通过螯合镍提高从Zn-Ni硫酸盐溶液中水解沉淀锌的选择性
来源期刊:中国有色金属学报(英文版)2018年第12期
论文作者:Mostafa Aghazadeh-Ghomi Javad MOGHADDAM Naghi Parvini Ahmadi
文章页码:2566 - 2573
关键词:锌;镍;水解沉淀;分离;螯合物;平衡分析
Key words:zinc; nickel; hydrolytic precipitation; separation; chelate; equilibrium analysis
摘 要:研究锌/镍摩尔比为20:1的锌-镍硫酸盐溶液中锌的选择性沉淀过程。将0.5 mol/L NaOH溶液滴加到含0、0.01、0.02、0.03和0.04 mol/L乙二胺四乙酸盐(EDTA)作为螯合剂的锌-镍硫酸盐溶液中。利用Visual MINTEQ软件进行沉淀途径的平衡分析。平衡分析结果表明,少量EDTA的存在可以防止镍在碱性条件下沉淀,而对锌的沉淀没有任何不利影响。鉴于此,溶液中仅存在0.03 mol/L EDTA、pH=9.0时,锌的沉淀率超过90%,沉淀产物中Zn含量约为50%,而Ni含量仅为0.11%。为了更完全地分离锌和镍,沉淀后继续搅拌120 min。沉淀产物的X射线衍射分析表明,沉淀相为Zn4(OH)6SO4·4H2O。
Abstract: The selective precipitation of zinc from zinc-nickel sulfate solution with the Zn/Ni molar ratio of 20:1 was studied. Dropwise addition of 0.5 mol/L NaOH solution into the zinc-nickel sulfate solution containing 0, 0.01, 0.02, 0.03 and 0.04 mol/L ethylene diamine tetraacetate (EDTA) as a chelating agent was done. The equilibrium analysis of precipitation pathway was performed using Visual MINTEQ program. The equilibrium analysis showed that the presence of small amounts of EDTA can prevent nickel precipitation in alkaline conditions without any negative effect on zinc precipitation. On this basis, more than 90% of zinc could be precipitated as a product with about 50% Zn and only 0.11% Ni at pH=9.0 merely as a result of the presence of 0.03 mol/L EDTA in the solution. The stirring time of 120 min after precipitation was found to be essential for more complete separation. The X-ray diffraction studies on the precipitate revealed that the precipitated phase was Zn4(OH)6SO4.4H2O.
Trans. Nonferrous Met. Soc. China 28(2018) 2566-2573
Mostafa Aghazadeh-Ghomi1, Javad Moghaddam2, Naghi Parvini Ahmadi3
1. Department of Materials Engineering, Azarbaijan Shahid Madani University, Tabriz, Iran;
2. Materials and Metallurgical Engineering department, University of Zanjan, Zanjan, Iran;
3. Faculty of Materials Engineering, Sahand University of Technology, Tabriz, Iran
Received 4 December 2017; accepted 4 May 2018
Abstract: The selective precipitation of zinc from zinc-nickel sulfate solution with the Zn/Ni molar ratio of 20:1 was studied. Dropwise addition of 0.5 mol/L NaOH solution into the zinc-nickel sulfate solution containing 0, 0.01, 0.02, 0.03 and 0.04 mol/L ethylene diamine tetraacetate (EDTA) as a chelating agent was done. The equilibrium analysis of precipitation pathway was performed using Visual MINTEQ program. The equilibrium analysis showed that the presence of small amounts of EDTA can prevent nickel precipitation in alkaline conditions without any negative effect on zinc precipitation. On this basis, more than 90% of zinc could be precipitated as a product with about 50% Zn and only 0.11% Ni at pH=9.0 merely as a result of the presence of 0.03 mol/L EDTA in the solution. The stirring time of 120 min after precipitation was found to be essential for more complete separation. The X-ray diffraction studies on the precipitate revealed that the precipitated phase was Zn4(OH)6SO4.4H2O.
Key words: zinc; nickel; hydrolytic precipitation; separation; chelate; equilibrium analysis
1 Introduction
The elements Zn and Ni are found together in various resources such as zinc industry residues [1-4], spent Ni-metal hydride batteries [5-7], spent catalysts [8] and electronic scrap [9]. Different methods including cementation [10-12], solvent extraction [13-16], ion exchange [17,18] and chemical precipitation [8,19,20], each with own limitations and problems, may be used to separate these two metals. However, selective precipitation of zinc and nickel via hydrolytic precipitation is not efficient due to their close precipitation pH [21,22].
