
Semi-solid processing of hypereutectic A390 alloys using novel rheoforming process
M. TEBIB1, J. B. MORIN1, F. AJERSCH2, X. GRANT CHEN1
1. Université du Québec à Chicoutimi, Chicoutimi, QC, G7H 2B1, Canada;
2. ?cole polytechnique de Montréal, Montréal, QC, H3C 3A7, Canada
Received 13 May 2010; accepted 25 June 2010
Abstract: The feasibility of semi solid processing of hypereutectic A390 alloys using a novel rheoforming process was investigated. A combination of the swirl enthalpy equilibration device (SEED) process, isothermal holding using insulation and addition of solid alloy during swirling was introduced as a novel method to improve the processability of semi solid slurry. The effects of isothermal holding and the addition of solid alloy on the temperature gradient between the centre and the wall and on the formation of α(Al) particles were examined. In additional tests, phosphorus and strontium were added to the molten metal to refine the primary and eutectic silicon structure to facilitate semi solid processing. The results show that the combination of the SEED process with two additional processing steps can produce semi-solid A390 alloys that can be rheoprocessed. The microstructure reveals an adequate amount of non-dendritic α(Al) globules surrounded by liquid, which greatly improves the processability of semi-solid slurry.
Key words: semi-solid forming; rheoforming process; hypereutectic A390 alloys; microstructure
1 Introduction
Hypereutectic A390 alloys (Al-17Si-4CuMg) have generated significant interest in the recent years, due to their attractive properties, such as low coefficient of thermal expansion, high wear resistance, high strength and hardness[1-3], and are widely used in automotive and aerospace industry. Typical applications are: pistons, cylinder blocks and AC compressors[3]. However, the semi-solid processing of A390 alloys presents considerable difficulties due to the segregation of large primary silicon particles, the wide range of solidification and a large proportion of eutectic, resulting in poor processability of the semi-solid slurry[4].
Semi solid metal (SSM) processing is a relatively new technology for near-net-shape manufacturing, which was discovered in early 1971 by SPENCER[5]. The most common process routes are the thixoforming, where the prefabricated billet is reheated to the semi solid state prior to casting and rheoforming generally starts with molten metal that is processed directly into semi solid slurry by controlled cooling resulting in an appropriate non-dendritic structure (slurry on demand)[5].
It has been reported that thixoforming was successfully used to produce hypereutectic A390 semi solid cast parts[6-8]. In this process, the partial remelting and isothermal holding promoted the separation of α(Al) and Si eutectic phases and increased the amount of non-dendritic α(Al), resulting in a suitable microstructure for semi solid slurry[9]. However, thixoforming is not the most economical route for semi solid processing.
Recently, new concepts of rheoforming have been explored because of its many obvious advantages. It consists of a simple process step from liquid metal to slurry with a large capability to use all possible material sources (primary, secondary, selected process and recycle scraps)[10-12]. However, semi solid processing of hypereutectic A390 alloy by rheoforming presents certain difficulties such as a small processing temperature window, a high heat of fusion of silicon, a long solidification time and segregation of primary silicon particles. These factors lead to the development of innovative methods to achieve acceptable semi solid microstructure of rheoformed hypereutectic alloys. SAHA et al[4] have introduced two novel methods using diffusion solidification as a means to extract heat from the slurry. The two concepts are: 1) mixing hypoeutectic alloy with hypereutectic alloy at different temperatures and 2) cooling the liquid hypereutectic alloy with the addition of solid particles. To date, there are still only few publications on the rheoforming of hypereutectic Al-Si alloys.
The swirl enthalpy equilibration device (SEED) process is a slurry-on-demand process that involves the application of mechanical mixing (swirling), which allows the extraction of a controlled amount of heat from the molten alloy to generate a semi-solid mixture[12]. This method is well adapted to the processing of A356/A357, 6061 and 206 semi solid alloys. In this study, a modification of the SEED process, including isothermal holding in an insulated mould and the addition of solid alloy during swirling, is employed to improve the processability of the semi solid slurry. The effects of isothermal holding and the addition of solid alloy on the semi solid microstructure on the temperature gradient within the slurry are investigated. The impact of phosphorus and strontium on the primary and eutectic silicon structure is also explored.
