Effects of cathodic component of current on porosity and hardness characteristics of micro plasma oxidation(MPO) coatings on aluminum alloy
Samir Hamid Awad, QIAN Han-cheng(钱翰城)
(School of Mechanical Engineering, Chongqing University, Chongqing 400044, China)
Abstract: Micro plasma oxidation(MPO) has recently been investigated as a novel, rapid and effective means to provide modified surfaces with improved properties of load bearing and wear resistance on light alloys particularly aluminum alloys. MPO is a multifactor-controlled process, these factors must be controlled to produce high quality coatings. The main research emphasis in MPO coating development over the past years seems to be the attainment of higher hardness levels and thick coatings. The porosity of MPO coating is the most complex phenomenon affecting the distribution, levels and the measurements of the hardness; and it is controlled by suitable selection of important parameters such as the electrical conditions. Ceramics coatings were synthesized on Al substrate by MPO to examine the effects of adding a cathodic phase alternated with anodic-cathodic current on the porosity and hardness characteristics of coatings by X-ray diffraction(XRD), scanning electron microscopy(SEM), and microhardness tester. The coatings produced by the combined mode are more dense and less porous than that by the anodic-cathodic mode. Microhardness test shows that the coatings produced by the combined mode exhibit both the highest hardness, and less reduction percentage in hardness with increasing the coatings thickness. These improvements become more significant for the polished and thicker coatings.
Key words: micro plasma oxidation(MPO); aluminum alloys; hardness; porosity CLC number: TG178
Document code: A
1 INTRODUCTION
Despite the great achievements made in improvement of wear resistance properties of aluminum alloys, their applications are limited where a heavy surface load bearing is required[1]. Previous coatings applied to aluminum alloys, for example, by traditional processes such as hard anodizing and thermal spraying, have suffered from the low load support from the underlying material and/or insufficient adhesion, which reduces their durability[2]. Although TiN-, CrN- or DLC-coated aluminum alloys using various physical vapour deposition(PVD) methods can achieve a high hardness, in practice they often exhibit poor performance under mechanical loading since the coatings are usually too thin to support the heavy load and protect the substrate in the contact conditions[2]. A novel advancement in this area is the single and duplex coatings prepared by micro plasma oxidation(MPO)[2-4]. The MPO technology has recently been studied as a rapid, novel and effective means to provide thick and hard ceramic coatings with excellent load bearing and wear resistance on light alloys particularly aluminum alloys[2]. The MPO technology, also called plasma electrolyte oxidation, spark anodisation, micro arc oxidation(MAO) or micro discharge oxidation, has been developed in the former Soviet Union as an advanced technology for routine anodizing[5]. In MPO, the aluminum material is immersed as an anode in an equeous solution containing modifying elements(e.g.SiO2), and voltage greater than breakdown voltage of an oxide film(typically 400-600V) is applied between the anode and the cathode[2, 6, 7]. It is characterized by multiple arcs moving rapidly over the treated surface. The excellent properties of MPO coatings are of particular interest to the components of textile machine, aerospace and engineering equipment, biomedical devices and machine building[8]. The main research emphasis in MPO coating development over the past years seems to be the attainment of higher hardness levels and thick coatings in order to improve the load-bearing capacity. Consequently, the load-bearing capacity is the most important factor for MPO coating as a single coating or a pre-coating in the duplex coatings. Desirable combination of good wear resistance and load-bearing capacity as well as corrosion resistance can be obtained through the duplex coatings combining PVD or plasma assisted chemical vapour deposition(PACVD) thin coatings on MPO coating[1, 9]. Generally, the surface morphology of MPO coatings is characterized by a porous structure due to the local thermal action of the sparks and high temperature sintering in the micro arc zone[8]. The pores give the outer ceramic layers: a high wear rate and friction coefficient, low hardness, non-uniform distribution of the hardness and also uncertainty measurements for microhardness[2, 6, 7].
Several benefits could be gained through controlling or decreasing porosity, such as improved phase composition with high hardness and good wear resistance for heavy load-bearing application; and ceramic layers for lubrication requirements and duplex coatings[10-12].
