
Effect of rare earth (RE) on diffusion of aluminum atoms in aluminizing
ZHANG Wei(张 伟)1, YE Xiao-feng(叶晓枫)2, PANG Bi-jun(庞碧君)3, ZHOU Si-kai(周思凯)1
1. Department of Materials Engineering, Luoyang College of Technology, Luoyang 471003, China;
2. Department of Mathematics and Information Sciences,
North China Institute of Water Conservancy and Hydroelectric Power, Zhengzhou 450008, China;
3. Department of Mathematics and Information Sciences, Luoyang Normal University,
Luoyang 471003, China
Received 28 July 2006; accepted 15 September 2006
Abstract: The RE-aluminized coating and pure aluminized coating on 20 carbons steel were prepared by hot dip aluminizing method at 740 ℃. After diffusion treatment at 850 ℃ for 4 h, the distribution of aluminum and lanthanum elements in the coating was analyzed with energy disperse spectroscopy(EDS) and electron probe microanalyses(EPMA), and the lattice parameter of α-Fe in the matrix of the coating was measured precisely by X-ray diffractometer(XRD). The results show that RE permeates into the aluminized coating, leads to lattice disturbance and increases the depth of the aluminized coating. On the basis of the results, the expression of the diffusion coefficient of Al atoms is derived from the diffusion flow, and the effect of the high vacancy concentration and high concentration gradient of vacancies on the diffusion of Al atoms was analyzed by establishing the kinetics model of the vacancy mechanism of diffusion. The results show that the high vacancy concentration and high concentration gradient of vacancies in the RE-aluminized processes are the main reason why the diffusion coefficient of Al atoms in RE-aluminizing is bigger than that in pure aluminizing.
Key words: hot dip aluminizing; diffusion treatments; rare earth elements; lattice disturbance; kinetics model; vacancy concentration
1 Introduction
The chemical heat treatments of adding rare earth (RE), such as RE-carburizing and carbonitriding, RE-nitriding and nitrocarburizing, RE-boronizing and boron-aluminizing, can not only enhance the diffusion coefficient of permeated metal atoms, but also increase the depth of the coating and improve the synthetic properties of the surface for treated steels[1-5]. But so far, their mechanism is not very clear. Many experiments showed that there were definite enrichments of RE elements on the coating and RE elements could be detected on the grain boundary and insides of crystal grain[6-9]. The positron annihilation tests indicated that the RE permeating into steel could enhance vacancy defects of the surface for treated steels, too[10-11]. In a previous investigation, the authors expressed that RE permeating into pure iron caused the lattice parameter to produce place distortion, and resulted in strain energy and the number of vacancies increased by 0.191 9 eV and 1.2×10-6, respectively[12]. Therefore, some scholars inferred that the main reason why RE accelerated the permeating velocity of the chemical heat treatment was related to the increasing number of crystal defects (such as vacancy and dislocation), owing to the permeation of RE atom. However, no report yet demonstrated whether RE permeated into the diffusion layer of hot-dip aluminized steel or increased the depth of the aluminized in RE-aluminizing.
The main mechanism of substitution diffusion is the vacancy mechanism. The atom realizes the directional flow by exchanging the position with the vacancy. In pure aluminizing processes, there is an equilibrium concentration of vacancy on the surface for treated steels. In RE-aluminizing processes, if the big size RE atoms permeate into the steel and cause lattice distortion, it will necessarily result in the increase of the number of crystal defects and forms the vacancy concentration and concentration gradient of vacancies in a definite depth of the surface for treated steels, which is far higher than the equilibrium concentration. In the present paper, the distributions of La and Al elements in the hot dip RE-aluminized and pure aluminized coating diffused- treat at 850 ℃ were analyzed with energy disperse spectroscopy(EDS) and electron probe microanalyses (EPMA), and the lattice parameter of α-Fe in the matrix of the coating was measured accurately with X-ray diffractometer(XRD). On the basis of the result, the effect of the vacancy concentration and concentration gradient of vacancies in the coating on the diffusion coefficient was analyzed by establishing the kinetics model of vacancy mechanism. It pointed out the main reason why the diffusion coefficient of Al atoms in RE-aluminizing is higher than that in pure aluminizing. Thus, the physical behavior of RE accelerating the process of the chemical heat treatment was further revealed.
