J. Cent. South Univ. (2012) 19: 30-35
DOI: 10.1007/s11771-012-0968-7
Effects of oxidation treatment on properties of SiO2f/SiO2-BN composites
LI Duan(李端), ZHANG Chang-rui(张长瑞), LI Bin(李斌),
CAO Feng(曹峰), WANG Si-qing(王思青), YANG Bei(杨备), LIU Kun(刘坤)
State Key Laboratory of Advanced Ceramic Fibers & Composites, College of Aerospace & Materials Engineering, National University of Defense Technology, Changsha 410073, China
? Central South University Press and Springer-Verlag Berlin Heidelberg 2012
Abstract: The silica fiber reinforced silica and boron nitride-based composites (SiO2f/SiO2-BN) were prepared firstly via the sol-gel method and then the urea route, and the effects of oxidation treatment on the component, structure, mechanical and dielectric properties of the composites were investigated. The results show that the oxidation treatment at 450 °C will not impair the structure of boron nitride, and carbon is the main impurity with the excessive urea. The density of SiO2f/SiO2-BN composites is 1.81 g/cm3, and the flexural strength and elastic modulus are 113.9 MPa and 36.5 GPa, respectively. After oxidation treatment, the density varies to 1.80 g/cm3, and the flexural strength and elastic modulus are decreased to 58.9 MPa and 9.4 GPa, respectively. The mechanical properties of the composites are severely damaged, but they still exhibit a good toughness. The composites show excellent dielectric properties with the dielectric constant and loss tangent being 3.22 and 0.003 9, respectively, which indicates that the oxidation treatment is ineffective to improve the dielectric properties of SiO2f/SiO2-BN composites.
Key words: radome; boron nitride; urea route; composites; oxidation treatment; wave-transparent structure; mechanical properties; dielectric properties
1 Introduction
In recent years, continuous fiber reinforced ceramic matrix composites have received considerable attention for structural and functional applications, of which the silica fiber reinforced silica composites (SiO2f/SiO2) are especially attractive due to their low dielectric constant and loss tangent, high ablation resistance, thermal shock damage resistance and chemical stability [1-4]. With these excellent properties, SiO2f/SiO2 composites have been widely used in the field of high temperature wave-transparent structures, like an electromagnetic window or a radome [5-7].
However, a severe drawback of SiO2f/SiO2 composites prepared by sol-gel method is their high porosity, which in turn leads to low density, low strength and low rain erosion resistance, as well as the poor moisture-proof ability, and this to a large extent limits their applications on the aircrafts of higher Mach numbers [1, 2, 4].
As one of the most important nitride ceramics, boron nitride (BN) possesses a number of highly desirable properties [8-10]. Its high thermal stability (as high as 2 800 °C in a non-oxidizing atmosphere and up to 900 °C in the air), fine dielectric properties over a wide temperature range, excellent thermal shock resistance, coupled with its good corrosion resistance, make it a promising material to overcome the above- mentioned problems. To bring BN particles to SiO2f/SiO2 composites is considered as a useful method to increase the density and decrease the porosity of the material, which may make a good structural integrity. In addition, SiO2 grains will be coated by the BN particles, leading to the improvement of the moisture-proof ability.