Particularly, current is one of the most important parameters affecting coating qualities[7], hardness and porosity properties. Furthermore, modified alternating current or unbalanced AC, i.e. an alternating current with different amplitudes to the positive(anodic Ia) and negative(cathodic Ic) components, e.g. Ic/Ia=1[13], Ic/Ia=0.6[7], Ic/Ia=1.5[14] has gained increasing attention recently to optimize the coatings quality, due to its superior characteristics as compared with DC and pulsed types[7]. Indeed, research on adding a cathodic phase alternated with anodic-cathodic current[13,15], has proved the activity of cathodic component in producing coatings of greater uniformity.
However, until the present, the research on the influence of adding a cathodic phase alternated with anodic-cathodic current on the characteristics of pores and porosity is not ample; and its influence on the distribution and levels of the hardness at different thickness has not been investigated. In view of the above, it is essential to carry out a further investigation on these issues. In this work, we used a cathodic phase(the C-phase) alternated with anodic-cathodic current(AC) with Ic/Ia=1 to investigate its effects on pores characteristics and hardness distribution of MPO coating.
2 EXPERIMENTAL
A capacitance power source that sequentially fed asymmetric alternating voltage(50Hz) and negative voltage to the bath during preset periods of time was used. By use of this circuit, two voltage modes, i.e. anodic-cathodic(AC), cathodic(C) and AC-C can be alternated. In this circuit, a set of high voltage capacitors redistributes electrical energy according to the total resistance of the cell in the positive and negative half-cycles. By varying the capacitance of the source in both half-cycles, the ratio of amplitudes of positive and negative current can be independently adjusted(Fig.1). MPO treatments were carried out under the conditions where the current density during the cathodic phase was jc=2.4A/dm2 and it was ja=jc=20A/dm2 during AC mode, for which Ic/Ia=1.0. The durations of the phases were τAC=2s and τC=0.78s.
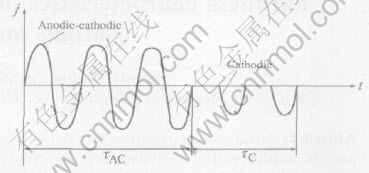
Fig.1 Shape of anodic-cathodic plus cathodic current
The substrates of aluminum alloy(1.2%-2 % Cu, 2%-3 % Mg, 5%-6 %Zn, and Al balance) with 25mm in diameter and 3mm in thickness were ground to a center line average roughness Ra (4.2±0.6)μm, cleaned with detergent and then washed with distilled water before subjecting to the oxidation process. Coatings were fabricated by use of a home-made micro plasma oxidation MPO unit. As shown in Fig.2, the unit consists of an insulated electrolyte bath, a high voltage AC power supply, a stainless steel container with a sample-holder used as an electrolyte cell, stirring and cooling systems. One output of the power supply was connected to the bath; the other was connected to the sample immersed in the electrolyte. The electrolyte was prepared from a solution of sodium silicate in distilled water with other additives. During the oxidation process, the electrolyte was mixed and cooled to prevent heating over 30℃. The treatment times were chosen to produce total coating thickness of(nominally) 70,120 and 180μm. The thickness of the MPO coating was measured with a ED-300 coating thickness equipment. The deposition process was stopped when the coating thickness came to an appropriate value, thus the as-deposited coatings on the Al alloy specimen were obtained. Afterwards, the coated Al alloy specimen was detached from the sample-holder, flushed with water and dried in warm air; and was used for measurements and tests. The as-deposited coatings were polished with SiC paper to remove 40% of the whole thickness of the coatings as polished coating samples. The coating hardness was measured using a Mitutoyo MVK G1 microhardness tester with Vickers indenter at a load of 0.5N. A scanning electron microscope(SEM) KYKY 1000 B was employed for the observation of the surface and cross-section morphology of the coatings. An energy-dispersive spectroscopy(EDX) attachment PVV 1830 Phillips was used for elements quantitative analysis.
