铝酸钙炉渣浸出过程的二次反应机理
来源期刊:中国有色金属学报2015年第4期
论文作者:孙会兰 王 波 张建新 宗书凤 刘佳佳
文章页码:1334 - 1340
Key words:calcium aluminate slag; secondary reaction; alumina; leaching
摘 要:SiO2以γ-2CaO·SiO2的形式存在于铝酸钙炉渣中,γ-2CaO·SiO2比β-2CaO·SiO2稳定,但是在氧化铝溶出过程中它仍然可以被碳酸钠溶液分解,并引起二次反应。利用XRD研究铝酸钙炉渣二次反应的程度和机理。结果表明,γ-2CaO·SiO2的分解率随着浸出时间和碳酸钠浓度的增加而上升,主要二次反应产物为水化石榴石和钠硅渣的混合物。溶液中SiO2的浓度随着溶出温度的上升先增加而后降低。XRD分析表明,低温下二次反应的产物是水化石榴石,而高温下水化石榴石则会转变为钠硅渣。
Abstract: SiO2 in calcium aluminate slag exists in the form of γ-2CaO·SiO2 which is more stable than β-2CaO·SiO2. However, it is decomposed by sodium carbonate solution during leaching process, leading to the secondary reaction. The extent of secondary reaction and reaction mechanism of calcium aluminate slag were studied using XRD. The results show that the decomposition rate of γ-2CaO·SiO2 increases with the increase in leaching time and sodium carbonate concentration. The main products of secondary reaction are the mixture of hydrogarnet and sodium hydrate alumina-silicate. SiO2 concentration rises firstly and then drops with the increase of leaching temperature. XRD results indicate that the stable product of secondary reaction at low temperature is hydrogarnet. But hydrogarnet is transformed into sodium hydrate alumina-silicate at high temperature.
Trans. Nonferrous Met. Soc. China 25(2015) 1334-1340
Hui-lan SUN1,2, Bo WANG1,2, Jian-xin ZHANG1, Shu-feng ZONG1, Jia-jia LIU1
1. School of Materials Science and Engineering, Hebei University of Science and Technology, Shijiazhuang 050018, China;
2. Hebei Key Laboratory of Material Near-net Forming Technology, Hebei University of Science and Technology, Shijiazhuang 050018, China
Received 12 March 2014; accepted 26 January 2015
Abstract: SiO2 in calcium aluminate slag exists in the form of γ-2CaO·SiO2 which is more stable than β-2CaO·SiO2. However, it is decomposed by sodium carbonate solution during leaching process, leading to the secondary reaction. The extent of secondary reaction and reaction mechanism of calcium aluminate slag were studied using XRD. The results show that the decomposition rate of γ-2CaO·SiO2 increases with the increase in leaching time and sodium carbonate concentration. The main products of secondary reaction are the mixture of hydrogarnet and sodium hydrate alumina-silicate. SiO2 concentration rises firstly and then drops with the increase of leaching temperature. XRD results indicate that the stable product of secondary reaction at low temperature is hydrogarnet. But hydrogarnet is transformed into sodium hydrate alumina-silicate at high temperature.
Key words: calcium aluminate slag; secondary reaction; alumina; leaching
1 Introduction
With the significantly increasing alumina production capability in recent years, the discrepancy between the supply and the demand of the materials of bauxite in China is increasingly serious [1]. One of the methods to solve this discrepancy is to explore and utilize new alumina-containing materials [2-4]. Calcium aluminate process (lime sintering process) is a method to leach alumina from iron-bearing bauxite, fly ash and alumina-containing slags [5-7]. In this process, iron- bearing bauxite is sintered with limestone at 1500 °C. The main phases of the sinter are 12CaO·7Al2O3 and γ-2CaO·SiO2. The alumina of 12CaO·7Al2O3 is easily leached into sodium carbonate solution.
The crystal form of 2CaO·SiO2 of calcium aluminate slag is γ type [8], but that of soda-lime sinter is β type [9]. The different crystal structures of dicalcium silicate have different influences on alumina leaching of sinters. β-2CaO·SiO2 of soda-lime sinter will react with NaOH, Na2CO3 and NaAl(OH)4. Then, sodium hydrate alumino-silicate and hydrogarnet are formed [10-13]. In this way, Al(OH)4- and Na+ are leached out from sinter and then precipitated into the red mud again. Thus, alumina leaching rate of sinter decreased. This is called the secondary reaction. Secondary reaction will cause the alumina loss of the leaching process. And the decomposed silicon dioxide will come into the crude liquor and complicate the desilication system.