2 Experimental
The substrate specimens were 20 carbons steel with the following composition (mass fraction, %): C 0.20, Si 0.27, Mn 0.50, P 0.04, S 0.04 and Fe balance. Rectan- gular specimens were cut to the dimensions of 50 mm×30 mm×3 mm by a water-cooled cutting machine. The surface of the specimens was finished to 0.45 mm in Ra using a grinding machine. Commercial grade pure aluminum with purity of 99.7% and RE aluminum alloy with 0.5% La were heated in a silica crucible using a box furnace, respectively, and used as the molten aluminum bath, respectively. The temperature of the molten aluminum bath was controlled within ±2 ℃.
Prior to hot dip aluminizing, the substrate specimens were initially degreased by immersion to an aqueous solution of 20% NaOH at 80 ℃ for 10 min and rinsed with alcohol. Subsequently, they were pickled in a dilute solution of 5% HCl at 80 ℃ for 3 min and fluxed in an aqueous solution of 200 g/L ZnCl2, 100 g/L NH4Cl, 10 g/L NaF and 5 g/L NaCl at 80 ℃ for 10 min. Finally, they were dried at 150-180 ℃ for 3 min, dipped immediately into molten aluminum bath at 740 ℃ for 3 min, raised from molten aluminum bath and cooled to room temperature in air. The hot-dipped specimens were further diffused at 850 ℃ for 4 h in air furnace and then cooled to room temperature in furnace.
3 Results
3.1 La distribution in coating
Fig.1 shows La distribution in the RE-aluminized coating after diffusion treatments with EPMA analysis. It can be seen from Fig.1 that the La content in the coating gradually decreases from the outer surface to inside and the permeating depth of La elements is far larger than the depth of aluminized coating. It undoubtedly proves that RE permeates into the matrix of steel and the permeating rate of RE element is quicker than that of Al element.

Fig.1 Distributions of La content in RE-aluminized coating
3.2 Al distribution in coating
Al distribution in the aluminized coating with RE and without RE after diffusion treatments is shown in Fig.2. It can be seen from Fig.2 whether adding RE or not, Al content on the outer surface and the regularity of Al distribution in the coating are same, but the permeating depth of Al element in RE-aluminized coating increases by about 30%-40%, which indicates that RE increases the depth of the coating and enhances the diffusion coefficient of Al atoms.

Fig.2 Distribution of Al content in aluminized coating with RE and without RE
3.3 Change of lattice parameter in α-Fe
Table 1 shows the measurement results with X- ray diffraction and calculated value of the lattice parameter of α-Fe in the matrix, which is close to the aluminized coating of the RE-aluminized and pure aluminized specimen after diffusion treatments. It can be seen from Table 1 that the lattice parameter of α-Fe with RE is bigger than that of α-Fe without RE. That is to say, after aluminizing with RE, the big size RE atoms permeating into the coating cause lattice distortion. Therefore, there must be a large number of crystal defects at a certain depth of the coating, which will result in a high vacancy concentration and a high concentration gradient of vacancies will appear in the coating[12-13].
Table 1 Measurement results with X-ray diffraction and calculated value of lattice parameter of α-Fe in matrix (λ=0.154 056 nm)

3.4 Kinetics model of Al atom diffusion
In diffusion process of hot dip aluminized steel, Al and La atoms on the outer surface coating diffuse to the inside and Fe atoms of the matrix diffuse to the outside. Because La content in the RE-aluminized coating is very low, it is generally accepted that the surface of treated steel forms the aluminized coating of Al-Fe alloys with high Al concentration.
It is assumed that there is only a one-dimensional flow for the object researched and the forward direction of the X axis perpendicular to the atomic plane is the diffusion direction, in which Al and vacancy concentration decrease and Fe concentration increases, as shown in Fig.3. The distance of the adjacent atomic plane is d and the dotted line in Fig.3 is an imaginary atomic plane. Based on the vacancy mechanism of substitution diffusion, we suppose that the metal atom exchanges position with the vacancy between atomic planes Ⅰ and Ⅱ. JⅠ→Ⅱ is the flow rate from atomic plane Ⅰ to atomic plane Ⅱ and JⅡ→Ⅰ is the flow rate from atomic plane Ⅱ to atomic plane Ⅰ.