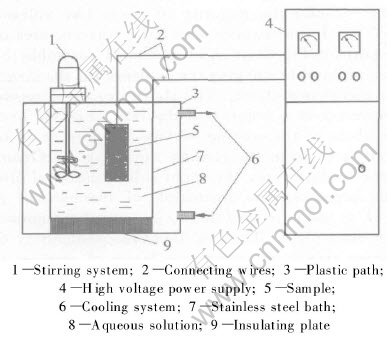
Fig.2 Schematic diagram of micro plasma oxidation
3 RESULTS AND DISCUSSION
3.1 Porosity characterization
Fig.3 shows SEM micrographs of MPO coatings formed by AC-C and AC modes. As shown in Fig.3(a), the coating produced by AC-C mode is more dense and less porous than that by anodic-cathodic mode. A clear observation of these differences can be found in the fractured cross-sectional micrographs of coatings(see Fig.4). The MPO coating consists of a dense-inner layer(compact layer) and a porous and non-uniform outer layer(loose top layer). Unfortunately, the dense-inner layer could not be distinguished in AC mode coating. However, it should be obvious that the outer layer in AC-C mode coating is more dense and less porous than that in the AC coating. The XRD spectra of unpolished and polished coatings showed that all MPO coatings had a structure consisting of a mixture of α-Al2O3 and γ-Al2O3 and a few of other impurities. Due to the very little contents of impurities, we roughly proposed that MPO coatings only consisted of α -Al2O3 and γ-Al2O3. The relative contents of α-Al2O3(density 3.99g/cm3) and γ-Al2O3(density 3.60g/cm3) were calculated on the basis of the integration of diffraction intensity of peak (113) α -Al2O3 and peak (400) γ -Al2O3 of the XRD spectra[15]. Accordingly, the mass fraction of α-Al2O3 in the coating can be calculated also.

Fig.3 SEM micrographs of 180μm-thick MPO coatings
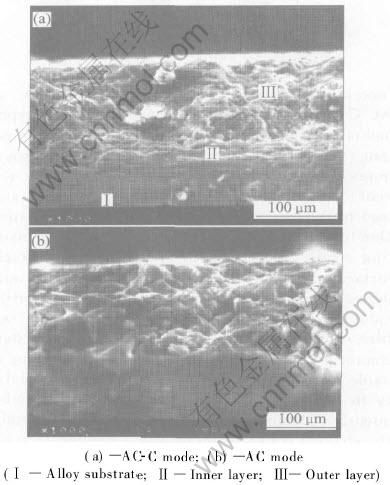
Fig.4 SEM micrographs of cross-section of 180μm-thick MPO coatings
Fig.5 shows the α-Al2O3 content in the as deposited and 40% polished coatings. The density of pores was measured by analyzing the SEM micrographs obtained at 1000 times magnification, which were then magnified a further 500 times using Adobe Photo Deluxe Software. Afterwards, with NIH image 1.62 area of each pore was measured and the density of the pores, e.g. the average area of pores per square centimeter area was then calculated. Fig.6 shows the pores density as a

Fig.5 α-Al2O3 content in MPO coatings
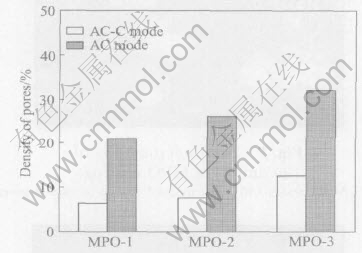
Fig.6 Pores density in MPO coatings
function of coating thickness obtained in AC and AC-C mode. Prior to a discussion of the experimental results reported in this study, it is important to clarify the phenomenological mechanism for pores formation in MPO coating due to its relevance to the present work. Briefly, several steps lead to the formation of pores in the MPO coating. Due to the early anodisation process, a thin insulating oxide coating with many pores is quickly formed on the aluminum alloy surface at the initial deposition stage. As the voltage reaches a critical value(breakthrough voltage), pores in the oxide film are broken and a number of discrete discharge channels (or spark discharges) are formed in the oxide film as a result of loss of its dielectric stability in a region of low conductivity[8, 16]. Subsequently, plasma is generated and the material in the channel is heated-up by generated electron avalanches up to temperatures of 2000℃[17]. Concurrently, owing to the high temperature, aluminum and alloying elements are melted out of the substrate, enter the channel and get oxidized. Then, these oxidized Al are ejected from the channel into the coating surface in contact with the electrolyte, and solidified rapidly leaving the sharp and distinctly visible boundaries and pancake-like structure or a structure contains large lamellar circular pieces around the majority of pores like volcano top[8, 17]. In our experiments, the adjacent area of the pores in Fig.3(b) and Fig.7 much resemble the trace of melting and give the evidence for the structure mentioned above. The above processes repeat themselves at a number of discrete locations over the whole of the coating surface, leading to an overall increase in the coating thickness[12]. Owing to the cooling rate, the rapid solidification of alumina promotes the formation of meta-stable γ-Al2O3 at the pore wall due to its immediate contact with electrolyte. The low thermal conductivity of alumina causes the underlying layers of coatings to become heated and thus further transformation of the initially formed γ-Al2O3 to the much harder α-Al2O3[6, 8].