In our research program on the recovery of metal values from the residue of zinc hydrometallurgical process known as cold filter cake [3], we obtained a zinc-nickel sulfate solution with Zn/Ni molar ratio of about 20:1. The equilibrium simulation revealed that the majority of zinc from the solution should precipitate below nickel precipitation pH. Owing to the development of local high pH zone around the added precipitant agent, precipitation of a part of nickel cannot be prevented in practice [23]. To overcome this problem, we explored an additive with three criteria: (1) the additive should suppress the precipitation of Ni in alkaline pH values; (2) the additive should not prevent the precipitation of Zn; (3) the needed amount of additive should be as low as possible.
Preliminary investigation on the influences of several complexing agents was done using Visual MINTEQ 3.1 program. The chelating agent ethylene diamine tetraacetate, EDTA, was selected as the most effective additive. EDTA is a hexadentate chelating agent. This means that one molecule of EDTA can bind to a metal cation through six coordinate bounds therefore forms very strong complexes with metal cations. This feature is utilized not only in the field of analytical chemistry, but also widely used in detergent, textile, paper and even food industries. Numerous works have been published in the field of metal polluted soil remediation using EDTA and other chelators [24-27]. EDTA can efficiently solubilize heavy metals from contaminated soils without any negative impact on soil properties. The development of processes to release metal cations from high stability metal chelate which allows chelator to be reused, has attracted a lot of interest [28-32]. Theoretical analysis of the complexed metal precipitation as hydroxide and suppressing effect of chelating agent on the precipitation of metals has been made [33,34]. The precipitation of chelated heavy metals from effluents has been studied also [35,36].
The aim of the present work is to restrain Ni precipitation by means of chelation with the minimum possible amount of EDTA in order to obtain a high purity zinc precipitate. The precipitation process was simulated with Visual MINTEQ 3.1 program and its results were utilized for prediction of experimental results and interpretation of observations.
2 Experimental
2.1 Equilibrium analysis
The optimum conditions for separation of two metals via selective precipitation may be explored by the prediction of the precipitation process pathway. The Visual MINTEQ 3.1 program with the ability to solve several problems in one run (using Multi-problem/Sweep menu), was employed to thermodynamic analysis of the solution. The solution temperature was assumed to be 25 °C. The pH and ionic strength of solution were not fixed on specific values but were allowed to be calculated. The extended Debye-Hückel equation was chosen for performing activity corrections. The initial composition for all solutions was defined as: 0.3 mol/L Zn2+, 0.015 mol/L Ni2+ (equal to Zn:Ni mole ratio of 20:1) and, 0.315 mol/L  . In addition, needed amount of Na+ and EDTA4- were added to initial composition of corresponding solutions in order to simulate the use of Na2EDTA as chelator. Initial volume of solutions was assumed as arbitrary value of 0.1 L. A solution of 0.5 mol/L NaOH was defined as titrant. The new equilibrium state of the system after each step of titrant addition (0. 6 mL of 0.5 mol/L NaOH in each step), including volume, pH, amount of precipitated solids and concentration of species, was computed via program and recorded in a new data row in the output spreadsheet. The Microsoft Excel was employed to further processing of obtained data from Visual MINTEQ 3.1 program and to graphs construction. The equilibrium constant of formation of aqueous species of zinc and nickel as well as the solubility products of all solid phases that Visual MINTEQ uses them in its calculations, are given in our previous work [23].
. In addition, needed amount of Na+ and EDTA4- were added to initial composition of corresponding solutions in order to simulate the use of Na2EDTA as chelator. Initial volume of solutions was assumed as arbitrary value of 0.1 L. A solution of 0.5 mol/L NaOH was defined as titrant. The new equilibrium state of the system after each step of titrant addition (0. 6 mL of 0.5 mol/L NaOH in each step), including volume, pH, amount of precipitated solids and concentration of species, was computed via program and recorded in a new data row in the output spreadsheet. The Microsoft Excel was employed to further processing of obtained data from Visual MINTEQ 3.1 program and to graphs construction. The equilibrium constant of formation of aqueous species of zinc and nickel as well as the solubility products of all solid phases that Visual MINTEQ uses them in its calculations, are given in our previous work [23].
2.2 Procedure
A synthetic stock solution of 0.3 mol/L zinc and 0.015 mol/L Ni with the Zn:Ni molar ratio of 20:1 was prepared by dissolving requisite quantity of their analytical grade sulfate salts in distilled water. The pH of solution was adjusted to 4.0 by dropping diluted sulfuric acid solution. Solid salt of disodium EDTA, C10H14N2Na2O8·2H2O (Merck, Germany) was added into the stock Zn-Ni solution to prepare E1 to E4 solutions (see Table 1). Freshly prepared 0.5 mol/L NaOH solution was used as precipitant. All the chemicals used were in analytical grade (Merk, Germany) and were utilized without further purification.