It has been reported that γ-2CaO·SiO2 is inert in sodium aluminate solution, and it normally does not react with other phases [14]. But our former research indicates that partial γ-2CaO·SiO2 will be decomposed and cause secondary reaction in the solution with high sodium carbonate concentration [15]. The secondary reaction of γ-2CaO·SiO2 is seldom reported. Therefore, in this work, the effects of sodium carbonate concentration, leaching time and leaching temperature on the decomposition of γ-2CaO·SiO2 were studied and the secondary reaction mechanism was discussed.
2 Experimental
2.1 Materials
CaCO3, Na2CO3, NaOH and SiO2 used in the experimental studies are analytical pure reagents. Al(OH)3 used in the experiment is an industrial pure reagent.
2.2 Equipments
Muffle furnace, gas shielded MoSi2 furnace, magnetic stirring constant temperature water bath, X-ray diffraction analyzer (PANalytical PW3040/60) and spectrophotometer (722S) were used.
2.3 Smelting of calcium aluminate slag
The smelting experiments were carried out in a gas shielded MoSi2 furnace and the vessel was a graphite crucible. The smelting temperature was 1500 °C and the holding time was 1 h. After smelting, the melt was cooled at the speed of 5 °C/min, and it was taken out at the temperature of 400 °C. After grinding, the particle size of the slag is less than 74 μm.
The calcium aluminate slag is synthesized by chemical reagents. Its m(Al2O3)/m(SiO2) (the mass ratio of Al2O3 to SiO2) and n(CaO)/n(Al2O3) (the mole ratio of CaO to Al2O3, excluding the CaO of 2CaO·SiO2) are 1.3 and 1.7, respectively. Table 1 shows the chemical composition of the slag and Fig. 1 shows the XRD results of the slag. XRD result indicates that the main phases in the slag are 12CaO·7Al2O3 and γ-2CaO·SiO2, whose contents are 46.57% and 52.96%, respectively according to Rietveld analysis. Pure 12CaO·7Al2O3 and γ-2CaO·SiO2 are synthesized at 1500 °C by chemical reagents separately.
Table 1 Chemical composition of slag (mass fraction, %)
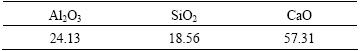

Fig. 1 XRD pattern of calcium aluminate slag
2.4 Leaching of calcium aluminate slag
The sodium aluminate solution obtained from the slag was treated according to the carbonization precipitation process. Circulating mother liquid was used to leach new calcium aluminate slag. The conditions of the leaching solution are: caustic alkali concentration of 7 g/L, αk=1.6 (mole ratio of Na2O to Al2O3) and liquid- solid ratio of 4.5 mL/g.
2.5 Methods of analysis
The contents of Al2O3, SiO2, CaO and Na2O in samples and filtrate were analyzed by XRF. Alumina leaching rate is calculated according to Eq. (1).
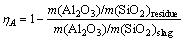 (1)
(1)
where ηA is alumina leaching rate; m(Al2O3) is the mass of Al2O3 in slag or residue; m(SiO2) is the mass of SiO2 in slag or residue.
Phase components of calcium aluminate slag and leaching residues were identified by X-ray diffraction (PANalytical PW3040/60). The concentration of SiO2 in leaching solution was determined with silico- molybdenum blue spectrophotometer. Thus, the decomposition property of γ-2CaO·SiO2 (Δρ(SiO2)) during alumina leaching process is defined as the concentration change of SiO2 after alumina leaching.
3 Results and discussion
3.1 Effect of sodium carbonate concentration on decomposition of γ-2CaO·SiO2
The reaction between 12CaO·7Al2O3 and Na2CO3 (Eq. (2)) can realize the recovery of alumina from calcium aluminate slag. Meanwhile, γ-2CaO·SiO2 will also react with Na2CO3, leading to the decomposition of dicalcium silicate (Eq. (3)) and the loss of alumina extracted into solution (Eq. (4)-(6)). Therefore, the effect of sodium carbonate concentration (abbr., Na2Oc) on decomposition characteristics of γ-2CaO·SiO2 was studied. The results are shown in Fig. 2.
