Trans. Nonferrous Met. Soc. China 25(2015) 329-334
Solvent extraction of rubidium and cesium from salt lake brine with t-BAMBP-kerosene solution
Shi-ming LIU, He-hui LIU, Yun-jing HUANG, Wei-jun YANG
College of Chemistry and Chemical Engineering, Hunan University, Changsha 410082, China
Received 24 March 2014; accepted 11 July 2014
Abstract: The residues of salt lake brine from which potassium had been removed were used to extract Rb+ and Cs+ together with a sulphonated kerosene (SK) solution of 1.0 mol/L 4-tert-butyl-2-(α-methylbenzyl) phenol (t-BAMBP). Rb+ and Cs+ were enriched and separated effectively by precipitating Mg2+ before extraction and by scrubbing out K+ and Na+ repeatedly before stripping. The effects of the volume ratio of organic phase to aqueous extraction phase (O/A), alkalinity of aqueous phase (c(OH)-), interference from K+ and Mg2+, and ratio the volume of organic phase to aqueous scrubbing phase (O/A′) were investigated. The experimental brine was extracted optimally by 5-stage extraction with 1.0 mol/L t-BAMBP in SK, c(OH-)=1 mol/L, and O/A=1:1. The scrubbing yield of rubidium was only about 10.5% when the extraction solvent was washed 3 times with 1×10-4 mol/L NaOH at O/A′=1:0.5. After 5-stage countercurrent extraction, the final extraction yields of Rb+ and Cs+ reached 95.04% and 99.80%, respectively.
Key words: salt lake brine; t-BAMBP; extraction; rubidium ion; cesium ion
1 Introduction
Rare metals rubidium and cesium are playing increasingly important roles in the fields of new energy, new materials, night-vision equipments, fiber optic telecommunication systems and metal-ion catalysts [1-3]. Due to the highly similar properties, rubidium and cesium usually coexist in minerals. There are many large hinterland salt lakes worldwide containing abundant rubidium and cesium but often at trace concentrations (about 0-20 mg/L) [4]. K+, Na+, Mg2+, Li+ and other ions also coexist in salt lake brine, the concentrations of which are thousands or even hundreds of thousands times that of Rb+ and Cs+. Moreover, these ions have very similar physical and chemical properties to those of Rb+ and Cs+ [5,6], so it remains hard to separate trace Rb+ and Cs+ economically from salt lake brine and to purify them.
Two methods, i.e., adsorption [7,8] and solvent extraction [9,10], have been widely used to enrich and separate low-concentration Rb+ and Cs+ hitherto. For the solvent extraction method, substituted phenols, such as 4-sec-butyl-2(α-methylbenzyl) phenol (BAMBP) and 4-tert-butyl-2 (α-methylbenzyl) phenol (t-BAMBP), are highly practically valuable due to high selectivities toward Rb+ and Cs+ and easy stripping [11-13]. But up to now, phenolic extraction solvents have seldom been used to extract Rb+ and Cs+ from salt lake brine which contains high concentrations of other ions.
The samples of salt lake brine from Qinghai Province (China) and the intermediate liquids in potassium fertilizer or in lithium carbonate manufacturing process using salt lake brine as raw material were analyzed. The residual brine from potassium extracting system had Rb+ and Cs+ at the concentrations 8-10 times those in the original brine. Especially, the contents in the brine samples from East or West Taijnar Lake (Qinghai Citic Guoan Technology Development Co., Ltd.) were the highest. The concentrations of Rb+ and Cs+ rose to 20.0 mg/L and 2.0 mg/L, respectively, whereas they were only 2.3 mg/L and 0.35 mg/L respectively in the original brine. A new process was proposed in this work to extract Rb+ and Cs+ from salt lake brine with t-BAMBP. Magnesium was first precipitated before extraction. Meanwhile, potassium and sodium were washed before stripping. Finally, Rb+ and Cs+ were enriched, and they were then separated easily by further extraction to give pure compounds.
2 Experimental
2.1 Reagents
t-BAMBP (98% GC purity) was obtained from Beijing Realkan Seprtech Co., Ltd. Sulphonated kerosene (SK) was prepared with concentrated sulfuric acid. All other reagents were analytical reagents and used as received.
The residual liquid with potassium products eliminated was obtained from East Taijnar Plant (Citic Guoan Technology Development Co., Ltd., China) and used as the study object. The concentrations of cations are: ρ(Rb+)=20.0 mg/L, ρ(Cs+)=2.0 mg/L, ρ(Na+)=19.39 g/L, ρ(Mg2+)=28.38 g/L, ρ(K+)=2.772 g/L and ρ(Li+)=0.685 g/L; pH: 6.