Table 1 Initial compositions of synthetic solutions

The experimental work was planned based on previously done equilibrium analysis. Two series of precipitation tests were performed; partial and full precipitation of selectively precipitable Zn. The partial precipitation tests were done with the addition of 75 mL of 0.5 mol/L NaOH into the 100 mL of Zn-Ni sulfate solutions containing 0, 0.01, 0.02, 0.03 and 0.04 mol/L EDTA (Table 1). With the selected volume of NaOH solution, the final pH of solutions will never reach precipitation pH of Ni, based on the equilibrium analysis. Hence, for all solutions in these experiments, coprecipitation of Ni along with Zn is not expected theoretically. After determining the optimum concentration of EDTA and the time required for the reaction to approach equilibrium, the full precipitation tests were carried out by increasing the pH of the solution up to 9.0. At this pH value the precipitation of maximum amount of Zn is expected.
All the experiments were carried out at (25±0.5) °C in a Pyrex beaker in a thermostatically controlled water bath equipped with a digitally controlled thermometer. In each precipitation test, 100 mL of corresponding solution was transferred into the beaker. The solution was agitated with a magnetic stirrer at the rotational rate of 150 r/min. The needed volume of 0.5 mol/L NaOH solution was dosed drop-wisely using a burette. Then, the mixture was left to stir for specified time or post-precipitation stirring time (PPS time) and filtered through quantitative filter paper, subsequently. The yielded filtercake was repulped in 250 mL hot distilled water and was stirred for 15 min at stirring rate of 150 r/min. Then, the mixture was filtered again and left to dry at room temperature for one week. Finally, the yielded solid product was subjected to chemical and phase structure analysis.
Zn and Ni contents of precipitates and filtrates were determined with AA240 atomic absorption spectrometer, (AAS) Varian (Australia). To probe the mineralogical state of Zn in the precipitates, X-ray diffraction using X’Pert Pro diffractometer (XRD-D8 ADVANCED- BRUKERS AXS model, Germany), with a copper anode Kα radiation in a wavelength (λ) of 1.546  was utilized.
was utilized.
3 Results and discussion
3.1 Equilibrium analysis
The equilibrium analysis on the influence of EDTA presence in the solution was performed using Visual MINTEQ 3.1. In brief, it was revealed that the precipitation of Zn as Zn4(OH)6SO4 is thermo- dynamically more favorable than that of other compounds like Zn(OH)2 and ZnO, and for Ni the Ni(OH)2 was predicted as precipitating solid at the lack of chelating agent condition. Accordingly, the precipitated amounts of Zn and Ni by the addition of hydroxyl ions were computed (Fig. 1). It is seen in Fig. 1 that the presence of EDTA in the solution restrains Ni precipitation strongly. In the absence of EDTA, complete precipitation of Ni occurs at the pH value higher than about 8 while, with 0.01 mol/L EDTA, the precipitation of Ni never reaches 40% and with EDTA concentration higher than 0.02 mol/L the precipitation of Ni is not possible even at very alkaline conditions. On the other hand, the precipitation curves for Zn do not affect so much and its precipitation starts at pH 6 regardless of EDTA content. The maximum amount of Zn that can be precipitated, decreases slightly with the addition of EDTA.
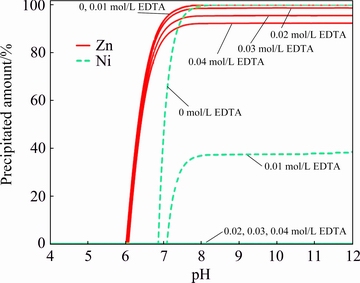
Fig. 1 Precipitated amount of Zn and Ni as function of pH during addition of 0.5 mol/L NaOH into 0.3 mol/L Zn, 0.015 mol/L Ni solutions containing 0, 0.01, 0.02, 0.03 and 0.04 mol/L EDTA (Simulated in Visual MINTEQ 3.1)
Although EDTA forms stable chelates with both Zn and Ni but the stability of Ni-EDTA chelate is higher compared with Zn-EDTA chelate according to thermodynamic data given in Table 2. Zn and Ni aqueous species in the solution compete to be chelated with EDTA. Due to the lower amount of Ni compared with Zn (Zn/Ni mole ratio of 20:1) and higher stability of Ni-EDTA chelate, nearly all Ni transforms to its chelated forms in contrast to Zn.
