
Microwave synthesis and characterization of new red long afterglow phosphor Sr3Al2O6: Eu
ZHANG Ping(张 平)1, XU Ming-xia(徐明霞)1, ZHENG Zhen-tai(郑振太)1, 3,
SUN Bo(孙 波)2, ZHANG Yan-hui(张艳辉)2
1. School of Materials Science and Engineering, Key Laboratory for Advanced Ceramics and Machining Technology of Ministry of Education, Tianjin University, Tianjin 300072, China;
2. Department of Materials Chemistry, College of Chemistry, Nankai University, Tianjin 300071, China;
3. Hebei University of Technology, Tianjin 300130, China
Received 10 April 2006; accepted 25 April 2006
Abstract: Nanoparticles of red long afterglow phosphor Sr3Al2O6: Eu2+ were prepared by microwave irradiation method at a power of 680 W and a processing time of 15 min. The phosphors nanoparticles were characterized by X-ray diffraction (XRD), scanning electron microscopy (SEM) and Fluorescence spectrophotometer techniques. The results reveal that the samples are composed of single Sr3Al2O6 phase. The resultant nanoparticles show small size (80-100 nm) and spherical shape. The excitation and emission spectra indicate that excitation broad band chiefly lies in visible range and the nanoparticles emit much strong light at 612 nm under around 473 nm excitation. And the long afterglow phosphorescence of Sr3Al2O6 doped with Eu2+ was observed in the dark with naked eye after the removal of the excitation light. The effect of Eu2+ doping concentrations of the samples on the emission intensity is studied systematically. Furthermore, the microwave method requires a very short heating-time and the energy consumption.
Key words: Sr3Al2O6; red long afterglow; phosphor; microwave irradiation method; nanoparticles
1 Introduction
Long lasting phosphor is a kind of energy-storing material. The material can absorb the visible lights, store the energy, and then release the energy as visible light which leads to a long lasting afterglow in the darkness. Recently, strontium aluminate phosphors activated by europium have attracted much attention since they show excellent properties such as high quantum efficiency[1], long persistence of phosphorescence and good stability[2, 3] when compared with sulfide phosphorescent phosphors. These properties result in a wide application of the material in many fields[4].
Strontium aluminates activated by Eu2+ with long afterglow, such as SrAl2O4 (Eu2+, Dy3+) and Sr4Al14O25 (Eu2+, Dy3+), have been studied extensively[5, 6]. By alternating the crystal structures of the matrix in which Eu2+ ions reside, visible light emitting with different wavelength was obtained. Examples that had been reported are emission at 510 nm for SrAl2O4 (Eu2+, Dy3+) and 490 nm for Sr4Al14O25 (Eu2+, Dy3+), respectively [7, 8]. However, long lasting behavior of Eu2+ in Sr3Al2O6 has not been reported yet.
Conventional synthesis of strontium aluminate phosphors demand controlled heating at high temperatures and long processing times, which often results in inhomogeneous products with low surface area. Many investigations suggested that heating treatment is an important factor for controlling size and crystalline structure of the products. Therefore, it is necessary to search for new methods to produce nanoparticles and to control their size and morphology.
Microwave irradiation as a heating method has found a number of applications in chemistry[9]. Applications of microwave irradiation in preparation of nanoparticles have been reported in recent years[10]. This method has the advantages of short reaction time, production of small particles and high purity.
In this work, for the first time, a new kind of red long afterglow Sr3Al2O6: Eu2+ nanoparticles was prepared by microwave irradiation method under a weak-reducing atmosphere. This method shows advantages of short heating time, simplicity, small size, and high purity. We also present the dependence of photo-luminescence of red Sr3Al2O6: Eu2+ upon doped-Eu2+ concentration.