12CaO·7Al2O3+12Na2CO3+33H2O=14NaAl(OH)4+12CaCO3+10NaOH (2)
2CaO·SiO2+2Na2CO3+H2O=2CaCO3+Na2SiO3+2NaOH (3)
1.7Na2SiO3+2NaAl(OH)4+(n-2.3) H2O=Na2O·Al2O3·1.7SiO2·nH2O+3.4NaOH (4)
3Ca(OH)2+2NaAl(OH)4=3CaO·Al2O3·6H2O+2NaOH (5)
3CaO·Al2O3·6H2O+nNa2SiO3=3CaO·Al2O3·nSiO2·(6-2n)H2O+2nNaOH+nH2O (6)
The results indicate that the Δρ(SiO2) concentration increases obviously when Na2Oc concentration increases from 40 g/L to 80 g/L. The increasing rate of Δρ(SiO2) decreases as the Na2Oc concentration continuously increases.
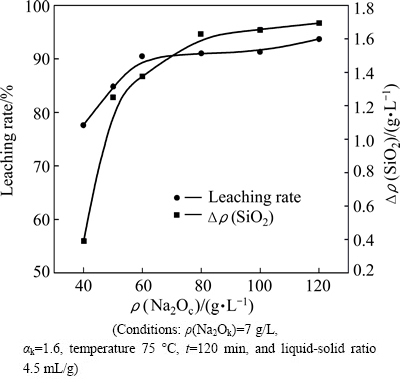
Fig. 2 Effect of Na2Oc on leaching rate of slag and decomposability of γ-2CaO·SiO2
The values of Δρ(SiO2) vary from 0.4 g/L to 1.7 g/L, indicating that the decomposition of γ-2CaO·SiO2 is serious when Na2Oc concentration is up to 80 g/L.
The change trend of alumina leaching rate under different Na2Oc concentrations is similar to that of Δρ(SiO2). When liquid-solid ratio is 4.5 mL/g, calcium aluminate slag will consume Na2Oc at approximately 50-60 g/L according to Eq. (2). Therefore, the alumina leaching rate increases from 77.59% to 90.49% under different Na2Oc concentrations. Further extension of Na2Oc has little effect on the improvement of alumina leaching ratio.
3.2 Effect of Na2Ok concentration and αk on decomposition of γ-2CaO·SiO2
In order to avoid the effect of sodium carbonate on decomposition of γ-2CaO·SiO2, its concentration is fixed at 7 g/L in this section. The effects of Na2Ok concentration and αk on the alumina leaching rate of calcium aluminate slag and the decomposition of γ-2CaO·SiO2 are shown in Fig. 3 and Fig. 4. It can be seen that the leaching rates of samples are less than 21% under these conditions. This is because the Na2Oc is not enough to react with 12CaO·7Al2O3 according to Eq. (2). The Δρ(SiO2) concentration increases when Na2Ok concentration (Fig. 3) and αk (Fig. 4) increase. The maximum values of Δρ(SiO2) concentration are 0.75 g/L and 0.47 g/L when Na2Ok concentration is 50 g/L (Fig. 3) and αk is 11 (Fig. 4). Both of the two values are lower than 1.69 g/L which is obtianed when Na2Oc concentration is 120 g/L (Fig. 2). These results indicate that Na2Ok concentration and αk have important effect on the decomposability of γ-2CaO·SiO2, but they are not the main factors compared to Na2Oc.