Table 2 Formation reactions of M-EDTA chelate and their lg K values (K is the stability constant of a chelate)

The separation potential may be defined as the maximum amount of Zn that can be precipitated before Ni precipitation. Accordingly, in the case of the solutions E0 and E1, the precipitation potential can be determined by drawing a vertical line from pH where Ni commences to precipitate to intersect the Zn curve. The separation potential for the solutions with 0.02, 0.03 and 0.04 mol/L EDTA where Ni precipitation does not occur, simply is the maximum of Zn precipitation curve. Table 3 shows the separation potential values extracted from Fig. 1. Although the complete precipitation of Zn is suppressed as a result of the presence of EDTA, but the separation potential for EDTA-containing solutions is still higher than that of EDTA-free solution. Moreover, for solutions E0 and E1 the total potential of separation is not accessible owing to the risk of Ni precipitation at higher pH. In fact, the base addition should be stopped early to prevent Ni precipitation.
Table 3 Separation potential of Zn from 0.3 mol/L Zn, 0.015 mol/L Ni solutions containing different amounts of EDTA
To scrutinize the chemistry of the solution, the distribution of chemical species in the solution was calculated using Visual MINTEQ 3.1 program. Figures 2 and 3 show the speciation of solutions during precipitation process respectively for the solutions E0 and E3. It is seen that before precipitation (pH<6), the predominant aqueous species for Zn and Ni in the EDTA-free solution are: Zn2+, ZnSO4(aq),  , Ni2+ and NiSO4(aq) (Fig. 2) At the same condition, the addition of 0.03 mol/L EDTA does not make serious changes in the distribution of Zn species. However, in the case of Ni, the presence of EDTA results in very deep changes in distribution of Ni species so that NiHEDTA- and NiEDTA2- become predominant species at pH<3 and pH>3, respectively (Fig. 3). During the precipitation of Zn from EDTA-containing solution, the contribution of chelated forms of Zn remains near 4% regardless of pH, while the amount of unchelated species decreases with pH so that in the final stage of precipitation the chelated Zn becomes predominant aqueous species. Therefore, it can be concluded that the chelated Zn species never participate in precipitation reaction (Fig. 3).
, Ni2+ and NiSO4(aq) (Fig. 2) At the same condition, the addition of 0.03 mol/L EDTA does not make serious changes in the distribution of Zn species. However, in the case of Ni, the presence of EDTA results in very deep changes in distribution of Ni species so that NiHEDTA- and NiEDTA2- become predominant species at pH<3 and pH>3, respectively (Fig. 3). During the precipitation of Zn from EDTA-containing solution, the contribution of chelated forms of Zn remains near 4% regardless of pH, while the amount of unchelated species decreases with pH so that in the final stage of precipitation the chelated Zn becomes predominant aqueous species. Therefore, it can be concluded that the chelated Zn species never participate in precipitation reaction (Fig. 3).
An important point that is found from Ni speciation (Fig. 3) is the presence of some unchelated aqueous species of nickel, i.e., Ni2+ and NiSO4(aq) at pH<7.

Fig. 2 Distribution of chemical species as function of pH during addition of 0.5 mol/L NaOH into EDTA-free 0.3 mol/L Zn, 0.015 mol/L Ni solutions (Contribution of omitted species is less than 1%)
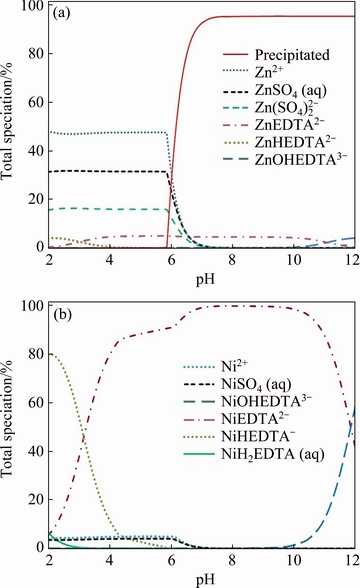
Fig. 3 Distribution of chemical species as function of pH during addition of 0.5 mol/L NaOH into 0.3 mol/L Zn, 0.015 mol/L Ni and 0.03 mol/L EDTA solution (Contribution of omitted species is less than 1%)
Precipitation of these unchelated species of nickel is not possible from the thermodynamics viewpoint due to their little activity. However, they may play an indirect role in the contaminating solid product as a result of entrapment and adsorption processes. Figure 4 shows the contribution of Ni2+ in the solutions E1 to E4. For example, about 10% of Ni in the 0.02 mol/L EDTA containing solution E2 is as Ni2+. According to Fig. 1 precipitation of Ni from solutions E2, E3 and E4 is not expected but owing to the high level of Ni2+ in the solution E2, it is reasonable to utilize higher concentrations of EDTA.