2 Experimental
The experimental setup is shown in Fig.1. It consists of an insulated steel crucible, a device for adding solid pieces of alloy and the SEED apparatus. The process steps to produce the semi solid slurry are illustrated in Fig.2. First, approximately 1.5 kg alloy was heated to the liquid state at 750 °C in a 3 kg capacity refractory crucible, using an electrical resistance furnace. The molten metal was then carefully poured at 670 °C into a crucible of 85 mm in diameter and 250 nm in depth. The molten metal was then swirled using the SEED process with the addition of solid alloy pieces. After swirling, the melt was held isothermally for 10 s. Two K-type thermocouples were placed in the crucible prior to pouring, one at the center and the other near the wall of the crucible, to monitor the temperature gradient in the slurry during solidification. The tips of the thermocouples were positioned at 70 mm from the
bottom of the crucible. Finally, the semi-solid slurry was demoulded from the crucible.

Fig.1 Schematic of apparatus used in novel rheoprocessing method
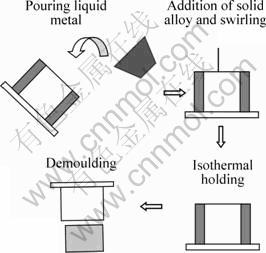
Fig.2 Illustration of steps involved in process
A commercial A390 alloy was used to produce the semi solid material. Its chemical composition is listed in Table 1. During swirling, two different quantities of solid alloys were added to the molten metal. The first addition of A390 has the same composition as the melt in the crucible, followed by a second addition of A356 solid alloy with the chemical composition shown in Table 1. In other tests, phosphorus or phosphorus combined with strontium was added to the liquid metal to refine the primary Si and modify the eutectic Si of the alloys.
Table 1 Chemical composition of alloys used in present work (mass fraction, %)

After demoulding, the semi solid slurry was rapidly quenched in water. Metallographic specimens were cut from the quenched slurry and polished using standard metallographic procedure. The microstructure was examined using optical microscopy. A CLEMEX JS-2000 optical image analyser was used to quantify the volume fraction of α(Al) and the particle size of the primary Si particles.
3 Results and discussion
3.1 Effect of processing parameters
Fig.3 compares the temperature profiles at the wall and at the center of the slurry using the conventional SEED process and the modified method. The crucible and pouring temperatures were fixed at 50 °C and 670 °C, respectively, in the tests. Fig.3(a) shows the thermal history of the slurry using the SEED process. It can be seen that the initial measured temperatures are 619 °C and 592 °C at the centre of the melt and at the mid height of the crucible wall, respectively. The radial temperature gradient during the initial stage of cooling is very large because of the temperature difference between the mould and the liquid alloy. This temperature gradient decreases rapidly while swirling for the first 50 s as the temperature of the crucible mould increases. After 100 s, the temperature at the centre remains almost constant, whereas the temperature at the wall continues to slowly decrease, resulting in a temperature gradient of 6 °C at the final stage.

Fig.3 Temperatures measured at wall and at center during production of semi solid slurries: (a) Conventional SEED process; (b) Modified method
The temperature profile using the modified method is shown in Fig.3(b). After pouring, the initial temperature difference between the wall and the centre is small due to the insulation of the crucible. It can be seen that after the solid alloy addition (at 50-60 s), the temperature gradient almost disappears. The results of Fig.3(b) clearly illustrate the effectiveness of the insulation of the crucible and the addition of the solid alloy with swirling which allows high heat extraction from the centre of the slurry. Consequently, the temperature of the slurry becomes much more uniform for an extended period of time from 100 to 320 s in the semi solid range at 560 °C. At the final stage, the temperature difference in the slurry is only 0.5 °C, compared with 6 °C for the conventional SEED process.