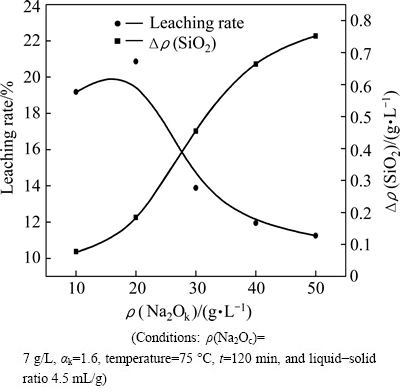
Fig. 3 Effect of Na2Ok concentration on leaching rate of slag and decomposability of γ-2CaO·SiO2
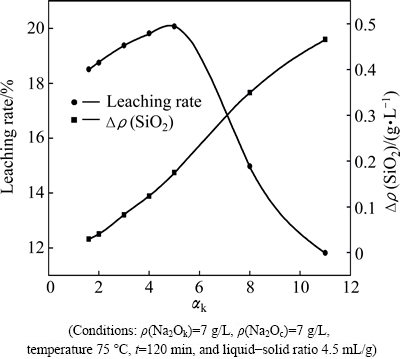
Fig. 4 Effect of αk on leaching rate of slag and decomposability of γ-2CaO·SiO2
3.3 Effect of leaching time on decomposition of γ-2CaO·SiO2
The effect of leaching time on the alumina leaching rate of calcium aluminate slag and the decomposition of γ-2CaO·SiO2 is shown in Fig. 5. It can be seen that the leaching rate and the Δρ(SiO2) concentration increase with increasing leaching time. When leaching time is up to 80 min, its effect becomes little. The inflection point of leaching ratio does not appear under this condition because the reaction extent of secondary reaction is lower than that of leaching reaction.
3.4 Effect of leaching temperature on decomposition of γ-2CaO·SiO2
Figure 6 shows alumina leaching rate and decomposability under different leaching temperatures. The Δρ(SiO2) concentration increases to 1.73 g/L when leaching temperature is 80 °C, and then decreases subsequently. It has been reported that reaction temperature rise will enhance the equilibrium solubility of silica solution. It indicates that the solubilised silica is transferred into the residue when leaching temperature is higher than 80 °C. There are two transferred forms of silica in residue: sodium hydrate alumino-silicate and hydrogarnet. Both will cause the loss of alumina leached into solution.

Fig. 5 Effect of leaching time on leaching rate of slag and decomposability of γ-2CaO·SiO2
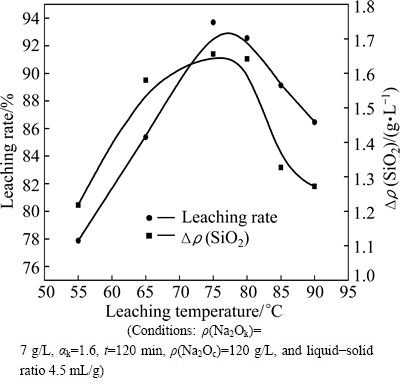
Fig. 6 Effect of leaching temperature on leaching rate of slag and decomposability of γ-2CaO·SiO2
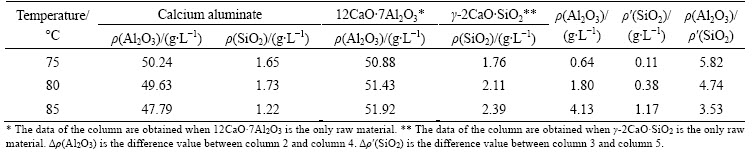
The inverted V trend of alumina leaching rate also proves the existence of the secondary reaction. The maximum value of leaching rate is 93.69% at the inflection point (75 °C). The results of different leaching temperatures are shown in Table 2. The leaching experiments of pure 12CaO·7Al2O3 and γ-2CaO·SiO2 are also carried out separately under the same conditions. The molecular formulas of sodium hydrate alumino- silicate and hydrogarnet are Na2O·Al2O3·1.7SiO2·nH2O and 3CaO·Al2O3·nSiO2·(6-2n)H2O (n≈0.1), respectively. Thus, the mass ratios of Al2O3 to SiO2 in sodium hydrate alumino-silicate and hydrogarnet are 1.0 and 17, respectively.
From the ratio of Δρ(Al2O3) and Δρ′(SiO2), it can be concluded that the products of secondary reaction are the mixture of sodium hydrate alumino-silicate and hydrogarnet. With the increase of leaching temperature, hydrogarnet tends to be transformed into sodium hydrate alumino-silicate. The amount of secondary reaction becomes large and then alumina leaching rate decreases obviously when leaching temperature is up to 85°C. The further study of secondary reaction mechanism is discussed in the next section.