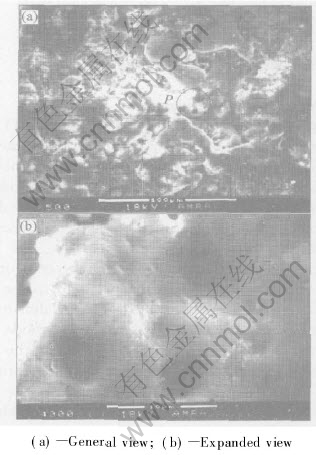
Fig.7 SEM micrographs of pore morphology in outer layer of 120μm-thick MPO coating formed by AC mode
An explanation for the features obtained experimentally can now be proposed.
As the coating thickness increased, the percentage of α-Al2O3 increased(Fig.5) and the pores density increased also[2](Fig.6). This can be acceptable in consideration of the fact that an increase in coating roughness caused by the pores with increasing of coating thickness was found in the earlier works[7, 8, 16, 17]. However, we believe that the coating roughness mostly can be attributed to the non-uniformity observed for pores in MPO coating in the works mentioned above[7, 17]. Briefly, during the progress of MPO coating deposition, the bigger spark spots gradually disappear, and make the ceramic coating continue growing towards and impacting the Al substrate. Afterwards, a α-Al2O3 compact layer containing γ-Al2O3 in the pore wall is formed and the pore diameter is decreased. Therefore, the α-Al2O3 content of coating grows towards the interface between the coating and Al substrate with increasing oxidation time. In other meaning, although the total coating thickness increases with increasing treatment time, the thickness increase is mainly provided by dense inner layer in the initial stage of the process and by the porous outer layer in the later stages. Thus, the coating composed of the α-Al2O3 compact layer and the γ-Al2O3 loose layer with pores is obtained, while the pore diameter decreases with extended deposition. This account of a thick ceramic coating from the inner to the surface. Thus, it was noticeable that in the thinner coating, the α-Al2O3 fraction of the as deposited and 40% polished coatings were only slightly different(Fig.5). The increase in α-Al2O3 with increasing the coating thickness became more significant for the polished coatings.
It is interesting to note that the contents of α-Al2O3 in the as deposited and 40% polished coatings fabricated by AC-C mode are higher than that by AC mode(Fig.5). Also, the porosity density in the coatings fabricated by AC-C mode is lower than that by AC mode(Fig.6). This is expectable in consideration of the observed differences in the structure of outer layers(Fig.4).
A possible explanation of the noted modification is that cathodic pulses in the AC-C mode serve to interrupt the formation of localized discharges arcs thereby permit the portions of the surface region near the discharges to cool. In other words, cathodic pulses interrupt pores formation and induce the re-conversion of soluble compounds into metal oxide, leading to pores sealing. Accordingly, the method has produced thicker coatings of greater uniformity and higher densities than that produced by AC mode, and by the conventional process that includes gradually increasing the voltage until a spark discharge occurs[10]. Generally, the anodic current serves to convert the insoluble metal oxide layer to water-soluble compounds such as hydroxides. The dissolution of such compounds into the electrolyte fluid depletes the oxide layer and slows the spread of the coating over the surface region[12].