3.2 Selective precipitation
To investigate the influence of EDTA in the solution on the selectivity of hydrolytic precipitation, series of experiments were conducted. In these experiments 75 mL of 0.5 mol/L NaOH solution was added dropwise into 100 mL of Zn-Ni solutions. The procedure would not provide conditions for Ni precipitation even in the EDTA-free solution based on Visual MINTEQ 3.1 analysis and observed final pH of solutions which was always around 6.4 (precipitation pH of Ni).

Fig. 4 Content of Ni2+ as function of pH during addition of 0.5 mol/L NaOH into 0.3 mol/L Zn, 0.015 mol/L Ni and 0.01 to 0.04 mol/L EDTA solutions

Fig. 5 Effect of EDTA concentration of solution on Zn/Ni molar ratio of precipitate
The Zn/Ni molar ratios in the yielded precipitates in the presence of different EDTA are given in Fig. 5. The Zn/Ni molar ratio reflects the degree of selectivity of Zn precipitation. Since the Zn/Ni molar ratio in the initial solution is equal to 20:1, any values greater than 20:1 for Zn/Ni molar ratio in the solid product show the existence of selectivity for Zn precipitation. It is seen that EDTA could largely restrain Ni precipitation. Although the precipitation of Ni at the used condition is not theoretically possible, but the yielded precipitates never were free of Ni as seen in Fig. 5. At least four mechanisms can be proposed for Ni entrance in the precipitate: (1) Ni precipitation as hydroxide within the local high pH zone developed around the NaOH solution droplets; (2) Entrapment of Ni contained solution within the precipitate; (3) Adsorption and surface precipitation; (4) Substitution of Ni ions in the crystalline lattice of precipitation product of Zn.
The mechanism (1) is the most important mechanism of Ni co-precipitation for solutions E0 and E1 due to their vulnerability to Ni precipitation at pH higher than 7. The mechanism in the solutions E2, E3 and E4 is ineffective, yet they were contaminated with some Ni (Fig. 5). The presence of Ni in the precipitate of solution E2 may be attributed to high level of unchelated species in the solution particularly Ni2+ which have high potential to contaminate the precipitate. Likewise, the similar levels of selectivity for solutions E3 and E4 (Fig. 5) may be attributed to their close levels of Ni2+ given in Fig. 4.
The maximum separation of Zn was reached using 0.03 mol/L EDTA so there is no need to higher amounts of EDTA. The chemical analysis and Zn/Ni molar ratio of yielded precipitates are given in Table 4. The Ni content of precipitate was decreased from 1.19% to 0.22% merely as a result of the presence of 0.03 mol/L EDTA in the solution; actually, the Ni impurity in the product was decreased more than 5-fold.
In order to employ the maximum chelating power of EDTA, the obtained slurry after precipitation process was left on the stirrer for various PPS durations. The results are given in Fig. 6. The Ni content of precipitate was decreased considerably with increasing PPS time. Namely, Ni that had been entered into the precipitate, for any reason, could return largely into the solution. The yielded precipitate from the solution E3 after PPS time of 120 min showed only 0.13% Ni (Table 4).
Table 4 Compositions and Zn/Ni molar ratios of solid products precipitated in different conditions


Fig. 6 Effect of stirring time after precipitation (PPS) on Zn/Ni molar ratio of precipitate
Knowing the optimum conditions of selective precipitation, the addition of NaOH solution was continued until precipitation of the maximum attainable amount of Zn (full precipitation). For this, the pH of the solution E3 was increased up to 9.0 based on precipitation curves given in Fig. 2 and left to agitate for 120 min. The chemical composition of yielded precipitate at pH of 9.0 is given in Table 4. The precipitate with Zn/Ni molar ratio of 411:1 is surprisingly purer than the product of partial precipitation. The lower level of Ni impurity can be attributed to the different speciations of Ni in the solution at the final pH of partial and full precipitation of solution E3. Figure 3 shows that at pH lower that 7, some Ni2+ and NiSO4(aq) are present along with NiEDTA2- but at pH of 9.0 the amount of Ni2+ and NiSO4(aq) are negligible. It is reasonable that a small size species such as Ni2+ can more easily contaminate the precipitate than a very large size species like NiEDTA2-. The measurement of volume of the filtrate and wash water and mass of the precipitate allowed us to calculate the distribution of Zn and Ni in the feed solution, precipitate, filtrate and wash water which are given in Fig. 7. The values are obtained by multiplying the concentration of zinc and nickel of each phase by the volume or mass of that phase. Zn recovery and Ni loss in the precipitate were calculated as 90.57% and 4.54% respectively based on the given values for Zn and Ni mass of precipitate in Fig. 7.