Urea route is a smart method to fabricate the nitride ceramics because of its low cost, convenience and feasibleness as well as its possibility to prepare nanomaterials, which has attracted an increasing number of researches on it during these years, and many kinds of nitride ceramics and composites have been synthesized such as BN, TiN, Fe3Mo3N, SiC/BN and ZrO2/BN [10-15]. LII et al [10] have prepared turbostratic boron nitride (t-BN) films on carbon fibers and graphite substrates by dip-coating in methanolic boric acid and urea solutions followed by nitriding in an ammonia flow at 1 000 °C. GOMATHI et al [11-12] have obtained nanoparticles of BN, TiN and NbN as well as the ternary metal oxynitrides like Fe3Mo3N by heating mixtures of H3BO3, TiCl4, NbCl5 and the corresponding metal oxides with urea in the temperature range of 850-1 000 °C. SUN et al [13] have synthesized TiN powders by reactive ball milling of titanium powders and urea at room temperature. SiC/BN nanocomposite powders have been fabricated via a chemical reaction of boric acid and urea on the surface of SiC particles in a nitrogen gas by WANG et al [14]. In the research of LI et al [15], 3Y-ZrO2/BN ceramic composites with nano-sized BN have been obtained via in situ reaction between boric acid and urea on the surface of 3Y-ZrO2 particles in nitrogen gas. However, until now, there have been few reports about the silica fiber reinforced silica and boron nitride-based (SiO2f/SiO2-BN) composites via the urea route. In our previous work [16], urea and boron acid were used to prepare SiO2f/SiO2-BN composites, which exhibited superior mechanical and dielectric properties. Whereas, the carbon produced by urea during the high preparation temperature would be adverse to the properties of the composites. One of the effective methods to remove carbon is the oxidation treatment above 400 °C, which may in turn cause some bad effects on the composites.
In this work, based on the preparation of SiO2f/SiO2-BN composites, the effects of oxidation treatment on the component, structure, mechanical and dielectric properties of the composites were primarily investigated.
2 Experimental
2.1 Raw materials
Silica fibers used in the present work were produced by Feilihua Quartz Glass Corporation (Jingzhou, China) with the following characteristics: purity ≥99.95%, density 2.2 g/cm3, tensile strength 1.7 GPa, elastic modulus 78 GPa and diameter 6-8 μm. The fibers were woven into 2.5-dimensional fabric with a fiber volume fraction about 45% by Beijing FRP Research and Design Institute (Beijing, China). Boron acid (H3BO3, AR, Sinopharm Chemical Reagent Co., Ltd, Shanghai, China) and urea (CO(NH2)2, AR, Sinopharm Chemical Reagent Co., Ltd, Shanghai, China) were also used.
2.2 Preparation process
As the starting materials, SiO2f/SiO2 composites were prepared by the sol-gel method based on the above-mentioned silica fiber fabric and silica sol.
SiO2f/SiO2-BN composites were fabricated via the urea route as follows. Firstly, the solution for infiltration was made by mixing CO(NH2)2 and H3BO3 at a molar ratio of 3:2 with ethanol to a total volume of 500 mL. Secondly, SiO2f/SiO2 composites were infiltrated with the solution in vacuum at about 70 °C for 5 h. Finally, the composites were dried and heated in flowing nitrogen at about 900 °C for 2 h.
To discuss the effects of oxidation treatment on the properties of SiO2f/SiO2-BN composites, they were heat-treated in the air at 450 °C for 2 h and then cooled to room temperature.
2.3 Measurement and characterization
Thermogravimetric-differential thermal analysis of boron nitride was conducted using a thermal analyzer (TG-DTA, Rigaku Thermoflex, DT-40, Japan) in oxygen atmosphere with a flow rate of 40 mL/min. The samples were heated at 10 °C/min to the final temperature of 1 250 °C. An investigation of bondings was performed via Fourier transform infrared spectrometer (FT-IR, Avatar 360, Nicolet Instrument Corp., Wisconsin, USA) on discs pressed from composite powders mixed with KBr. X-ray diffractometer (XRD, D8 Advance, Bruker/Axs Corp., Germany) was employed to examine the crystalline phase and its preferred orientation using Cu Kα radiation. The bulk density of composites was calculated from the mass to volume ratio. Three-point flexural testing was performed on a computer controlled universal testing machine (WDW-100, Changchun Research Institute of Testing Machines, China) with a span of 30 mm and crosshead speed of 0.5 mm/min carried out on specimens with a dimension of 3 mm× 4 mm×35 mm. Five specimens were used to calculate the average values. The fracture surface of the composites was examined by scanning electron microscope (SEM, FEI Sirion 200, Holland). The dielectric properties (dielectric constant e and loss tangent tand) were evaluated in Kα band range at room temperature by a resonant cavity method using the TE01δ mode. It was examined using a vector network analyzer (Hewlett- Packard, Hp8720ES) with 1 Hz resolution. The size of the specimens was 15.8 mm×7.9 mm×(5-10) mm.
