV A A, DUARTE A P. Evaluation of giant reed as a raw-material for paper production [J]. Appita Journal, 2007, 60(5): 410-415.
[17] BASSO M, CUKIERMAN A. Wastewater treatment by chemically activated carbons from giant reed: Effect of the activation atmosphere on properties and adsorptive behavior [J]. Separation Science and Technology, 2006, 41(1): 149-165.
[18] MAVROGIANOPOULOS G, VOGLI V, KYRITSIS S. Use of waste water as a nutrient solution in a closed gravel hydroponic culture of giant reed (Arundo donax) [J]. Bioresource Technology, 2002, 82(2): 103-107.
[19] STOLTZ E, GREGER M. Accumulation properties of As, Cd, Cu, Pb and Zn by four wetland plant species growing on submerged mine tailings [J]. Environmental and Experimental Botany, 2002, 47(3): 271-280.
[20] LU Ru-kun. Analytical methods of soil agricultural chemistry [M]. Beijing: Agriculture Science and Technology Press of China, 1999: 1-227. (in Chinese)
[21] TAKAHASHI-IWANAGA H. Comparative anatomy of the podocyte: A scanning electron microscopic study [J]. Microscopy Research and Technique, 2002, 57(4): 196-202.
[22] LEI M, CHEN T B, HUANG Z C, WANG Y D, HUANG Y Y. Simultaneous compartmentalization of lead and arsenic in co-hyperaccumulator Viola principis H. de Boiss.: An application of SRXRF microprobe [J]. Chemosphere, 2008, 72(10): 1491-1496.
[23] KOLESIK P, MILLS A, SEDGLEY M. Anatomical characteristics affecting the musical performance of clarinet reeds made from Arundo donax L. (Gramineae) [J]. Annals of Botany, 1998, 81(1): 151-155.
Foundation item: Project(20507022) supported by the National Natural Science Foundation of China
Received date: 2009-09-10; Accepted date: 2010-04-09
Corresponding author: GUO Zhao-hui, PhD, Associate professor; Tel: +86-731-88836442; E-mail: zhguo@mail.csu.edu.cn
(Edited by YANG You-ping)