Although, the recovery of Ni and remaining Zn from the filtrate is beyond the scope of the present work, it is worth noting that, the issue of releasing of chelated heavy metals from their chelated forms with the aim of re-using the chelator and the recovery of metals has been studied in the literature in abundance [29,36-39].
The hydrolytic product of selective precipitation of Zn according to the aforementioned procedure was submitted to XRD analysis. The XRD pattern of the precipitate, shown in Fig. 8, corresponds to the database diffraction pattern of Zn hydroxide sulfate tetrahydrate, Zn4(OH)6SO4.4H2O. Besides, Zn content of precipitate (Table 4) is close to the nominal Zn content of Zn4(OH)6SO4.4H2O (49.16%). The formation of Zn4(OH)6SO4.4H2O from zinc sulfate solutions and its dehydration to Zn4(OH)6SO4 at 113-140 °C are reported in Ref. [40].

Fig. 7 Distribution of Zn and Ni before and after precipitation of 100 mL solution E3 at pH of 9.0

Fig. 8 XRD pattern of precipitated product from solution E3 at pH of 9.0 along with database diffraction pattern of zinc hydroxide sulfate tetrahydrate
4 Conclusions
1) The pH range of selective precipitation and maximum amount of selectively precipitated Zn were determined using Visual MINTEQ 3.1 analysis before experimental work.
2) Chelating Ni with EDTA was proved to be the proper solution for the Ni coprecipitation problem both theoretically and practically.
3) The presence of 0.03 mol/L EDTA does not change the distribution of Zn species so much but it is quite sufficient to convert almost all Ni to the chelated forms.
4) Partial precipitation of zinc with the addition of 75 mL of 0.5 mol/L NaOH into 100 mL of 0.3 mol/L Zn, 0.015 mol/L Ni solution showed that the presence of 0.03 mol/L EDTA in the solution can decrease the Ni impurity 5 times.
5) Stirring the mixture after precipitation for 120 min caused further increase of purity. Precipitation at pH of 9.0 could recover more than 90% Zn and yield a precipitate composed of about 50.90% Zn as Zn4(OH)6SO4.4H2O and only 0.11% Ni.
Acknowledgments
Authors would like to express their sincere thanks to Zanjan Zinc Khalessazan Industries, Iran, for making available feed material and AAS analysis.
References
[1] SAFARZADEH M S, MORADKHANI D, OJAGHI-ILKHCHI M, GOLSHAN N H. Determination of the optimum conditions for the leaching of Cd-Ni residues from electrolytic zinc plant using statistical design of experiments [J]. Separation and Purification Technology, 2008, 58: 367-376.
[2] MORADKHANI D, RASOULI M, BEHNIAN D, ARJMANDFAR H, ASHTARI P. Selective zinc alkaline leaching optimization and cadmium sponge recovery by electrowinning from cold filter cake (CFC) residue [J]. Hydrometallurgy, 2012, 115-116: 84-92.
[3] AGHAZADEH-GHOMI M, MOGHADDAM J, AHMADI-PARVINI N. A new approach to zinc–nickel separation using solution alkalinization method: Application to a zinc plant residue [J]. Rare Metals, 2016: 1-7.
[4] BEHNAJADY B, BALESINI A, MOGHADDAM J. A new approach to the optimisation of zinc electrolyte cold purification process by Taguchi’s method [J]. Canadian Metallurgical Quarterly, 2014, 53: 333-339.
[5] ZHANG P, YOKOYAMA T, ITABASHI O, WAKUI Y, SUZUKI T M,INOUE K. Hydrometallurgical process for recovery of metal values from spent nickel-metal hydride secondary batteries [J]. Hydrometallurgy, 1998, 50: 61-75.
[6] BERTUOL D A, BERNARDES A M, TENORIO J A S. Spent NiMH batteries—The role of selective precipitation in the recovery of valuable metals [J]. Journal of Power Sources, 2009, 193: 914-923.
[7] WU F, XU S M, LI L Y, CHEN S Z, XU GXU J M. Recovery of valuable metals from anode material of hydrogen-nickel battery [J]. Transactions of Nonferrous Metals Society of China, 2009, 19: 468-473.
[8] LIAO J, HU H, FU W, LI S,CHEN Q. A hydrometallurgical route to produce ZnO nanoparticles and NiO strips from the spent Ni/ZnO catalyst [J]. Hydrometallurgy, 2012, 121-124: 107-115.