3 Results and discussion
3.1 Effects of oxidation treatment on component and structure of composites
It has been already reported that the main process for the synthesis of BN by using boric acid and urea can be written as follows [10, 12, 14]:
2CO(NH2)2(s)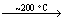 NH3(g)+
NH3(g)+
H2N-CO-NH-CO-NH2(s) (1)
2H3BO3(s)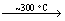 B2O3(s)+3H2O(g) (2)
B2O3(s)+3H2O(g) (2)
B2O3(1)+2NH3(g)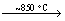 2BN(s)(amorphous)+
2BN(s)(amorphous)+
3H2O(g) (3)
So, we can see that boron nitride prepared at 900 °C is amorphous. Figure 1 shows the TG-DTA curves of boron nitride powders heated in oxygen atmosphere. The curves give the information concerning the mass loss and heat flow corresponding with the oxidation of boron nitride. It is distinct that the oxidation of boron nitride takes place at about 860 °C and ends at about 1 080 °C, which is a exothermal process. The mass loss is -22.7%, showing the increase in mass of BN, which can also be seen in the reaction (4). Besides, the latter mass loss at about 1 200 °C is probably due to the volatilization of boron oxide (B2O3) as well as the errors of the instrument. Therefore, the oxidation of boron nitride does not work at 450 °C.
4BN(s)+3O2(g)→2B2O3(s)+2N2(g) (4)
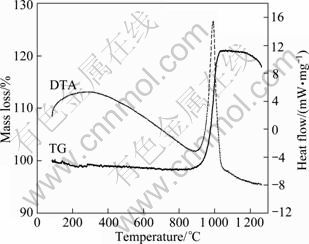
Fig. 1 TG-DTA curves of boron nitride powders in oxygen atmosphere
The FT-IR spectra of SiO2f/SiO2-BN composites before and after oxidation treatment are shown in Fig. 2. With the strong and broad absorption peaks located at 1 400 and 790 cm-1 belonging to B—N stretching and bending vibration, respectively, the existence of boron nitride is indicated. Other peaks at about 1 120 and 480 cm-1 are attributed to the vibration of [SiO4]4- groups, implying the existence of SiO2. With the oxidation treatment, all the above-mentioned peaks become a little sharper probably owing to the reduction of some impurities as well as a better ceramization and crystallization.
From the XRD patterns of SiO2f/SiO2-BN composites in Fig. 3, the characteristic diffraction peaks of h-BN located at 26.7° (002) and 42.7° (100) are obvious, while the characteristic diffraction peak of SiO2 located at 22.4° (101) is also observed. The low intensity and broadness of the above-mentioned peaks indicate a non-crystalline structure of BN and SiO2. Besides, there is no distinct difference between the as-received composites and the treated one, except for a little increase of the intensity of the peak at 26.7°. No other peaks can be seen.
All of the results show that boron nitride obtained is amorphous, and the oxidation treatment at 450 °C will not impair its structure.
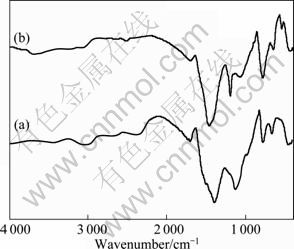
Fig. 2 FT-IR spectra of SiO2f/SiO2-BN composites: (a) As-received; (b) Treated
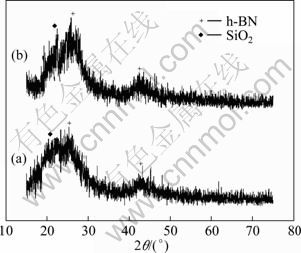
Fig. 3 XRD patterns of SiO2f/SiO2-BN composites: (a) As-received; (b) Treated
3.2 Effects of oxidation treatment on mechanical properties of composites
The mechanical properties of SiO2f/SiO2 and SiO2f/SiO2-BN composites are shown in Table 1, and the load-displacement curves are illustrated in Fig. 4. As can be seen, SiO2f/SiO2-BN composites have a density of 1.81 g/cm3, a flexural strength of 113.9 MPa and an elastic modulus of 36.5 GPa, which are increased by 7.74%, 12.1% and 76.3% compared with SiO2f/SiO2 composites, respectively. However, the oxidation treatment at 450 °C makes the flexural strength of the composites sharply decrease to 58.9 MPa, while the elastic modulus to 9.4 GPa, which suggests a severe degradation to the materials. From Fig. 4(a), we can see some non-linear saw-toothed fluctuation in the vicinity of the maximum load followed by a ladder-like decrease, showing that the as-received SiO2f/SiO2-BN composites have an excellent toughness and can absorb more fracture energy, compared with the arc-like curve in Fig. 4(b) exhibiting a little toughness of the treated composites.