Supposing CAl(x) and Cv(x) indicate Al atom concentration and vacancy atom concentration, respec-

Fig.3 Schematic diagram of metal diffusion
tively, and supposing they are all the continuous function of distance x. Al atom concentration and vacancy atom concentration of the atomic plane shown on the dotted line in Fig.3 are CAl and Cv, respectively.
On atomic plane Ⅰ:
Al atom concentration is 
Vacancy concentration is 
On atomic plane Ⅱ:
Al atom concentration is 
Vacancy concentration is 
We assume further that Pv(η, ξ) shows the probability that vacancies jump from an atomic plane (Al mole fraction is ξ) to the adjacent atomic plane (Al mole fraction is η), and PvAl(η, ξ) shows the probability that the vacancies on atomic plane where Al mole fraction is ξ, exchange the position with Al atoms on the atomic plane where the Al mole fraction is η. Pv(η, ξ) and PvAl(η, ξ) are all the continuous functions of η and ξ. On the position of dotted line in Fig.3, we develop P(η, ξ) into the Taylor’s series concerning η=ξ=CAl. Then, the probabilities whose vacancies jump from the atomic plane Ⅰ to atomic plane Ⅱ are as follows:
 (1)
(1)
The probabilities whose vacancies jump from the atomic plane Ⅱ to atomic plane Ⅰ are as follows:
 (2)
(2)
Based on vacancy mechanism, the net flow rate whose Al atoms jump from the atomic plane Ⅰ to the atomic plane Ⅱ is given as JAl.
 (3)
(3)
where Γ is the frequency of atomic vibration. According to Fick’s first law,
 (4)
(4)
Substituting the Eqn.(3) into the Eqn.(4), then the diffusion coefficient DAl can be written as
 (5)
(5)
Since  , the above Eqn.(5)
, the above Eqn.(5)
indicates that the main factors affecting Al atoms diffusion are the concentration gradient of Al atoms, the frequency of atomic vibration, vacancy concentration and concentration gradient of vacancies as well as the probability that vacancies exchange position with Al atoms.
4 Discussion
4.1 Frequency of atomic vibration Γ
According to Ref.[13], Γ=vzCvf. In diffusion process of the hot dip pure aluminized coating, the vacancy concentration in the coating is an equilibrium concentration. In diffusion process of the hot dip RE- aluminized coating, RE atoms with the big atomic radius permeates fastly into the coating and results in lattice distortion, which forms a much bigger vacancy concentration than equilibrium concentration in the coating. So, RE-aluminizing has the high frequency of atomic vibration.
4.2 PvAl(CAl, CAl)
According to Ref.[14], 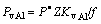 , where
, where  Q is the diffusion activation energy;
Q is the diffusion activation energy;  expresses that the given positions are the probability of vacancy. According to vacancy mechanism of diffusion,
expresses that the given positions are the probability of vacancy. According to vacancy mechanism of diffusion,  should be equal to vacancy concentration. Meanwhile, the high vacancy concentra- tion in the coating lowers the diffusion activation energy, which also causes PtAl(CAl, CAl) in RE-aluminizing to be higher than that in pure aluminizing. So, the diffusion coefficient in the RE-aluminizing is bigger, too.
should be equal to vacancy concentration. Meanwhile, the high vacancy concentra- tion in the coating lowers the diffusion activation energy, which also causes PtAl(CAl, CAl) in RE-aluminizing to be higher than that in pure aluminizing. So, the diffusion coefficient in the RE-aluminizing is bigger, too.
4.3 (dCv/dx)/(dCAl/dx)
In diffusion process of the hot dip pure aluminized coating, there is no concentration gradient of vacancies
in the coating. That is,  . According to
. According to
the results of above experiments and analysis (Fig.2 and Table 1), the crystal lattice of α-Fe in the matrix takes place distortion in RE-aluminizing process and there is the concentration gradient of vacancies, therefore,
 >0. So, the diffusion coefficient of Al
>0. So, the diffusion coefficient of Al
atoms in RE-aluminizing is bigger than that in pure aluminizing.