This long period of constant temperature results in the successful rheoprocessing of A390 alloy by the separation of α(Al) and Si eutectic phases and an increase of the volume fraction of the non-dendritic α(Al) phase. The resulting slurry can easily be demoulded, is self supporting and can be easily sliced as shown in Figs.4(a) and 4(b), indicative of the good processability of the alloy using this method.

Fig.4 Semi solid slurries self-supported (a) and easy to cut (b) using novel method
3.2 Microstructures of rheoprocessed alloys
Processing of hypereutectic Al-Si alloys, such as A390 using the SEED process results in a hard solid layer at the solidification surface with a high liquid content zone at the center of the ingot. These ingots are difficult to demould and are not self supporting, which makes them unsuitable for further die casting.
Fig.5 shows the microstructures in the center part of semi solid slurries produced by the conventional SEED process and by the modified method. All slurries were quenched at 560 °C. Slurries obtained by the conventional SEED process show large primary Si particles mostly in the form of clusters, few dendrite-rosette α(Al) particles and large amount of eutectic liquid (Fig.5(a)). Using the novel method, slurries show a significant increase of volume fraction of globular α(Al), resulting in an acceptable microstructure with rheological properties suitable for subsequent rheocasting (Fig.5(b)). In the novel method, the SEED process provides good mechanical mixing and the insulation reduces the heat loss at the wall. Also, the addition of solid alloy at the centre reduces the heat flux from the molten alloy to the crucible wall and extracts the high heat of fusion released by the precipitation of the primary silicon particles. The addition of solid metal also contributes to the massive nucleation of α(Al). This combined effect promotes the separation of α(Al) and Si eutectic phases and increases the amount of globular α(Al).
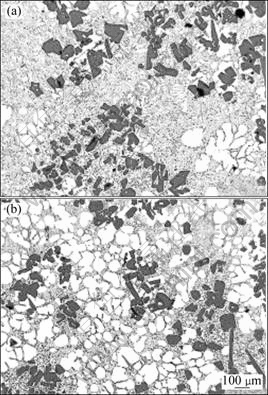
Fig.5 Microstructures of semi solid A390 obtained using conventional SEED process (a) and modified SEED process (b)
Fig.6 shows the effect of different solid alloy additions on the microstructure of semi solid slurries prepared with the novel method. It can be seen from Figs.6(a) and (b) that the amount of solid alloy addition has a significant effect on the microstructure. As the amount of A390 alloy increases from 50 g to 85 g, the solid fraction of α(Al) increases. The results show that the addition of small amount of solid alloy removes the heat from the liquid at a rapid rate, which promotes the nucleation of α(Al). The isothermal holding at the temperature near the eutectic point enhances the separation of α(Al) and Si eutectic phases, and therefore, the Al in the solution can easily precipitate on the existing α(Al). Fig.6(c) presents the microstructure of the combination of adding A390 and A356 during swirling. The addition of two different compositions of solid alloys significantly increases the volume fraction of the α(Al) particles, compared with the results obtained with the addition of A390 only (Figs.6(a) and (b)). The increase of the volume fraction of α(Al) is due to the
nucleation and growth of α(Al) by the dissolution of aluminum phase from the A356 solid alloy and by subsequent diffusion solidification[4].
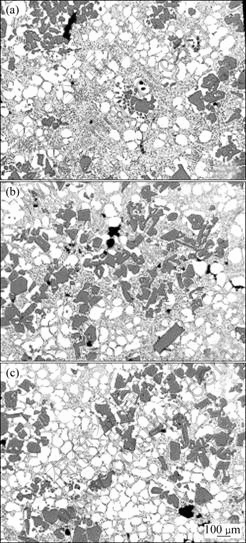
Fig.6 Effect of amount of addition of solid alloy on microstructure of A390 slurry: (a) 50 g A390; (b) 85 g A390; (c) 50 g A390+42 g A356
The effects of the addition of phosphorus and strontium on the microstructure of semi solid A390 slurries are shown in Fig.7. Primary silicon in untreated A390 alloy generally takes the form of coarse platelets with an irregular morphology as shown in Figs.4 and 5. After the addition of 8.8×10-5 P (mass fraction) in the form of a Cu-8%P master alloy, the size and the morphology of primary silicon particles are changed to a fine blocky shape with a considerable decrease in size (Fig.7(a)). It should be noted that during the preparation of the slurry, the fine primary silicon particles also have a tendency to agglomerate. It was previously reported that phosphorus can react with the liquid aluminum to form aluminum phosphide, AlP, which has a crystal structure very similar to that of silicon[14]. It is evident that primary Si nucleates heterogeneously on the AlP particles, which promotes the refinement of primary silicon. However, the phosphorus has no effect on the eutectic silicon which remains in a lamellar plate like form (Fig.7(b)).