Table 1 Mechanical properties of SiO2f/SiO2 and SiO2f/SiO2- BN composites
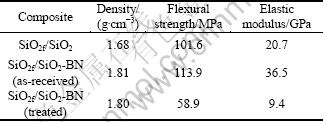
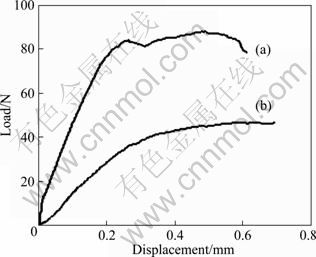
Fig. 4 Load-displacement curves for SiO2f/SiO2-BN composites: (a) As-received; (b) Treated
It can also be seen from the SEM photographs of the fibers which have debonded from the matrix during fracture (see Fig. 5). Both of the composites have a distinct fiber pull-outs, which suggests a non-brittle failure behavior. This will improve the toughness, damage tolerance and reliability of the composites. Furthermore, from Fig. 5(b) and Fig. 5(d), significant shattering of BN and SiO2 matrices can be observed, leaving mass residual matrix debris on the fracture surface, and the amount of the matrices adhesion to the pulled-out fibers varies from the as-received composites to the treated ones.
Typically, the behavior of a composite is determined by the combination of retained fiber strength, matrix properties, and the properties of the fiber/matrix interface [17-18]. As to continuous fiber reinforced ceramic- matrix composites which contain a high modulus matrix, their mechanical behavior is strongly influenced by the fiber/matrix interfacial characteristics. So, it is necessary to design them with moderate interface combined with fiber pull-out, fracture and interface debonding to absorb more fracture energy during deformation [2, 7, 17-18]. In this work, adding BN matrix appears to be well matched with the silica fibers and silica matrix, which can effectively prevent the micro-cracks deflection, allow for effective load transfer between fibers and matrix, as well as keep a relatively fine fiber/matrix interface [9]. With oxidation treatment, the flexural strength and elastic modulus of SiO2f/SiO2-BN composites are reduced to 51.7% and 25.8%, respectively. The degradation of the mechanical properties is probably due to the existence of oxygen, which could accelerate the reaction between BN and silica fibers so as to cause more debris during fracture (see Figs. 5(c) and (d)). However, the toughness of the composites avoids degrading much but retains a little owing to the dispersed BN particles.

Fig. 5 SEM photographs of fracture surfaces of SiO2f/SiO2-BN composites: (a), (b) As-received; (c), (d) Treated
3.3 Effects of oxidation treatment on dielectric properties of composites
The dielectric properties of a material can be mainly described by two important parameters: the dielectric constant ε and the loss tangent tanδ, which can be expressed by two formulas as [19]
ε=ε′-jε′′ (5)
tanδ=ε′′/ε′ (6)
The real part (ε′) correlates with polarization while the imaginary part (ε′′) represents dielectric loss, and the loss tangent (tanδ) predicts the ability of the material to convert the absorbed electromagnetic energy into heat. So, the ideal low dielectric constant and loss tangent are necessary to a wave-transparent material used in high speed aircrafts for microwave transmission, with their values being no more than 4.0 and 0.01 at the band of 0.3-300 GHz range, respectively [5, 20].