3.5 Mechanism of secondary reaction of calcium aluminate slag
3.5.1 Principle of secondary reaction
About 30% of sinter produced during soda-lime sintering process is β-2CaO·SiO2, which will be decomposed and cause the loss of alumina. The phenomenon mentioned above is called “secondary reaction” [16]. There are three main phases as NaOH, Na2CO3 and NaAl(OH)4 in sodium aluminate solution. But the dominant factors of the decomposition of dicalcium silicate have not been determined at present. These opinions may be described as follows [17-20].
Table 2 Liquid results of leaching solution at different temperatures
1) The decomposition of dicalcium silicate is mainly caused by NaOH. And the decomposition reaction is shown in Eq. (7).
β-2CaO·SiO2+2OH-+2H2O=2Ca(OH)2+H2SiO42- (7)
2) The decomposition of dicalcium silicate is mainly caused by Na2CO3. And the decomposition reaction is shown in Eq. (8):
β-2CaO·SiO2+2CO32-+2H2O=2CaCO3+H2SiO42-+2OH- (8)
3) The decomposition of dicalcium silicate is mainly caused by NaAl(OH)4. And the decomposition reaction is shown in Eq. (9):
3(2CaO·SiO2)+4Al(OH)4-+6H2O+2OH-=2(3CaO·Al2O3·6H2O)+3H2SiO42- (9)
Therefore, β type dicalcium silicate can be decomposed by the phases mentioned above under proper conditions. CHEN et al [17] has studied the thermomechanics of the decomposition reaction with NaOH, Na2CO3 and NaAl(OH)4. The results indicate that the stability of dicalcium silicate in the solution is decreased in the following sequence: NaOH, NaAl(OH)4, Na2CO3. That is to say, Na2CO3 has the strongest decomposability on β-2CaO·SiO2. Furthermore, BI [14] believed that dicalcium silicate could be decomposed thoroughly under pure Na2CO3 solution.
Our research is focused on lime sintering process which has two differences compared with soda-lime sintering process. Firstly, the crystal structure of dicalcium silicate of the former process is γ type with low activity; but that of the latter process is β type with high activity. Secondly, the sodium carbonate concentration in the former process is greater than that in the latter process, but the concentrations of caustic alkali and sodium aluminate show the contrary trend. Interestingly, γ-2CaO·SiO2 is more stable, but Na2CO3 has the stronger decomposability in lime sintering process. Therefore, the mechanism of the secondary reaction between lime sintering process and soda-lime sintering process is different.
3.5.2 Mechanism of secondary reaction of calcium aluminate slag
XRD analysis of leaching residues formed under different conditions is used to study the mechanism of the secondary reaction. The XRD patterns are shown in Figs. 7 and 9.
The XRD results (Figs. 7-9) show that there are large amounts of CaCO3 and γ-2CaO·SiO2 in leaching residues under different conditions. Small quantity of 12CaO·7Al2O3 remains in the residues. The product of secondary reaction is only sodium hydrate alumino- silicate when sodium carbonate concentrations are 50 g/L and 100 g/L, respectively (Fig. 7). Hydrogarnet is formed when leaching time is 20 min, but it will be transformed into sodium hydrate alumino-silicate with leaching time increasing (Fig. 8, Eq. (10)). Sodium hydrate alumino- silicate and hydrogarnet are formed at 55 °C, but the latter disappears when the leaching temperature is up to 80 °C (Fig. 9).
3CaO·Al2O3·xSiO2·(6-2x)H2O+3Na2CO3+xH2O=3CaCO3+2NaAl(OH)4+xNa2SiO3+2(2-x)NaOH (10)
According to Eq. (10), NaAl(OH)4 will be formed in this reaction especially when silica saturation of hydrogarnet is low. And the macro phenomenon of the reaction is the increase in alumina leaching rate. This is another reason for the increase of leaching rate with increasing leaching time (Figs. 5 and 8).