2 Experimental
The powders (reagents) of SrCO3 (AR), Al(OH)3 (AR) and Eu2O3 (99.99%) were used as raw materials for the preparation of red phosphor samples of Sr3Al2O6: Eu2+. The powders were weighted and ground in an agate mortar for 1 h for homogeneous mixing. The mixed powders were placed in a small alumina crucible inside a large covered alumina crucible. The space between the small and the large crucible was filled with powdered Fe2O3 and active carbon as heating medium and reducing agent. Then the crucibles were placed in a domestic microwave oven with a microwave frequency of 2 450 MHz. Microwave irradiation was carried out at power of 680 W for synthesis time of 15 min. Then, the red Sr3Al2O6: Eu2+ nanoparticles were obtained.
The crystalline structure of the phosphors powders was analyzed by X-ray diffractometer (Rigaka D/Max 2 500 v/pc, Japan). The morphology and size of phosphors powders were observed using scanning electron microscopy (SEM) (HITACHI X-650). The excitation and emission spectra were recorded on the powder samples using a fluorescence spectrophotometer (SPEX F111 AI). The decay curve of afterglow was measured using the brightness meter (ST-86LA). All the measurements were performed at room temperature.
3 Results and discussion
The XRD pattern for the phosphors prepared by the microwave method is shown in Fig.1. The XRD pattern of the pure cubic structure for Sr3Al2O6 is essentially the same as that of the literature (PDF No. 24-1187). No peak of any other phase is detected. These
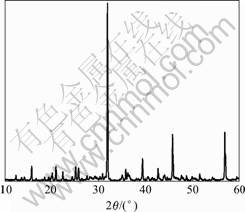
Fig.1 X-ray diffraction pattern of Sr3Al2O6: Eu2+ synthesized by microwave irradiation (680 W, 15 min)
results confirm that the irradiation of microwave provides a satisfactory condition for the formation of single phase Sr3Al2O6 within a quite short time of 15 min. According to the XRD phase analysis, little amount of doped rare earth ions (Eu2+) have almost no effect on the Sr3Al2O6 phase composition.
Fig.2 shows the scanning electron microscope images of Sr3Al2O6: Eu2+ synthesized by the microwave irradiation method at 680 W for 15 min. In the case of the microwave irradiation method, the particles with the sizes of 80-100 m in diameter are highly dispersed. And the particle morphology is of spherical shape. The reason is that the Sr3Al2O6: Eu2+ nanoparticles are synthesized in a quite shorter time with uniform heating in microwave oven. Moreover, the heating treatment requires a very short heating time and energy consumption.

Fig.2 SEM images of Sr3Al2O6: Eu2+ powders prepared by microwave irradiation (680 W, 15 min)
Fig.3 shows the excitation and emission spectra of Sr3Al2O6: Eu2+ phosphor prepared by microwave irradiation method. It is observed that the excitation spectra of phosphor nanoparticles show a broad band from 400 nm to 575 nm (λem=612 nm). The visible light (λex=473 nm) excited the phosphor nanoparticles at room

Fig.3 Excitation and emission spectra of Sr3Al2O6: Eu2+ prepared by microwave heating
temperature yields high bright red luminescence (λmax =612 nm). The bandwidth is quite large (70 nm), indicating only one luminescent centre, which is Eu2 + luminescence and excitation result from transitions between the 4f65d1 and 4f7 electron configurations. The Eu3 + emission peak has not been shown in this emission spectra, indicating that the active carbon can effectively deoxidize the Eu3 + to Eu2 +.
The effect of the doped-Eu2+content in Sr3Al2O6: Eu2+ on the relative luminescent intensity at 612 nm is shown in Fig.4. The highest intensity was found at a doped-Eu2+ concentration of about 5%(in mole fraction). Higher Eu2+ concentration in nanoparticles leads to quenching the luminescent intensity. The reason is that the microwave method is the ease and efficiency with which doping of activators in the host lattice is accomplished. Higher dopant concentrations in the host lattice can increase the emission intensity of the phosphor powders.
