Effect of gene dosage and incubation temperature on production ofβ-mannanase by recombinant Pichia pastoris
来源期刊:中南大学学报(英文版)2019年第1期
论文作者:周洪波 唐诗哲 林福来 ZHENG Jia(郑甲)
文章页码:184 - 195
Key words:β-mannanase; gene dosage; qPCR; Pichia pastoris
Abstract: High-level expression of β-mannanase has been reported in Pichia pastoris under control of the GAP promoter. Two factors that strongly influence protein production and fermentation process development in Pichia pastoris protein expression system are gene dosage and cultivation temperature. The aim of this research was to improve the expression level of β-mannanase in Pichia pastoris by proper increasing the gene dosage and decreasing the culture temperature. To this end, a panel of strains harboring different copy numbers of β-mannanase gene were obtained by multiple zeocin concentration gradients screening, the influence of gene copy number on the expression of β-mannanase in Pichia pastoris X33 was investigated. With the constitutive GAP promoter, the four copies strain exhibited a 4.04-fold higher β-mannanase yield and a 1.83-fold higher total secretion proteins than the one copy strain, but an increase of the copy number above four resulted in a decrease of expression. Furthermore, the effects of culture temperature were studied in flask. The decreased culture temperature of four copies strain resulted in a 1.8-fold (26 °C) and 3.5-fold (22 °C) higher β-mannanase activity compared to that at 30 °C. A fed-batch strategy was successfully used for high cell-density fermentation and β-mannanase activity reached 2124 U/mL after cultivation for 72 h in a 5 L fermenter.
Cite this article as: TANG Shi-zhe, LIN Fu-lai, ZHENG Jia, ZHOU Hong-bo. Effect of gene dosage and incubation temperature on the production of β-mannanase by recombinant Pichia pastoris [J]. Journal of Central South University, 2019, 26(1): 184–195. DOI: https://doi.org/10.1007/s11771-019-3992-z.

J. Cent. South Univ. (2019) 26: 184-195
DOI: https://doi.org/10.1007/s11771-019-3992-z
TANG Shi-zhe(唐诗哲)1, LIN Fu-lai(林福来)1, ZHENG Jia(郑甲)1, ZHOU Hong-bo(周洪波)1, 2
1. School of Minerals Processing and Bioengineering, Central South University, Changsha 410083, China;
2. Key Laboratory of Biometallurgy of Ministry of Education, Changsha 410083, China
 Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2019
Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2019
Abstract: High-level expression of β-mannanase has been reported in Pichia pastoris under control of the GAP promoter. Two factors that strongly influence protein production and fermentation process development in Pichia pastoris protein expression system are gene dosage and cultivation temperature. The aim of this research was to improve the expression level of β-mannanase in Pichia pastoris by proper increasing the gene dosage and decreasing the culture temperature. To this end, a panel of strains harboring different copy numbers of β-mannanase gene were obtained by multiple zeocin concentration gradients screening, the influence of gene copy number on the expression of β-mannanase in Pichia pastoris X33 was investigated. With the constitutive GAP promoter, the four copies strain exhibited a 4.04-fold higher β-mannanase yield and a 1.83-fold higher total secretion proteins than the one copy strain, but an increase of the copy number above four resulted in a decrease of expression. Furthermore, the effects of culture temperature were studied in flask. The decreased culture temperature of four copies strain resulted in a 1.8-fold (26 °C) and 3.5-fold (22 °C) higher β-mannanase activity compared to that at 30 °C. A fed-batch strategy was successfully used for high cell-density fermentation and β-mannanase activity reached 2124 U/mL after cultivation for 72 h in a 5 L fermenter.
Key words: β-mannanase; gene dosage; qPCR; Pichia pastoris
Cite this article as: TANG Shi-zhe, LIN Fu-lai, ZHENG Jia, ZHOU Hong-bo. Effect of gene dosage and incubation temperature on the production of β-mannanase by recombinant Pichia pastoris [J]. Journal of Central South University, 2019, 26(1): 184–195. DOI: https://doi.org/10.1007/s11771-019-3992-z.