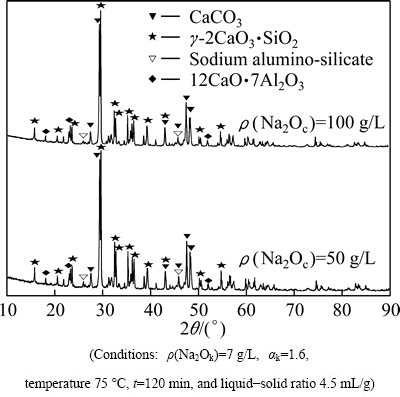
Fig. 7 XRD patterns of leaching residue with different Na2Oc concentrations
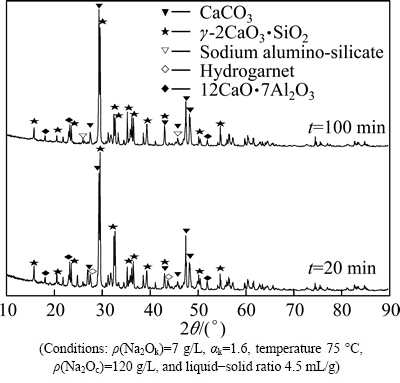
Fig. 8 XRD patterns of leaching residue with different leaching time

Fig. 9 XRD patterns of leaching residue at different temperatures
High reaction temperature could promote not only Eq. (10) but also Eq. (4). NaAl(OH)4 formed by Eq. (10) is less than that consumed by Eq. (4). Thus, alumina leaching ratio decreases when leaching temperature is higher than 75 °C (Fig. 6).
4 Conclusions
1) γ-2CaO·SiO2 is decomposed during alumina leaching process of calcium aluminate slag. But it is not stable in sodium carbonate solution. The occurrence of secondary reaction causes the loss of alumina from solution.
2) The dominant factor of the decomposition of dicalcium silicate is Na2CO3. The concentration of decomposed SiO2 increases to 1.71 g/L when the Na2CO3 concentration increases to 120 g/L under the studied conditions.
3) The products of secondary reaction are sodium hydrate alumino-silicate and hydrogarnet when leaching time is 20 min and leaching temperature is 55 °C. Hydrogarnet is transformed into sodium hydrate alumino-silicate with increasing leaching time and rising leaching temperature.
References
[1] GU Song-qing. Alumina production technology with high efficiency and low consumption from Chinese bauxite resource [J]. The Chinese Journal of Nonferrous Metals, 2004, 14(1): 91-97. (in Chinese)
[2] LIU Wan-chao, YANG Jia-kuan, XIAO Bo. Review on treatment and utilization of bauxite residues in China [J]. International Journal of Mineral Processing, 2009, 93(3): 220-231.
[3] LIU Kui, CHEN Qi-yuan, HU Hui-ping, DING Zhi-ying, YIN Zhou-lan. Characteristics of scales formed from pressure leaching of Yuanjiang laterite [J]. Hydrometallurgy, 2011, 109(1-2): 131-139.
[4] VESTOLA E A, KUUSENAHO M K N, RHI H M, TUOVINEN O H, PUHAKKA J A, PLUMB J J, KAKSONEN A H. Acid bioleaching of solid waste materials from copper, steel and recycling industries [J]. Hydrometallurgy, 2010, 103(1): 74-79.
[5] HAYNES R J, ZHOU Y F, NAIDU R. Recycling and use of wastes/co-products from the iron/steel and alumina industries [J]. International Journal of Environment and Waste Management, 2011, 8(1): 174-211.
[6] HUANG Zhao-hui, LIU Kai-qi, ZHAO Hong-wei, LI Zhuang. Study on extraction of alumina from sericite phyllite [J]. Key Engineering Materials, 2013, 544: 38-42.
[7] GRZYMEK J, DERDACKA-GRZYMEK A, KONIK Z, STOK A, IWANCIW J. The new way of alumina lixiviation from sinters containing 12CaO·7Al2O3 in J. Grzymek’s method [C]//Light Metals 1988. Warrendale: Minerals, Metals & Materials Society, 1988: 129-133.
[8] WANG Bo, YU Hai-yan, SUN Hui-lan, BI Shi-wen. Effect of material ratio on leaching and self-disintegrating property of calcium aluminate slag [J]. Journal of Northeastern University: Natural Science, 2008, 29(11): 1593-1596. (in Chinese)
[9] PADILLA R, SOHN H. Sodium aluminate leaching and desilication in lime-soda sinter process for alumina from coal wastes [J]. Metallurgical Transactions B, 1985, 16(4): 707-713.
[10] ZHANG Ran, ZHENG Shi-li, MA Shu-hua, ZHANG Yi. Recovery of alumina and alkali in Bayer red mud by the formation of andradite-grossular hydrogarnet in hydrothermal process [J]. Journal of Hazardous Materials, 2011, 189(3): 827-835.