2.2 Experimental design
The structure of extractant (t-BAMBP) is shown in Fig. 1.

Fig. 1 Structure of t-BAMBP
In alkaline solution, phenols extract alkali metal ions probably by cation exchange through the acidic hydrogen ions. Then the resulting phenolic salt with strong hydrophobicity (dissoluble in organic phase and insoluble in water phase) can be easily stripped by inorganic acid. The extraction reaction can be described as follows (where RH refers to phenol and M+ refers to Rb+ or Cs+) [14,15]:
aM++bRH+aOH-=[Ma·Rb·Hb-a]+aH2O (1)
Phenols are excellent extractants for alkali metals and have high selectivities for Rb+ and Cs+ over other elements in the same group. The extractabilities of alkali metals follow the descending order of Cs+>Rb+>K+> Na+>Li+. Rb+ and Cs+ extracted into the organic phase can be easily stripped by inorganic acid following the reaction below. At the same time, the extractant is recovered.
[Ma·Rb·Hb-a]+aH+=bRH+aM+ (2)
2.3 Analytical method
Rb+ and Cs+ were examined by flame atomic absorption spectrometry with PerkinElmer 700/800 atomic absorption spectrometer.
2.4 Experimental process
Mg2+ in the experimental brine was firstly removed with sodium hydroxide to eliminate its influence on extraction, and magnesium hydroxide precipitate was then separated by centrifugation as fire retardant or rubber stuffing. Then the alkalinity was adjusted, and the brine was extracted with t-BAMBP solution. Most of Rb+ and Cs+, a small amount of K+ and trace Na+ in the brine were extracted into the organic phase. RbCl and CsCl were readily prepared by washing the organic phase with 1.0×10-4 mol/L NaOH and by stripping with 2.0 mol/L HCl, and the stripping liquid was evaporated for crystallization. The technological process is described in Fig. 2.

Fig. 2 Technological process of extraction
3 Results and discussion
3.1 Extraction ability of t-BAMBP and effect of phase ratio
The extraction ability of t-BAMBP towards the simulated brine which only contained Rb+ and Cs+ (ρ(Rb+)=20.0 mg/L, ρ(Cs+)=2.0 mg/L) and the effect of phase ratio on extraction were investigated firstly (Fig. 3). The extraction efficiency (E) can be calculated from the following formula:
E=[M1/(M1+M2)]×100% (3)
where M1 is the amount of extracted ion in organic phase; M2 is the amount of the residue ion in aqueous phase.
As shown in Fig. 3, when SK was used as extractant alone (without t-BAMBP in it), no Rb+ or Cs+ was extracted. So SK was only a diluent. t-BAMBP showed high extraction capacity towards the simulated brine which only contained Rb+ and Cs+. The extraction yield of Rb+ was elevated with increasing the volume ratio of organic phase to aqueous phase (O/A), and that of Cs+ changed slightly because it had almost been completely extracted at low phase ratio. When O/A=2:1, the extraction efficiencies of Rb+ and Cs+ were 97.95% and nearly 100%, respectively. The original concentration of Rb+ was 10 times that of Cs+, so the extraction of Rb+ was crucial because Cs+ should be extracted first. Taking the cost of extraction into consideration simultaneously, O/A=1:1 was selected as the optimum phase ratio. The raw material of salt lake brine which contained other ions (e.g., alkali ions) at high concentrations may substantially reduce the extraction selectivities for Rb+ and Cs+.

Fig. 3 Effect of phase ratio (O/A) on extraction efficiency
3.2 Effect of extractant concentration
The effect of t-BAMBP on extraction was investigated by changing its concentration (0.5 mol/L, 1 mol/L) and the extraction phase ratio (O/A=0.25:1, 0.5:1 and 1:1). The results are given in Table 1.
Table 1 Effects of t-BAMBP concentration and phase ratio on extraction efficiency

As given in Table 1, K+ and Mg2+ interfered with the extraction of Rb+ and Cs+ significantly, with high extraction yields and maximum extractants. Hence, it is vital to evaluate the effects of the concentrations of K+ and Mg2+ on extraction. With rising phase ratio (O/A), the extraction yield of Rb+ increased, and the extraction rates of other ions reduced. With increasing the concentration of t-BAMBP, the extraction yield of Rb+ rose obviously, whereas the viscosity of organic phase and the cost of extraction were augmented simultaneously. Accordingly, 1.0 mol/L phenol and O/A=1:1 were optimum for the process in most cases.