3.2 Coating thickness and hardness
Measurements of the cross-sectional micro-hardness of coating indicated that the highest coating hardness at 0.5N load was HV2150 and HV1800 for coatings by AC-C and AC mode respectively(Fig.8). This difference may be due to the resulted improvement both in phase composition, and porosity characteristics exhibited by AC-C mode. However, the hardness differences can be also strongly attributed to the non-uniform distribution of pores that produced by AC mode. The above inference can be substantiated taking into account the results of other works. Furthermore, Yang et al[7] studied the effects of current density on the phase composition and microstructure of MPO coating, they found that the coatings deposited at high current density consisted of high α-Al2O3 content but the observed lowest hardness of these coatings was attributed to the non-uniform distribution of pores. Indeed, Nykyforchyn et al[14] used an asymmetrical anodic-cathodic current mode to deposit an oxide ceramic on different types of Al alloys in a 0.1% KOH solution, they observed the possibility of increasing porosity and gained coatings with higher density and microhardness than that produced by direct current method. They did not report the porosity characterization. However, in our view this can be expectable in consideration of the fact that in such case the pores had slight effect on the mechanical properties, probably, due to their uniform distribution and coating of low thickness. In comparison to aluminum alloy substrates with hardness around HV135, the hardness of coatings prepared by AC-C and AC mode reached rapidly to about HV 2150 and HV 1800 respectively at locations around 10μm for the interface of coating/substrate. However, the hardness values measured at 10μm distance from the interface proved that these values decreased as the coating thickness increased. This observation is in good agreement with an earlier study[2], while it shows negative results in comparing with the results from other study[8]. As the coating thickness increased, the maximum hardness within the coating reduced and the position of highest hardness moved away from the interface. Approximately, the position of the highest hardness of coatings prepared by AC mode was moved away more than that by AC-C mode for the 180μm thick coating. Generally, a decrease in hardness from the maximum, towards the coating surface, was observed; this may be due to both phase composition changes and a corresponding increase in coating porosity[2]. In addition, the reduction percentage in hardness for coatings prepared by AC-C mode was less than that by AC mode. As the coating thickness increased, the differences in hardness of coatings obtained by AC and AC-C mode increased; this may be due to the same reasons mentioned above. The relatively low non-uniform distribution of pores in the coatings fabricated by AC-C mode as compared with those by AC mode can be also another reason for the differences mentioned above.
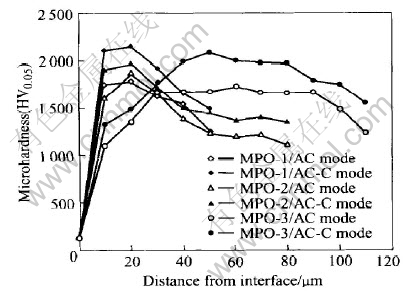
Fig.8 Distributions of hardness in MPO coatings
However, an accurate measurement of microhardness of coatings prepared by AC mode are relatively difficult due to non-uniformity of its porosity.
4 CONCLUSIONS
Based on the experiments mentioned above, it is clear that the controlled capacitor-based power source has been successfully used in this work to arrange two types of voltage modes, anodic-cathodic(AC) and AC-C for deposition of ceramic coatings on aluminum alloy. By means of adding a cathodic component of current alternated with anodic-cathodic current(AC-C), we can extensively change the uniformity of MPO coatings, microhardness profiles and porosity characteristics. Coatings fabricated by the combined mode are more dense and less porous than those by the anodic-cathodic mode. There is a non-uniform distribution of hardness for all the samples, which can be attributed to the non-uniform distribution of pores in the coatings. However, results shows that in comparison to the AC mode, the combined mode enables the coatings with higher hardness, less reduction percentage in hardness with increasing the coatings thickness and less non-uniform distribution of pores. These differences become more obvious for the polished and thicker coatings. The improvements obtained by AC-C mode can be attributed to the cathodic pulses in the AC-C mode, which serve to interrupt the formation of localized discharges arcs, and pores sealing by the re-conversion of soluble compounds into metal oxide.