[11] CHEN Bin, LI Xiao-bin, LIU Gui-hua. Behavior of SiO2 in the leaching process of alumina clinker with high concentration [J]. Journal of University of Science and Technology of Beijing, 2008, 15(5): 538-542.
[12] LIU Gui-hua, LI Xiao-bin, PENG Zhi-hong, ZHOU Qiu-sheng. Behavior of calcium silicate in leaching process [J]. Transactions of Nonferrous Metals Society of China, 2003, 13(1): 213-216.
[13] LI Xiao-bin, LIU Xiang-min, LIU Gui-hua, PENG Zhi-hong, LIU Ye-xiang. Study and application of intensified sintering processfor alumina production [J]. The Chinese Journal of Nonferrous Metals, 2004, 14(6): 1031-1036. (in Chinese)
[14] BI Shi-wen. Process of alumina production [M]. Beijing: Chemical Industry Press, 2006. (in Chinese)
[15] SUN Hui-lan, YU Hai-yan, WANG Bo, ZHOU Huai-min, TU Gan-feng, BI Shi-wen. Study on synthesis and decomposition property of γ-2CaO·SiO2 [J]. Mining and Metallurgical Engineering, 2008, 28(5): 59-63. (in Chinese)
[16] YU Hai-yan, PAN Xiao-lin, DING Ting-ting, ZHANG Wu, LIU Han, BI Shi-wen. Adsorption of sodium polyacrylate at interface of dicalcium silicate–sodium aluminate solution [J]. Transactions of Nonferrous Metals Society of China, 2011, 21(10): 2223-2226.
[17] CHEN Bin, LI Xiao-bin, XU Hua-jun, PENG Zhi-hong, LIU Gui-hua, ZHOU Qiu-sheng. Thermodynamic analysis of secondary reactions in the clinker leaching process [J]. Journal of Beijing University of Chemical Technology: Natural Science Edition, 2007, 34(2): 189-192. (in Chinese)
[18] LI Xiao-bin, XU Hua-jun, LIU Gui-hua, PENG Zhi-hong, ZHOU Qiu-sheng, LIU Yun-feng. Behavior of SiO2 during leaching process of alumina sinter [J]. The Chinese Journal of Process Engineering, 2006, 6(3): 431-434. (in Chinese)
[19] LIU Gui-hua, LIU Yun-feng, LI Xiao-bin, PENG Zhi-hong, ZHOU Qiu-sheng, XU Hua-jun. Reducing loss of soda in red mud in process of Bayer digestion [J]. The Chinese Journal of Nonferrous Metals, 2006, 16(3): 555-559. (in Chinese)
[20] XU Shuang. The secondary reaction in clinker digestion by sintering process [J]. Metal Materials and Metallurgy Engineering, 2008, 36(3): 56-59. (in Chinese).
孙会兰1,2,王 波1,2,张建新1,宗书凤1,刘佳佳1
1. 河北科技大学 材料科学与工程学院,石家庄 050018;
2. 河北科技大学 河北省材料近净成形技术重点实验室,石家庄 050018
摘 要:SiO2以γ-2CaO·SiO2的形式存在于铝酸钙炉渣中,γ-2CaO·SiO2比β-2CaO·SiO2稳定,但是在氧化铝溶出过程中它仍然可以被碳酸钠溶液分解,并引起二次反应。利用XRD研究铝酸钙炉渣二次反应的程度和机理。结果表明,γ-2CaO·SiO2的分解率随着浸出时间和碳酸钠浓度的增加而上升,主要二次反应产物为水化石榴石和钠硅渣的混合物。溶液中SiO2的浓度随着溶出温度的上升先增加而后降低。XRD分析表明,低温下二次反应的产物是水化石榴石,而高温下水化石榴石则会转变为钠硅渣。
关键词:铝酸钙炉渣;二次反应;氧化铝;浸出
(Edited by Yun-bin HE)
Foundation item: Project (51104053) supported by the National Natural Science Foundation of China; Project (E2012208047) supported by the Natural Science Foundation of Hebei Province, China
Corresponding author: Bo WANG; Tel: +86-311-81668705; E-mail: wangbo1996@gmail.com
DOI: 10.1016/S1003-6326(15)63732-3