1 Introduction
Mannans are one of the most abundant hemicelluloses polysaccharides present in nature, β-Mannanase (endo-1, 4-d-mannanase, EC 3.2.1.78) catalyzes the cleavage of β-1, 4-linked internal linkages of mannan and hetero-mannans. There are many potential applications of mannanases in pulp bleaching, coffee extract viscosity reducing, feed nutritional value improving, and oil drilling [1]. Various genes encoding β-mannanases have been cloned and sequenced, as well as expressed heterologous in Escherichia coli [2, 3], Bacillus subtilis [4, 5], and Saccharomyces cerevisiae [6]. In recent years, methylotrophic yeast Pichia pastoris is widely used as an efficient heterologous expression system to produce large scale of recombinant proteins. Genes of β-mannanase from different sources have been successfully cloned and expressed in Pichia pastoris system, such as from Rhizomucor miehei [7], Bacillus subtilis [8],Neosartorya fischeri [9], Aspergillus niger [10], and hindgut of termites [11].
To improve the efficiency and economy of enzymolysis with β-mannanases, means of genetic engineering was used to improve the catalytic properties and expression levels of enzyme. The copy number of the heterologous gene has been proven to be an important factor in high protein expression [12–15]. Such as heterologous expression levels of all four aquaporin isoforms strongly respond to an increase in recombinant gene dosage, independent of the amount of protein expressed from a single gene copy [16]. Nevertheless, increased copy number of foreign gene sometimes may cause negative influences on Pichia pastoris transformants, especially in the case of secretory expression, including reduced methanol consumption capacity and specific growth rate [17], decreased cell viability [18], increased instability of integrated foreign gene [19], or diminished cell secretory ability [20], which could result in decreased yield of heterologous protein. INAN and coworkers’ result indicated that increasing gene copy number enhanced transcript and expression of interest protein, but did not increase the secretion level of interest protein [21]. The effect of gene copy number on recombinant protein expression levels is unpredictable, increasing the copy number of the expression cassettes could have both positive and negative effects for expression of different proteins [22]. Increasing copy number might reasonably be expected to exert a knock-on effect on transcription and translation.
Protein expression is associated with transcript level in the cell, which is paralleled to the gene copy number in Pichia pastoris genome [23, 24]. VASSILEVA et al [24] demonstrated that there is no competition for any limiting transcription factors with the increasing of copy number (from 1 to 4). Nevertheless, with the increase of mRNA levels, the burden of protein translation pathway also increase, which results in the formation of unfold or misfold protein, secretion bottleneck and protein degradation [17, 18, 25–28], eventually leads to the decrease of protein expression levels.
Culture temperature is one of the most important factors affecting the recombinant protein production [29–31]. Some studies indicate that lowering the culture temperature can help to reduce cell death and target proteins degradation [32, 33]. JAHIC et al [34] lowered the fermentation temperature to 12.8 °C, and besides improvement of the protein expression, the protein proteolysis was reduced in Pichia pastoris cultivation [34]. In addition, culture temperature also can affect the correct protein folding pathway and maturation efficiency [35].
This investigation is aimed at the effects of gene dosage on the mRNA and protein expression levels of heterologous β-mannanase in Pichia pastoris but also the effects of fermentation temperature on the β-mannanase expression in Pichia pastoris. We also detect the mRNA expression levels for four strains which contain different gene copy number in the different culture phases and at the different fermentation temperatures.
2 Materials and methods
2.1 Strains and plasmids
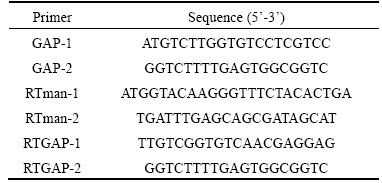
The Pichia pastoris X-33 strain was the host cell for expressing recombinant protein. The β-mannanase gene from Aspergillus niger CBS 513.88(accession No. XM001397260.1) was cloned into pGAPZαA vector, which was named pGAPZαA-man in previous work [36].
Escherichia coli strain DH5α was used for the heat-shock transformation of plasmids. Target gene standard plasmid T-man containing β-mannanase gene was constructed in previous work [46], vector PMD19-T from Takara was used for the standard plasmid construct.
2.2 Enzymes and reagents
Restriction endonuclease and T4 DNA ligase were from Takara. Premix Taq (ID: TSE003) and SYBR Green Real-time PCR Master Mix (ID: TSE202) were from Tsingke Co, Ltd., China. E.Z.N.A.TM Yeast DNA Isolation Kit, E.Z.N.A.TM Gel Extraction Kit, E.Z.N.A.TM Plasmid Miniprep Kit and E.Z.N.A.TM Yeast RNA Kit and Reverse One step RT-PCR kit were purchased from OMEGA. Real time PCR was performed in iCycler iQ Real-time PCR Detection System.