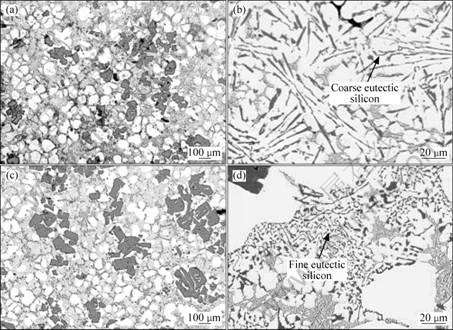
Fig.7 Microstructures of semi solid A390 with addition of 50 g A390 alloy + 42 g A356 alloy of solid metal (a), with 8.8×10-5 P (b) and with 8.8×10-5 P+2×10-4 Sr ((c) and (d))
Fig.7(c) shows the effect of the addition of both phosphorus and strontium to the molten metal. Examination of the resulting microstructure shows that the size and morphology of primary silicon particles are slightly affected. Most of the primary silicon remains coarse and has an irregular morphology, while the morphology of eutectic silicon changes considerably to a fine fibrous form (Fig.7(d)). This phenomenon is probably due to the interaction of the added elements. It was also previously reported that refinement of primary silicon and modification of silicon eutectic cannot be achieved simultaneously because the AlP particles are consumed by strontium to form Al2Si2Sr[14-15].
The evolution of the effective volume fraction of α(Al) with the addition of solid alloys is illustrated in Fig.8. It is clearly shown that the effective volume fraction of α(Al) particles in final semi solid slurries increases with the increase of the amount of solid alloy added as well as with the alloy composition. Fig.9 shows the effect of phosphorus and strontium addition on the particle surface area of primary silicon and α(Al). It can be seen that the size of primary silicon particle decreases with the addition of 8.8×10-5 P. The combination of phosphorus and strontium addition produces primary silicon particles that are only partially refined and remain in a size larger than that obtained by phosphorus refinement. It can be seen that, in all three cases, the size of α(Al) particles remains almost unchanged.
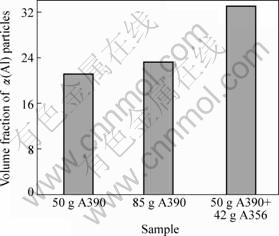
Fig.8 Effect of mass and composition of solid alloy on volume fraction of α(Al) particles
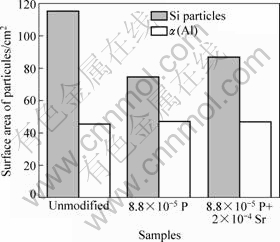
Fig.9 Effect of solid alloy (50 g A390+42 g A356) with P and Sr addition on particle surface area of primary silicon and α(Al)
4 Conclusions
1) A combination of the SEED process, isothermal holding using insulation and addition of solid alloy during swirling can be used to rheoprocess semi solid A390 alloy.
2) Isothermal holding using insulation reduces heat loss at the mould wall while the addition of solid alloy during swirling allows high extraction of latent heat at the center of the slurry, resulting in a decrease of the temperature gradient within the slurry and an increase of α(Al) volume fraction.
3) The addition of two different compositions of solid alloys (A390 and A356) significantly increases the volume fraction of non-dendritic α(Al) phases, which greatly improves the processability and rheological properties of semi solid A390 slurries.