REFERENCES
[1] Samir H A, QIAN Han-cheng, XIA Bo-cai, et al.Comments on processes of duplex coatings on aluminum alloys [J]. Journal of Central South University of Technology, 2004,11(3): 93-99.
[2]Nie X, Leyland A, Yerokhin L, et al. Thickness effects on the mechanical properties of micro-arc discharge oxide coatings on aluminum alloys [J]. Surf Coat Technol, 1999, 116-119 : 1055-1060.
[3]Dearnely A, Gummersbach J, Weiss H, et al. The sliding wear resistance and frictional characteristics of surface modified aluminum alloys under extreme pressure [J]. Wear, 1999, 225-229(1): 127-134.
[4]Dittrich K H, Krysmann W, Kurze P, et al. Structure and properties of ANOF layers [J]. Cryst Res &Tech, 1984,19(1) : 93-99.
[5]Voevodin A A, Yerokhin A L, Lyubimov V V, et al. Characterization of wear protective Al-Si-O coatings formed on Al-based alloys by micro-arc discharge treatment [J]. Surf Coat Technol, 1996, 86-87: 516-521.
[6]Yerokhin A, Nie X, Leyland A, et al. Plasma electrolysis for surface engineering [J]. Surf Coat Technol, 1999, 122: 73-93.
[7]YANG Guang-liang, LU Xian-yi, BAI Yi-zhen, et al. Effect of current density on the phase composition and microstructure properties of microarc oxidation coating [J]. Alloys & Compounds, 2002, 345(1-2): 196-200.
[8]Sundararajan G, Krishna L R. Mechanism underlying the formation of thick alumina coatings through the MAO coating technology [J]. Surf Coat Tehnol, 2003, 167: 269-277.
[9]Nie X, Wilson A, Leyland A, et al. Deposition of duplex Al2O3/DLC coatings on Al alloys for tribological applications using a combined micro-arc oxidation and plasma-immersion ion implantation technique [J]. Surf Coat Technol, 2000, 131: 506-513.
[10]Hradcovsky R J, Bayles S H. Coated Valve Metal Article Formed by Spark Anodizing [P]. US Patent 3956080.
[11]Samsonov V, Hiterer M. Process for Coating Metals [P]. US Patent 5616229.
[12]Yerokhin A, Voevodin A A. Method for Forming Coatings by Electrolyte Discharge and Coatings Formed Thereby [P]. US Patent 5720866.
[13]Markov G A, Slonova A I, Terleeva O P. Chemical composition, structure, and morphology of microplasma coatings [J]. Protection of Metals, 1997,33(3): 257-262.
[14]Nykyforchyn M, Klapkiv M D, Posuvailo V M. Properties of synthesized oxide-ceramic coatings in electrolyte plasma on aluminum alloys [J]. Surf Coat Technol, 1998,100-101: 219-221.
[15]XUE Wen-bin, DENG Zhi-wei, CHEN Ru-yi. Analysis of phase distribution for ceramic coatings formed by mi-croarc oxidation on aluminum alloy [J]. Am Ceram Soc, 1998, 81(5): 1365-1368.
[16]Boyle D, Collins D R, Popoola O O, et al. Surfacing of Aluminum Bodies by Anodic Spark Deposition [P]. US Patent 6245436.
[17]TIAN Jun, LUO Zhuang-zi, QI Xiao-jun, et al. Structure and antiwear behavior of micro-arc oxidized coatings on aluminum alloy [J]. Surf Coat Technol, 2002, 154: 1-7.
(Edited by YUAN Sai-qian)
Received date: 2004-06-06; Accepted date: 2004-09-20
Correspondence: Samir Hamid Awad, PhD candidate; Tel: +86-23-65102418; E-mail: samirhamidaw@yahoo.com