4.4 
In RE-aluminizing process, when Al concentration increases, vacancy concentration also increases. According to the definition of PtAl(η, ξ), when Al mole fraction η increases, the probability that vacancies jump from an atomic plane (Al mole fraction is ξ0) to the adjacent atomic plane (Al mole fraction is η) will decrease. When Al concentration ξ increases, the probability that vacancies jump from an atomic plane (Al mole fraction is ξ) to the adjacent atomic plane (Al mole fraction is η0) will increase. That is, PtAl(η, ξ0) is a decreasing function of η and PtAl(η0, ξ) is an increasing
function of ξ. Therefore,  >0. These
>0. These
also cause the diffusion coefficient of Al atoms in RE- aluminizing to be bigger than that of pure aluminizing.
5 Conclusions
1) In the diffusion process of hot dip RE-aluminized steel, RE permeates into the coating and increases the depth of the coating.
2) RE atoms permeating into the coating cause lattice distortion, which lowers the diffusion activation energy of Al atoms and enhances their diffusion coefficient.
References
[1] WEN Jiu-ba, LI Quan-an, ZHAO Wan-xiang WANG Zhi-hui. Investigation of properties of hot-dipped Ce-rich rare earth aluminum alloy upon conditions of corrosion environment [J]. Acta Scientiarum Naturalium Universitatis Sunyatseni, 2003, 42(3): 41-44.
[2] YAN Mu-fu, LIN Zhi-ru. Effect of rare earths on diffusion coefficient and transfer coefficient of carbon during carburizing [J].Rare Earths, 2001, 19(2):122-124.
[3] JIE Ze-sheng, XIA Li-fang, WANG Hai-bo, WU Yun-qi, HAN Zhi-tao. Effect of rare earths on kinetics of boron-aluminizing of armco iron and computer fitting [J]. Journal of the Chinese Rare Earth Society, 1999, 17(1): 50-53.
[4] WEN Jiu-ba, ZHANG Wei, LI Xiao-yuan. The influence of La on the corrosion resistance of hot-dip aluminized steel [J]. Materials Science Forum, 2005, 475/479: 3851-3854.
[5] AHMADI H, LI D Y. Beneficial effects of yttrium on mechanical properties and high-temperature wear behavior of surface aluminized 1045 steel [J]. Wear, 2003, 255: 933-942.
[6] JIE Ze-sheng. Effect of rare earth on B-Al permeating and computer kinetic simulation of permeation layer forming [J]. Trans Nonferrous Met Soc China, 1999, 9(4): 791-795.
[7] HUANG Qing-guo, ZHONG Hua-ren. Effect of RE on the microstructures and properties of hot-dip aluminized coatings [J]. Journal of Nanchang Institute of Aeronautical Technology, 1999, 13(2): 31-34.
[8] LV Zhen-jia, LIOU Zhi-ru. Surface effect of RE permeating into steel [J]. Journal of Shenyang Polytechnic University, 1989, 11(3): 1-12.
[9] YAN Mu-fu, LIN Zhi-ru, ZHU Fa-yi. Progress in rare earth thermochemical treatment [J]. Heat Treatment of Metals, 2003, 28(3): 1-6.
[10] LV Zhen-jia, LI Gong-ying, LIOU Zheng. Investigation of salt bath RE-B permeation at temperature of (α+γ) two-phase region on 45 carbon steel [J]. Journal of Shenyang Polytechnic University, 1987, 9(1): 1-11.
[11] JIE Ze-sheng, LI Dong-ha, XIN Ming-de, LI Qing-fen. Effect of rare earth on mechanism of B-Al permeating [J]. Journal of Aeronautical Materials, 2002, 22(3): 5-8.
[12] LIOU Zheng, LV Zhen-jia. Vacancy mechanism of surface treatment with rare earth [J]. Chinese Science Bulletin, 1991, 36(12): 951-954.
[13] LI Zhong-hu, LV Xiao-ping, XU Zong. Effect of vacancies concentration gradient on diffusion in double glow plasma surface alloying [J]. Journal of Applied Sciences, 2001, 18(2): 183-185.
[14] LIOU Guo-Xuen. Principle of Metallography [M]. Beijing: Metallurgy Industry Press, 1980: 274-285.
(Edited by LI Yan-hong)
Foundation item: Project(0511021600) supported by the Natural Science Foundation of Henan Province, China
Corresponding author: ZHANG Wei; Tel: +86-379-64909996; E-mail: weizhang57@163.com