4) Primary silicon in the semi solid microstructure can be refined by phosphorus additions. However, refinement of primary silicon and modification of eutectic silicon cannot be achieved simultaneously by phosphorus and strontium additions.
Acknowledgements
The authors would like to acknowledge the financial support provided by the Natural Sciences and Engineering Research Council of Canada (NSERC) and Rio Tinto Alcan through the NSERC Industrial Research Chair in Metallurgy of Aluminum Transformation at Université du Québec à Chicoutimi.
References
[1] LASA L, RODRIGUEZ-IBABE J M. Wear behaviour of eutectic and hypereutectic Al-Si-Cu-Mg casting alloys tested against a composite brake pad [J]. Materials Science and Engineering A, 2003, 363: 193-202.
[2] TIMMERMANS G, FROYEN L. Fretting wear behaviour of hypereutectic P/M Al-Si in oil environment [J]. Wear, 1999, 230: 105-117.
[3] KIM H J. Effect of calcium on primary silicon particles size in hypereutectic Al-Si alloys [J]. Materials Science and Technology, 2003, 19:915-918.
[4] SAHA D, APELIAN D, DADGUPTA R. Semi solid processing of hypereutectic alloys [J]. AFS Transactions, 2004, 112: 325-337.
[5] FAN Z. Semi solid metal processing [J]. International Materials Reviews, 2002, 47: 49-84.
[6] CHEN C M, YANG C C, CHAO C G. A novel method for net-shape forming of hypereutectic Al-Si alloys by thixocasting with powder performs [J]. Journal of Materials Processing Technology, 2005, 167: 103-109.
[7] KAPRANOS P, KIRKWOOD D H, ATKINSON H V, RHEINLANDER J T, BENTZEN J J, TOFT P T, DEBEL C P, LASLAZ G, MAENNER L, BLAIS S, RODRIGUEZ-IBABE J M, LASA L, GIORDANO P, CHIARMETTA G, GIESE A. Thixoforming of an automotive part in A390 hypereutectic Al-Si alloy [J]. Journal of Materials Processing Technology, 2003, 135: 271-277.
[8] BIROL Y. Cooling slop casting and thixoforming of hypereutectic A390 alloy [J]. Journal of Materials Processing Technology, 2008, 207: 200-203.
[9] WANG H, NING Z L, DAVIDSON C J, STJOHN D H, XIE S S. Thixotropic structure formation in A390 hypereutectic Al-Si alloy [C]//The 8th International Conference of Semi Solid Processing (S2P). Limassol, Cyprus, 2004: Paper #14-1.
[10] HAGA T, KAPRANOS P. Simple rheocasting processes [J]. Journal of Materials Processing Technology, 2002, 130/131: 594-598.
[11] BIROL Y. Internal cooling to produce aluminum alloy slurries for rheocasting [J]. Journal of Alloys and Compounds, 2009, 480: 365-368.
[12] JORSTAD J, APELIAN D. Hypereutectic Al-Si alloys: Practical casting consideration [J]. International Journal of Metalcasting, 2009, 3(3): 13-36.
[13] DOUTRE D, HAY G, WALES P, GABATHULER J P. SEED: A new process for semi solid forming [J]. Canadian Metallurgical Quarterly, 2004, 43(2): 265-272.
[14] HO C R, CANTOR B. Heterogeneous nucleation of solidification of Si in Al-Si and Al-Si-P alloys [J]. Acta Metall Mater, 1995, 43: 3231-3246.
[15] CHO Y H, LEE H C, OH K H, DAHLE A K. Effect of strontium and phosphorus on eutectic Al-Si nucleation and formation of β-Al5FeSi in hypereutectic Al-Si foundry alloys [J]. Metallurgical and Materials Transactions A, 2009, 39: 2435-2448.
(Edited by YANG Bing)
Corresponding author: X. GRANT CHEN; Tel: 1-418-545-5011 ext. 2603; E-mail: xgrant_chen@uqac.ca
DOI: 10.1016/S1003-6326(09)60368-X