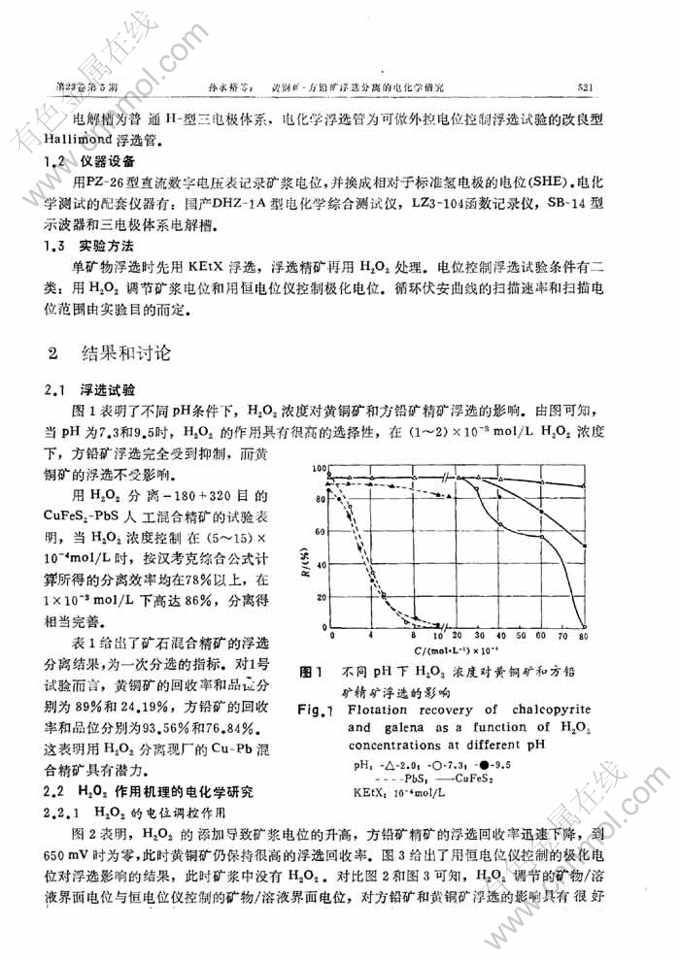
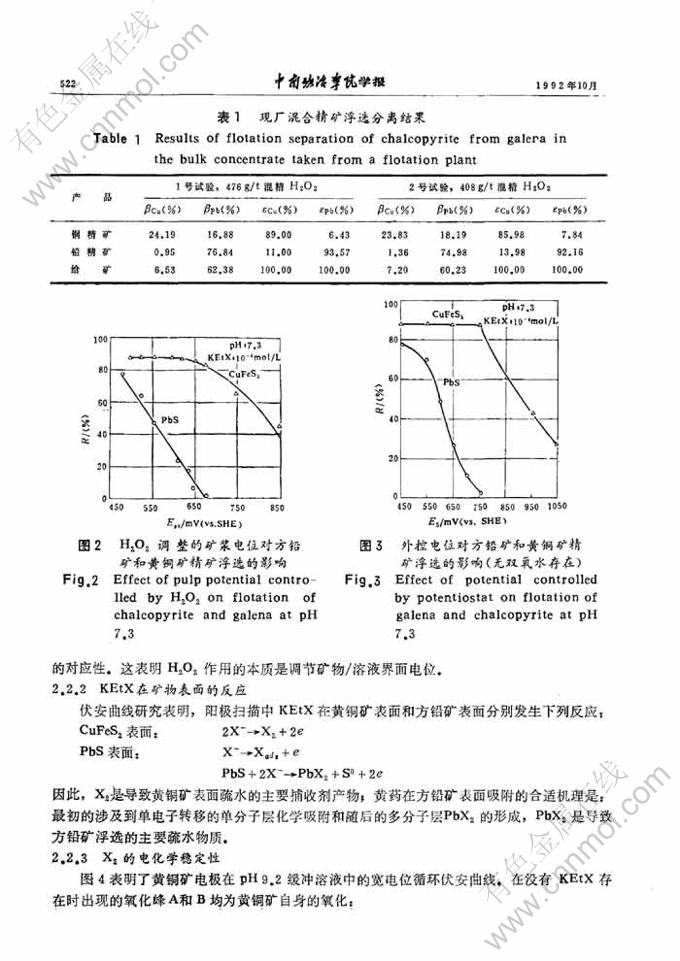
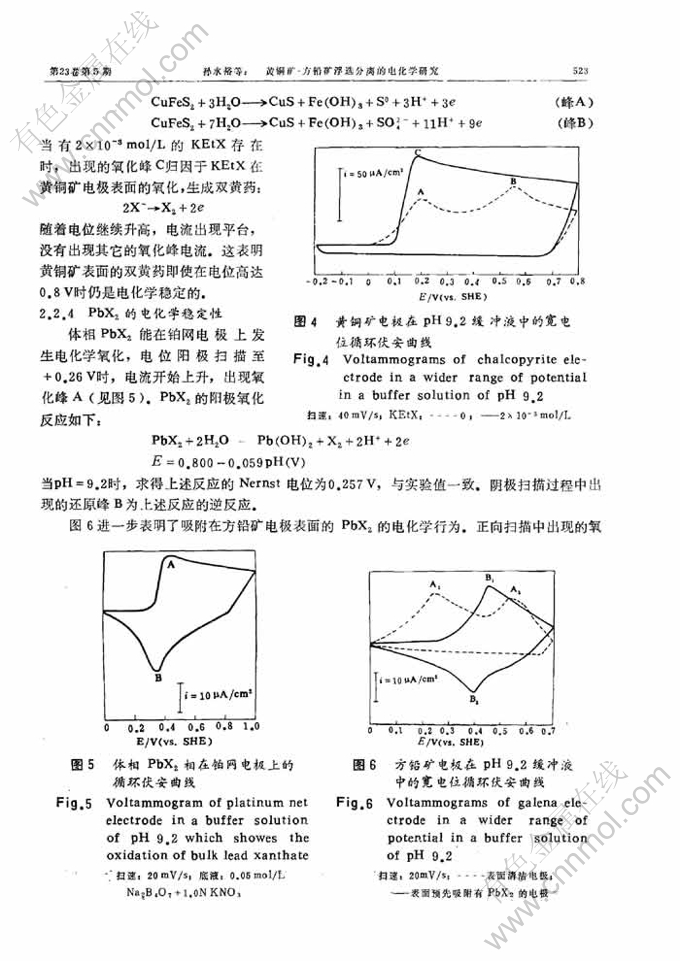
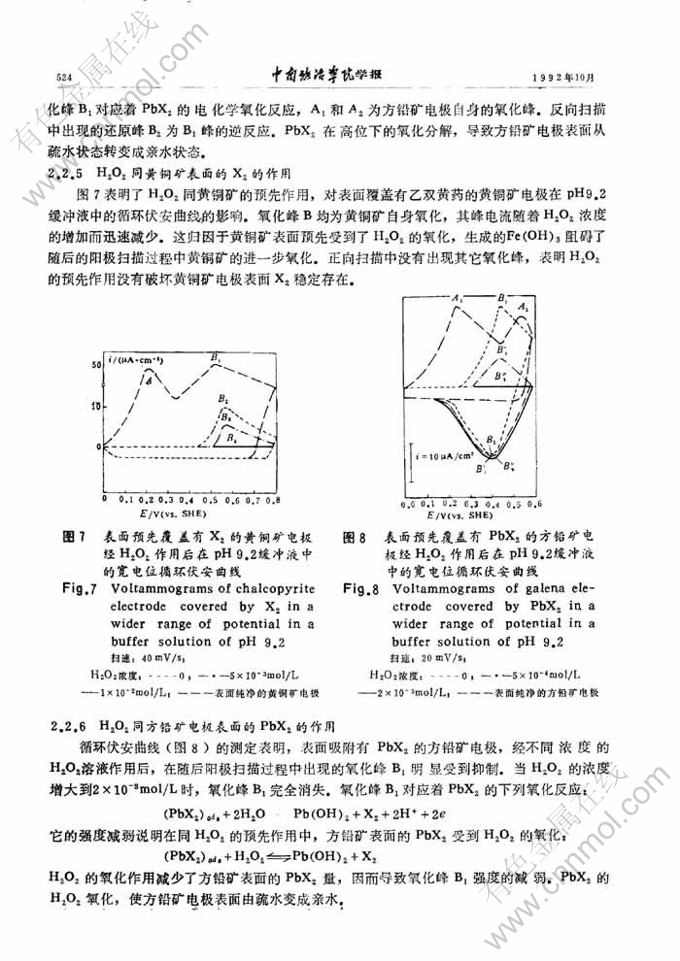
黄铜矿-方铅矿浮选分离的电化学研究
来源期刊:中南大学学报(自然科学版)1992年第5期
论文作者:孙水裕 王淀佐 李柏淡
文章页码:520 - 525
关键词:电化学; 浮选分离; 黄铜矿; 方铅矿
Key words:electrochemistry; flotation separation; galena; chalcopyrite
摘 要:选用双氧水作抑制剂,实现了人工和现厂的铜-铅混合精矿的选择性浮选分离。通过矿浆电位测量和循环伏安曲线测定,详细地研究了双氧水的抑制机理。研究结果表明,双氧水能够氧化分解方铅矿表面的乙基黄原酸铅,但未能氧化黄铜矿表面的双黄药,由此造成双氧水作用的选择性。
Abstract: The flotation separation of chalcopyrite from galena in bulk concentrateswas achieved using hydrogen peroxide (H2O2) as modifier. The collector-inducedflotation of galena was depressed in the presence of H2O2, but chalcopyrite floatedwell. The depression mechanism of galena flotation was investigated through themeasurement of potential and cyclic voltammetry. The results have shown thatlead ethyl xanthate was responsible for galena flotation and can be furtheroxidized and decomposed at higher potentials controlled by H2O2 and potentiostat.The oxidation and composition of PbX2 resulted in a change of galena surfacefrom hydrophobic to hydrophilic condition. On the other hand, dixanthogen wasresponsible for chalcopyrite flotation and was stable at higher potentials. Thedifference of electrochemical stability of PbX2 and X2 resulted in the flotationof chalcopyrite from galena.