3.3 Effect of Mg2+ and K+
The extraction efficiencies of Li+ and Na+ are much lower than those for Rb+ and Cs+ because their ionic radii are much smaller. However, K+ and Mg2+, which have similar ionic radii to those of Rb+ and Cs+, were bound to affect the extraction most significantly. After Mg2+ was precipitated with NaOH or K+ was removed with tartaric acid, the experimental brine was extracted with 1.0 mol/L t-BAMBP in SK when O/A=1:1.
A very small portion of Rb+ (3.6%) was precipitated together with Mg2+, after which the single-stage extraction yield of Rb+ reached 63.8%. On the contrary, a large amount of Rb+ (65.7%) was precipitated with potassium hydrogen tartrate during K+ elimination (without Mg2+ precipitation). Probably, Rb+ was impacted into the crystal lattice of K+ precipitate because of their similar physicochemical properties. Furthermore, the extraction yield of remaining Rb+ was very low after K+ precipitation, revealing that Mg2+ actually reduced the Rb+ extraction yield. Therefore, it is necessary to remove Mg2+ and to raise pH value of the brine before Rb+/Cs+ extraction. The by-product Mg(OH)2 can be used as fire resistant or rubber filler to reduce the costs remarkably.
3.4 Effect of c(OH-)-
Figure 4 shows the extraction efficiencies of various alkali metals in the experimental brine with 1.0 mol/L t-BAMBP by adjusting c(OH-) in the aqueous phase from 0 to 1.0 mol/L. The extraction efficiencies of Rb+ and Cs+ increased with rising c(OH-), and the extraction yield of Rb+ increased from 40.9% to 90.8% when c(OH-) rose to 1.0 mol/L.

Fig. 4 Effect of c(OH-) on extraction efficiency
The extraction selectivity of t-BAMBP solution (a weak acidic solvent) in alkaline condition is much higher than that in neutral or acidic condition, so alkalinity is critical for the extraction system. Extra Na+ or K+ may be introduced into the experimental brine because c(OH-) is adjusted by sodium hydroxide and potassium hydroxide. Comparably, high concentration of Na+ is tolerable because it exerts less effect on Rb+/Cs+ extraction. In this case, NaOH was chosen to precipitate Mg2+ and to adjust c(OH-) of the aqueous phase. According to the curves in Fig. 4, c(OH-)=0.5 mol/L was the most suitable.
3.5 Effect of scrubbing phase
As a weak acid, t-BAMBP can only extract Rb+ and Cs+ efficiently at high pH values, and it can be easily stripped with diluted acid [16]. To enrich Rb+ and Cs+ in the extractant and to scrub out Na+, K+ and other impurities as much as possible before stripping, the scrubbed extractant was stripped with HCl. Therefore, the effects of scrubbing phase and the volume ratio of organic phase to aqueous scrubbing phase (O/A′) were investigated. The results are given in Table 2.
Table 2 Effects of scrubbing phase and O/A′ ratio on Rb+ scrubbing yield

As shown in Table 2, the scrubbing yield of Rb+ decreased with increasing pH and decreasing O/A′. When c(NaOH)=1×10-4 mol/L and O/A′=1:0.5, the scrubbing yield of Rb+ was very low (E′(Rb+)=10.5%), and 57.7% of K+ and 69.4% of Na+ could be scrubbed from the organic phase. As a result, Rb+ and Cs+ were further enriched in the organic phase. In the presence of considerable Na+ and K+ in the extractant, the actual concentrations of Rb+ and Cs+ after scrubbing remained low and were further enriched by reusing the extractant and scrubbing the extractant repeatedly through multi-stage extraction (Fig. 2).
3.6 Multi-stage continuous countercurrent extraction
Based on the above results, NaOH solution was added in the experimental brine (250 mL) from which potassium compounds had been separated in the fertilizer plant, aiming to eliminate Mg2+. Then c(OH-) was adjusted to 0.5 mol/L after precipitates were removed by centrifugation, and the contents of alkali metal ions were analyzed by atomic absorption spectrometry. According to the distribution coefficients of these alkali metal ions in the experimental brine, 5-stage extraction was suitable for separating Rb+ and Cs+ from the brine. Therefore, 200 mL of brine in each bottle from which Mg2+ had been eliminated was extracted in 5-stage with 1.0 mol/L t-BAMBP+SK (O/A=1:1) by shaking the bottle. The extraction procedure is shown in Fig. 5(a) and the results are shown in Table 3. The organic phase that was mixed with E1 to E5 after extraction was scrubbed in 3-stage with 1×10-4 mol/L NaOH (O/A′=1:0.5, Fig. 5(b)). The organic phase that was mixed with E′1 to E′3 after scrubbing was stripped in 2-stage with 2 mol/L HCl (O/A′′=1:1). The stripping procedure is shown in Fig. 5(c) and the results are summarized in Table 4.