Table 2 gives the dielectric properties of the composites prepared. It is obvious that SiO2f/SiO2-BN composites exhibit excellent dielectric properties with dielectric constant of 3.32 and loss tangent of 4.0×10-3, which are similar to SiO2f/SiO2 composites. For the well-distributed composite, dielectric properties can be calculated according to LICHTENCKER’s logarithmic equation [21]:
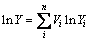 (7)
(7)
where Yi and Vi are the dielectric parameters (εi, tanδi) and volume fraction of every phase i, respectively. According to the reactions (2) and (3), SiO2f/SiO2-BN composites fabricated at 900 °C via urea route mainly contain the impurities of B2O3 and carbon. The characteristic peaks of B2O3 are not found in Figs.2 and 3, so we think that B2O3 is completely reacted by the excessive urea with a molar ratio of 3:2 (CO(NH2)2:H3BO3). Thus, carbon produced by excess urea is the main impurity. However, because of the similar crystal structure between hexagonal-boron nitride (h-BN) and graphite, their X-ray diffraction peaks usually appear almost at the same location [22], which makes the peaks both undistinguishable in Fig. 3.
Table 2 Dielectric properties of composites

Therefore, SiO2f/SiO2-BN composites mainly consist of silica fiber, silica, BN, fiber/ matrix interface and pores, probably as well as carbon. Carbon is of high dielectric constant and loss tangent, which is extremely harmful to the dielectric properties of the composites in view of Eq. (7). The oxidation treatment above 400 °C is feasible to remove carbon. However, the composites treated in air at 450 °C show a dielectric constant of 3.22 and loss tangent 3.9 (see Table 2), respectively, which are improved little compared with the composites as- received, and on the contrary the mechanical properties are severely damaged according to discussion in Section 3.2. So, the oxidation treatment is ineffective to improve the dielectric properties of SiO2f/SiO2-BN composites.
4 Conclusions
1) TG-DTA analysis of boron nitride in oxygen atmosphere shows that the oxidation treatment at 450 °C will not impair the structure of boron nitride. FT-IR and XRD results suggest that the amorphous BN and SiO2 are contained in SiO2f/SiO2-BN composites, while the main impurity is carbon with the excessive urea.
2) The density of SiO2f/SiO2-BN composites varies to 1.80 g/cm3 with the oxidation treatment at 450 °C, and the flexural strength and elastic modulus are decreased to 58.9 MPa and 9.4 GPa, respectively. The mechanical properties of the composites are severely damaged, but it still exhibits a good toughness.
3) With the dielectric constant and loss tangent being 3.22 and 0.003 9, respectively, the composites exhibit excellent dielectric properties, which indicates that the oxidation treatment is ineffective to improve the dielectric properties of SiO2f/SiO2-BN composites.
References
[1] NEIL J T, BOWEN L J, MICHAUD B E. Fused silica radome: US, 4949095 [P]. 1990-08-14.
[2] QI Gong-jin, ZHANG Chang-rui, HU Hai-feng. High strength three-dimensional silica fiber reinforced silicon nitride-based composites via polyhydridomethylsilazane pyrolysis [J]. Ceram Int, 2007, 33: 891-894.
[3] BRAZEL J P, FENTON R. ADL-4D6: A silica/silica composite for hardened antenna windows [C]// Proceedings of the 13th symposium on electromagnetic windows. Atlanta, Georgia: Georgia Institute of Technology, 1976: 9-16.
[4] MANOCHA L M, PANCHAL C N, MANOCHA S. Silica/silica composites through electrophoretic infiltration [J]. Ceram Eng Sci Proc, 2002, 23: 655-661.
[5] LI Xiang-ming, YIN Xiao-wei, ZHANG Li-tong, CHENG Lai-fei, QI Yuan-chen. Mechanical and dielectric properties of porous Si3N4-SiO2 composite ceramics [J]. Mater Sci Eng A, 2009, 500: 63-69.
[6] LI Bin, ZHANG Chang-rui, CAO Feng, WANG Si-qing, CAO Ying-bin, QI Gong-jin, JIANG Yong-gang. Effect of pyrolysis temperature on the properties of three-dimensional silica fiber reinforced nitride matrix composites [J]. J Mater Eng Perform, 2008, 17(1): 111-114.