Fig.5 shows the decay curve of afterglow at room

Fig.4 Effect of content of doped-Eu2+ on relative luminescent intensity at 612 nm for Sr3Al2O6: Eu2+ prepared by microwave heating (680 W, 15 min)

Fig.5 Decay curve of Sr3Al2O6: Eu2+ + prepared by microwave heating (680 W, 15 min, room temperature 25 ℃, excitation time 5 min, source conventional tricolor luminescent lamp
temperature after the excitation at 473 nm for 5 min. The decay characteristics of Sr3Al2O6: Eu2+ phosphor indicates that the decay process contains the rapid- decaying process and the slow-decaying one. The afterglow of phosphor nanoparticles, which allows the time to be recognized by the brightness meter (≥1 mcd/m2), lasts for over 180 s after the excited source is cut off.
4 Conclusions
The red long afterglow phosphor Sr3Al2O6: Eu2+ nanoparticles can be prepared by the microwave irradiation method in a weak reductive atmosphere of active carbon. The results show that the nanometer phosphors have pure cubic Sr3Al2O6 phase, with a spherical shape and diameters of 80-100 nm. The microwave method is very simple and has the advantages of short heating time, small particle size. With 473 nm light as an excitation source, all the prepared Sr3Al2O6: Eu2+ nanoparticles show bright red emission, and the highest luminescent intensity at 612 nm is found at a content of about 5% Eu2+ in mole fraction. An important result of the present work is that the long afterglow red phosphorescence was observed in Eu2+ doped Sr3Al2O6.
References
[1] SMETS B, RUTTEN J, HOEKS G, VERLIJSDONK. 2SrO?3Al2O3:Eu2+ and 1.29(Ba, Ca)O·6Al2O3:Eu2+ two new blue-emitting phosphors [J]. J Electrochem Soc, 1989, 136 (7): 2119-2123.
[2] PALILLA F C, LEVINE A K, TOMKUS M R. Fluorescent properties of alkaline earth aluminates of the type Mal2O4 activated by divalent europium [J]. J Electrochem Soc, 1968, 115 (6): 642-644.
[3] JIA W Y, YUAN H B, LU L Z, LIU H M, YEN W M. Phosphorescent dynamics in SrAl2O4: Eu2 +, Dy3 + single crystal fibers [J]. J Luminesc, 1998, 76: 424-428.
[4] MATSUZAWA T, AOKI Y, TAKEUCHI N, MURAYAMA Y. A new phosphorescent phosphor with high brightness SrAl2O4: Eu2+, Dy2+ [J]. J Electrochem Soc, 1996, 143: 2670-2673.
[5] WANG M Q, WANG D, LU G L. Research on fluorescence spectra and structure of single-phase 4SrO·7Al2O3:Eu2+ phosphor prepared by solid-state reaction method [J]. Mater Sci Eng B, 1998, 57: 18-23.
[6] HOLSA J, JUNGNER H, LASTUSUARI M, NITTYKOSKI J. Persistent luminescence of Eu2+ doped alkaline earth aluminates, MAl2O4: Eu2+ [J]. J Alloys Compd, 2001, 326: 323-324.
[7] LIN Y H, TANG Z L, ZHANG Z T. Preparation of long-afterglow Sr4Al14O25-based luminescent material and its optical properties [J]. Materials Letters, 2001, 51: 14-18.
[8] LIN Y H, ZHANG Z T, TANG Z L, ZHANG J Y, ZHENG Z S, LU X. The characterization and mechanism of long afterglow in alkaline earth aluminates phosphors co-doped by Eu2O3 and Dy2O3 [J]. Materials Chemistry and Physics, 2001, 70: 156–159.
[9] SUNDAR M S, GOYAL S. Microwave synthesis and characterization of doped ZnS based phosphor materials [J]. Mater Res Bull, 2001, 36: 1039-1043.
[10] ZHU J J, ZHOU M G. Preparation of CdS and ZnS nanoparticles using microwave irradiation [J]. Mater Lett, 2001, 47: 25-32.
(Edited by HE Xue-feng)
Foundation item: Project (50072014) supported by the National Natural Science Foundation of China
Corresponding author: XU Ming-xia; Tel:+86-22-27890489; Fax: +86-22-27404724; E-mail: xumingxia@tju.edu.cn, zp03270819@sina.com,
sunbqk@126.com