[9] PARK Y J, FRAY D J. Separation of zinc and nickel ions in a strong acid through liquid–liquid extraction [J]. Journal of Hazardous Materials, 2009, 163: 259-265.
[10] BOYANOV B, KONAREVA V, KOLEV N. Removal of cobalt and nickel from zinc sulphate solutions using activated cementation [J]. Journal of Mining and Metallurgy B: Metallurgy, 2004, 40: 41-55.
[11] MOGHADDAM J, SARRAF-MAMOORY R, ABDOLLAHY M, YAMINI Y. Purification of zinc ammoniacal leaching solution by cementation: Determination of optimum process conditions with experimental design by Taguchi’s method [J]. Separation and Purification Technology, 2006, 51: 157-164.
[12] RAHMAN H H A, WAHED E M A. Removal of nickel ions by cementation on zinc from NiSO4 solution in presence of accelerator non-toxic organic compounds [J]. Hydrometallurgy, 2012, 129: 111-117.
[13] REDDY B R, PRIYA D N. Process development for the separation of copper(II), nickel(II) and zinc(II) from sulphate solutions by solvent extraction using LIX 84 I [J]. Separation and Purification Technology, 2005, 45: 163-167.
[14] ZHANG X, LI X, CAO H, ZHANG Y. Separation of copper, iron (III), zinc and nickel from nitrate solution by solvent extraction using LK-C2 [J]. Separation and Purification Technology, 2010, 70: 306-313.
[15] BALESINI A, ZAKERI A, RAZAVIZADEH H,KHANI A. Nickel solvent extraction from cold purification filter cakes of Angouran mine concentrate using LIX984N [J]. International Journal of Minerals, Metallurgy, and Materials, 2013, 20: 1029-1034.
[16] MISHRA R K, ROUT P C, SARANGI K, NATHSARMA K C. Solvent extraction of zinc, manganese, cobalt and nickel from nickel laterite bacterial leach liquor using sodium salts of TOPS-99 and Cyanex 272 [J]. Transactions of Nonferrous Metals Society of China, 2016, 26: 301-309.
[17] ALYüZ B,VELI S. Kinetics and equilibrium studies for the removal of nickel and zinc from aqueous solutions by ion exchange resins [J]. Journal of Hazardous Materials, 2009, 167: 482-488.
[18] FRANCO P E, VEIT M T, BORBA C E, GONCALVES G D C, FAGUNDES-KLEN M R, BERGAMASCO R, DA SILVA E A, SUZAKI P Y R. Nickel(II) and zinc(II) removal using Amberlite IR-120 resin: Ion exchange equilibrium and kinetics [J]. Chemical Engineering Journal, 2013, 221: 426-435.
[19] SAMPAIO R M M, TIMMERS R A, KOCKS N, ANDRE V, DUARTE M T, van HULLEBUSCH E D, FARGES F, LENS P N L. Zn-Ni sulfide selective precipitation: The role of supersaturation [J]. Separation and Purification Technology, 2010, 74: 108-118.
[20] MACHADO M D, SOARES E V, SOARES H M V M. Selective recovery of copper, nickel and zinc from ashes produced from Saccharomyces cerevisiae contaminated biomass used in the treatment of real electroplating effluents [J]. Journal of Hazardous Materials, 2010, 184: 357-363.
[21] GIANNOPOULOU I, PANIAS D. Differential precipitation of copper and nickel from acidic polymetallic aqueous solutions [J]. Hydrometallurgy, 2008, 90: 137-146.
[22] INNOCENZI V, VEGLIO F. Recovery of rare earths and base metals from spent nickel-metal hydride batteries by sequential sulphuric acid leaching and selective precipitations [J]. Journal of Power Sources, 2012, 211: 184-191.
[23] AGHAZADEH-GHOMI M, MOGHADDAM J, AHMADI-PARVINI N. On the role of dilution on selective hydrolytic precipitation from bimetallic solutions: Zn-Ni sulfate solution [J]. Transactions of the Indian Institute of Metals, 2017, 70: 1697-1705.
[24] PETERS R W. Chelant extraction of heavy metals from contaminated soils [J]. Journal of Hazardous Materials, 1999, 66: 151-210.
[25] CHEN T C, MACAULEY E, HONG A. Selection and test of effective chelators for removal of heavy metals from contaminated soils [J]. Canadian Journal of Civil Engineering, 1995, 22: 1185-1197.