Fig. 5 Schemes of multi-stage continuous countercurrent extraction
Table 3 Results of 5-stage continuous countercurrent extraction
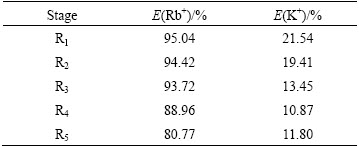
Table 4 Results of 2-stage continuous countercurrent stripping with HCl

The extracted organic phase was scrubbed in 3-stage with 1.0×10-4 mol/L NaOH. Most of K+ ions were eluted with E′(K+)=97.81%, and Rb+ ions were hardly removed with E′(Rb+)=14.81%. The scrubbed liquid phase should be extracted repeatedly to increase the concentrations of Rb+ and Cs+. When c(NaOH)=0.5 mol/L, the highest extraction efficiency of Rb+ reached 95.04% after 5-stage extraction, and the other cations (e.g., K+ and Na+) had lower extraction yields, while the extraction yield of Li+ was negligible and almost all of Cs+ ions were extracted. After the organic phase was stripped with 2.0 mol/L HCl, the stripping yields of Rb+ and Cs+ were 95.2% and 99.5%, respectively. After the aqueous stripping solution was evaporated, the contents of RbCl and CsCl in the crystals were 85.40% and 9.25%, respectively. This mixed compound can be further separated easily as pure RbCl and CsCl by extraction, and relevant studies are still ongoing in our group.
4 Conclusions
1) The conditions under which trace Rb+ and Cs+ were extracted from the salt brine with potassium removed previously in plant were optimized. NaOH solution was added in the experimental brine to eliminate Mg2+ firstly. Then c(OH-) was adjusted to 1.0 mol/L after Mg(OH)2 was removed by centrifugation. The liquid phase was extracted with 1.0 mol/L t-BAMBP+SK at O/A=1:1. By using single-stage extraction, the extraction efficiency of Rb+ reached up to 90.8%, and the stripping efficiency was up to 84.6%.
2) After 5-stage continuous countercurrent extraction, the final extraction efficiency of rubidium reached 95.04%, and the purity of rubidium chloride reached 85.40% after 3-stage scrubbing by using 1.0×10-4 mol/L NaOH and 2-stage stripping by using 2.0 mol/L HCl.
3) The extraction process proposed in this work can readily and economically be applied to extract trace Rb+ and Cs+ simultaneously from the brine of salt lakes in China or the brine containing copious other cations with potassium products removed. Hence, the method is highly valuable in practice.
References
[1] SLOBODIN B V, ISHCHENKO A V, SAMIGULLINA R F, YAGODIN V V, SHULGIN B V. Preparation and luminescent properties of rubidium and cesium vanadates [J]. Inorganic Materials, 2014, 50(2): 179-183.
[2] VDOVIC S, SARKISYAN D, PICHLER G. Absorption spectrum of rubidium and cesium dimers by compact computer operated spectrometer [J]. Optics Communications, 2006, 268(1): 58-63.
[3] YANG Wei-jun, FANG Cao, ZHOU Ji-cang, GUO Can-cheng, WAN Wei. Catalytic synthesis of perfluorolyethers [J]. Journal of Central South University, 2013, 20(3): 629-633.
[4] GUO Xiu-hong, ZHENG Mian-ping, LIU Xi-fang, NIE Zhen, PU Ling-zhong. Saline cesium resource and prospect of its exploitation and utilization in Tibet [J]. Journal of Salt and Chemical Industry, 2008, 37: 8-12. (in Chinese)
[5] DAN De-zhong, DU Gu, WAN Yu-ping. Indirect determination of cesium in brine by single-sweep oscillopolarography [J]. Electroanalysis, 1992, 4(5): 575-579.
[6] YANG Wei-jun, LIU Shi-ming, LI Yong-jin, HUANG Yun-jing, LUO Xi-sheng. Process analysis of Rb+ and Cs+ adsorption from salt lake brine by ammonium molybdophosphate composite material [J]. Advanced Materials Research, 2013, 785: 812-816.