[7] LI Bin, ZHANG Chang-rui, CAO Feng, WANG Si-qing, LI Jun-sheng, CHEN Bang. Effects of curing atmospheric pressure on properties of silica fiber reinforced silicon-boron nitride matrix composites derived from precursor infiltration and pyrolysis [J]. Mater Technol, 2007, 22 (2): 81-84.
[8] ECONOMY J, JUN C K, LIN R Y. Boron nitride-boron nitride composites: US, 4075276 [P]. 1978-02-21.
[9] YUAN Bo, LIU Ji-xuan, ZHANG Guo-jun, KAN Yan-mei, WANG Pei-ling. Silicon nitride/boron nitride ceramic composites fabricated by reactive pressureless sintering [J]. Ceram Int, 2009, 35: 2155-2159.
[10] LII Ding-fwu, HUANG Jow-lay, TSUI Li-jen, LEE Shaw-min. Formation of BN films on carbon fibers by dip-coating [J]. Surf Coat Technol, 2002, 150: 269-276.
[11] GOMATHI A, RESHMA S, RAO C N R. A simple urea-based route to ternary metal oxynitride nanoparticles [J]. J Solid State Chem, 2009, 182: 72-76.
[12] GOMATHI A, RAO C N R. Nanostructures of the binary nitrides, BN, TiN, and NbN prepared by the urea-route [J]. Mater Res Bull, 2006, 41: 941-947.
[13] SUN J F, WANG M Z, ZhAO Y C, LI X P, LIANG B Y. Synthesis of titanium nitride powders by reactive ball milling of titanium and urea [J]. J Alloy Compd, 2009, 482: L29-L31.
[14] WANG Xiang-dong, QIAO Guan-jun, JIN Zhi-hao. Preparation of SiC/BN nanocomposite powders by chemical processing [J]. Mater Lett, 2004, 58: 1419-1423.
[15] LI Yong-li, ZHANG Jiu-xing, QIAO Guan-jun, JIN Zhi-hao. Fabrication and properties of machinable 3Y-ZrO2/BN nanocomposites [J]. Mater Sci Eng A, 2005, 397: 35-40.
[16] LI Duan, ZHANG Chang-rui, LI Bin, CAO Feng, WANG Si-qing, CAO Ying-bin. Preparation and properties of SiO2f/SiO2-BN composites [J]. Acta Materiae Compositae Sinica, 2011, 28(3): 63-68. (in Chinese)
[17] COFER C G, ECONOMY J, XU Y R, ZANGVIL A, CURZIO E L, FERBER M K, MORE K L. Characterization of fiber/matrix interfaces in composites with a boron nitride matrix [J]. Compos Sci Technol, 1996, 56: 967-975.
[18] ZALDIVAR R J, RELLICK G S, YANG J M. Fiber strength utilization in carbon/carbon composites [J]. J Mater Res, 1993, 8: 501-511.
[19] LI Shu-qin, PEI Yu-chen, YU Chang-qing, LI Jia-lu. Mechanical and dielectric properties of porous Si2N2O-Si3N4 in situ composites [J]. Ceram Int, 2009, 35: 1851-1854.
[20] PAQUETTE D G. Method of making a radar transparent window material operable above 2000 °C: US, 5627542 [P]. 1997-05-06.
[21] KINGERY W D, BOWEN H K, UHLMANN D R. Introduction to ceramics [M]. 2nd edition, New York: John Wiley & Sons, 1976.
[22] LI Bin, ZHANG Chang-rui, WANG Si-qing, CAO Feng. Crystallization behaviors of carbon fiber reinforced BN-Si3N4 matrix composite [J]. Cryst Res Technol, 2007, 42(7): 648-651.
(Edited by YANG Bing)
Foundation item: Projects(50902150, 90916019) supported by the National Natural Science Foundation of China; Project (9140C8203040905) supported by the State Key Laboratory Foundation of China; Project(S100103) supported by the Graduate Innovation Foundation of National University of Defense Technology, China
Received date: 2010-12-22; Accepted date: 2011-05-11
Corresponding author: ZHANG Chang-rui, Professor, PhD; Tel: +86-731-84576433; E-mail: crzhang@nudt.edu.cn