[26] LEE C C, MARSHALL W D. Recycling of complexometric extractants to remediate a soil contaminated with heavy metals [J]. Journal of Environmental Monitoring, 2002, 4: 325-329.
[27] POCIECHA M, LESTAN D. Washing of metal contaminated soil with EDTA and process water recycling [J]. Journal of Hazardous Materials, 2012, 235: 384-387.
[28] DI PALMA L, FERRANTELLI P, MERLI C, BIANCIFIORI F. Recovery of EDTA and metal precipitation from soil flushing solutions [J]. Journal of Hazardous Materials, 2003, 103: 153-168.
[29] POCIECHA M, KASTELEC D, LESTAN D. Electrochemical EDTA recycling after soil washing of Pb, Zn and Cd contaminated soil [J]. Journal of Hazardous Materials, 2011, 192: 714-721.
[30] VOGLAR D, LESTAN D. Electrochemical separation and reuse of EDTA after extraction of Cu contaminated soil [J]. Journal of Hazardous Materials, 2010, 180: 152-157.
[31] POCIECHA M, LESTAN D. Novel EDTA and process water recycling method after soil washing of multi-metal contaminated soil [J]. Journal of Hazardous Materials, 2012, 201: 273-279.
[32] DEMIR A, KOLELI N. The sequential use of washing and an electrochemical reduction process for the remediation of lead-contaminated soils [J]. Environmental Technology, 2013, 34: 799-805.
[33] KELLY J J, SUTTON D C. Prediction and measurement of effect of chelating selectivity on precipitation reactions [J]. Talanta, 1966, 13: 1573-1585.
[34] TüNAY O, KABDASLI N. Hydroxide precipitation of complexed metals [J]. Water Research, 1994, 28: 2117-2124.
[35] KIM C, ONG S K. Recycling of lead-contaminated EDTA wastewater [J]. Journal of Hazardous Materials, 1999, 69: 273-286.
[36] JIANG S, FU F, QU J, XIONG Y. A simple method for removing
chelated copper from wastewaters: Ca(OH)2-based replacement- precipitation [J]. Chemosphere, 2008, 73: 785-790.
[37] ZENG Q, SAUVE S, ALLEN H, HENDERSHOT W. Recycling EDTA solutions used to remediate metal-polluted soils [J]. Environmental Pollution, 2005, 133: 225-231.
[38] LO I M, ZHANG W. Study on optimal conditions for recovery of EDTA from soil washing effluents [J]. Journal of Environmental Engineering, 2005, 131: 1507-1513.
[39] HONG P A, LI C, BANERJI S K, REGMI T. Extraction, recovery, and biostability of EDTA for remediation of heavy metal- contaminated soil [J]. Journal of Soil Contamination, 1999, 8: 81-103.
[40] MOEZZI A, CORTIE M B, MCDONAGH A M. Zinc hydroxide sulphate and its transformation to crystalline zinc oxide [J]. Dalton Transactions, 2013, 42: 14432-14437.
Mostafa Aghazadeh-Ghomi1, Javad Moghaddam2, Naghi Parvini Ahmadi3
1. Department of Materials Engineering, Azarbaijan Shahid Madani University, Tabriz, Iran;
2. Materials and Metallurgical Engineering department, University of Zanjan, Zanjan, Iran;
3. Faculty of Materials Engineering, Sahand University of Technology, Tabriz, Iran
摘 要:研究锌/镍摩尔比为20:1的锌-镍硫酸盐溶液中锌的选择性沉淀过程。将0.5 mol/L NaOH溶液滴加到含0、0.01、0.02、0.03和0.04 mol/L乙二胺四乙酸盐(EDTA)作为螯合剂的锌-镍硫酸盐溶液中。利用Visual MINTEQ软件进行沉淀途径的平衡分析。平衡分析结果表明,少量EDTA的存在可以防止镍在碱性条件下沉淀,而对锌的沉淀没有任何不利影响。鉴于此,溶液中仅存在0.03 mol/L EDTA、pH=9.0时,锌的沉淀率超过90%,沉淀产物中Zn含量约为50%,而Ni含量仅为0.11%。为了更完全地分离锌和镍,沉淀后继续搅拌120 min。沉淀产物的X射线衍射分析表明,沉淀相为Zn4(OH)6SO4·4H2O。
关键词:锌;镍;水解沉淀;分离;螯合物;平衡分析
(Edited by Xiang-qun LI)
Corresponding author: Javad Moghaddam; Tel: +98-243-3054364; Fax: +98-243-2383400; E-mail: moghaddam@znu.ac.ir, hastyir@yahoo.com
DOI: 10.1016/S1003-6326(18)64903-9