[7] JIA Li-ying, CHEN Xiao-qing, WEI Jun-ting, LIU Ying. Synthesis of graft-p-tert-butylphenol resin and its adsorbtion capability of rubidium [J]. Journal of Central South University of Technology: Natural Science, 2001, 32(1): 54-57. (in Chinese)
[8] GIBERT O, VALDERRAMA C, MICHAELA P, CORTINA J L. Evaluation of selective sorbents for the extraction of valuable metal ions (Cs, Rb, Li, U) from reverse osmosis rejected brine [J]. Solvent Extraction and Ion Exchange, 2010, 28(4): 543-562.
[9] NISAN S, LAFFORE F, POLETIKO C, SIMON N. Extraction of rubidium from the concentrated brine rejected by integrated nuclear desalination systems [J]. Desalination and Water Treatment, 2009, 8: 236-245.
[10] DIRACH J L, NISAN S, POLETIKO C. Extraction of strategic materials from the concentrated brine rejected by integrated nuclear desalination systems [J]. Desalination, 2005, 182(1-3): 449-460.
[11] LIU Xue-ying,YANG Jin-yu,CHEN Xiao-wei,ZHANG Qian. Extraction mechanism and thermodynamic functions of Rb+ with t-BAMBP [J]. Journal of Nuclear and Radiochemistry, 2007, 29(3): 151-155
[12] BRYAN J C, DELMAU L H, HAY B P, NICHOLAS J B, ROGERS L M, ROGERS R D, MOYER B A. Cesium recognition by supramolecular assemblies of 2-benzylphenol and 2-benzylphenolate [J]. Structural Chemistry, 1999, 10(3): 187-203.
[13] RAIS J, KRTIL J, CHOTIVKA V. Extraction and separation of 137Cs and 86Rb by means of 4-t-butyl-2(α-methylbenzyl)phenol [J]. Talanta, 1971, 18(2): 213-218.
[14] MACKENZIE P D, KING C J. Combined solvent extraction and stripping for removal and isolation of ammonia from sour waters [J]. Industrial and Engineering Chemistry Process Design and Development, 1985, 24(4): 1192-1200.
[15] DELMAU L H,BRYAN J C,HAY B P,ENGLE N L,SACHLEBEN R A,MOYER B A. Benzyl phenol derivatives: Extraction properties of calixarene fragments [J]. ACS Symposium Series, 2000, 757: 86-106.
[16] YANG Jin-yu, ZHANG Qian, LIU Xue-ying, CHEN Xiao-wei. Stripping of rubidium with inorganic acid solution from t-BAMBP load organic phase [J]. Chinese Journal of Rare Metals, 2007, 31(2): 228-231. (in Chinese).
t-BAMBP-煤油溶液萃取盐湖卤水中铷和铯离子
刘世明,刘和辉,黄云敬,阳卫军
湖南大学 化学化工学院,湖南 长沙 410082
摘 要:将工厂提钾后的盐湖卤水作为提取Rb+和Cs+的实验用卤水,将萃取剂t-BAMBP的磺化煤油溶液作为有机相进行萃取。在萃取之前预先沉淀出镁并作为一种产品,在反萃前再多次洗涤分离出大部分的K+ 和 Na+,最终使Rb+和Cs+得到有效富集和分离。研究油水相比(O/A)、水相的碱性(c(OH-)、K+ 和Mg2+的含量及洗涤油水相比(O/A′)对萃取过程的影响。最佳工艺条件为:1.0 mol/L的 t-BAMBP磺化煤油溶液,水相碱性c(OH-)=1 mol/L, 油水相比O/A=1:1。当用1×10-4 mol/L NaOH溶液洗涤萃取油相3次,洗涤油水相比O/A′=1:0.5时,铷和铯的洗脱率仅为10.5%。经过5级逆流萃取,最终铷和铯的萃取率分别达到了95.04% 与 99.80%。
关键词:盐湖卤水;t-BAMBP;萃取;铷离子;铯离子
(Edited by Yun-bin HE)
Foundation item: Project (20606008) supported by the National Natural Science Foundation of China; Project (11070210) supported by the Fundamental Research Funds for the Central Universities of China
Corresponding author: Wei-jun YANG; Tel: +86-731-88821449; E-mail: wjyang@hnu.edu.cn
DOI: 10.1016/S1003-6326(15)63608-1