2.3 Construction of standard plasmids
The E.Z.N.A.TM Yeast DNA Isolation Kit was used to extract genomic DNA of Pichia pastoris X-33. The GAPDH (glyceraldehyde-3-phosphate dehydrogenase) gene was amplified by PCR using specific primers GAP-1 and GAP-2 as shown in Table 2. The PCR products were purified and ligated into the PMD19-T vector for construction of standard plasmid.
Table 1 Strains and plasmids
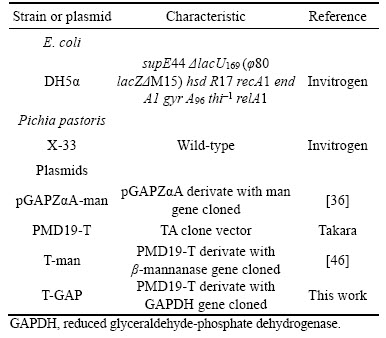
Table 2 Oligonucleotides used in this study
2.4 Genomic DNA extraction from Pichia pastoris
The genomic DNA was extracted with the E.Z.N.A.TM Yeast DNA Isolation Kit. RNA was removed from genomic DNA extraction by incubation with RNase A (Fermentas) at 20 °C for 12 h. The genomic DNA was quantified by absorbance measurements at 260 nm with NanoDrop ND-1000. Gel Electrophoresis method was applied to the analysis of the DNA samples.
2.5 Establishing double standard curve
Plasmid T-GAP and T-Man were respectively extracted with E.Z.N.A.TM Plasmid Miniprep Kit, and then the concentration of each plasmid were determined with NanoDrop ND-1000. Plasmid concentrations were converted into copy/μL and used as templates 104, 105, 106, 107, 108 copy/μL following dilutions and then detected by qPCR with primers RTman-1, RTman-2, RTGAP-1 and RTGAP-2 to establish double standard curves. Reaction conditions were carried out in 20 he ina the case without a wind μL volume and were comprised of SYBR Green Real- time PCR Master Mix (2×) (10 μL), F1 (10 μM, 1 μL), R1 (10 μM, 1 μL), template plasmid DNA (1 μL) and dH2O (7 μL). Reaction conditions consisted of a pre-denaturation step at 95 °C for 5 min followed by 40 cycles of 94 °C for 30 s,60 °C for 30 s and 72 °C for 45 s. Each DNA sample was determined in 3 replications and averaged the results.
2.6 Screening for different gene dosage transformants
The linearized plasmid pGAPZαB-man was transformed to Pichia pastoris X-33 by electroporation, the preparation of competent cells and electroporation were performed as the protocol from Invitrogen (invitrogen.com). The integration of target gene was verified by colony PCR using the universal primers pGAP-F and 3’AOX1 according to the instructions of the EasySelectTM Pichia Expression Kit (Invitrogen). YPD (1% w/v yeast extract, 2% w/v peptone, 2% w/v dextrose) agar plates containing 100, 200, 500, 1000, 2000 μg/mL zeocin, were used to select transformants harboring different copy number of target gene.
2.7 β-mannanase gene copy number detection
Real-time qPCR was performed with the iCycler iQ Real-time PCR Detection System. Taking 1 μL genomic DNA of the 12 transformants as templates, qPCR was performed with primer RTman-1, RTman-2, RTGAP-1 and TGAP-2. Thermal cycling conditions included a pre- denaturation step at 95 °C for 5 min followed by 40 cycles of 94 °C for 30 s, 60 °C for 30 s and 72 °C for 45 s. Each DNA sample was determined in 3 replications and averaged the results. Melting curve analyses were performed at the end of each qPCR assay to ensure the specificity. Ct values were substituted into the standard curve, and then calculated GAP and man gene starting template copy number in each DNA samples. Pichia pastoris genome contains only one copy of GAP gene [37], finally, the man/GAP copy number ratio was calibrated as the man gene copy number integrated into the genome of Pichia pastoris transformant.
2.8 Shake-flask culture and comparison of β-mannanase yield at different temperatures
Strains were grown in 50 mL YPD broth in 250 mL flask (200 r/min) for the expression of β-mannanase. In this study, two experimental groups were conducted. In the first experimental group, five transformants harboring 1, 3, 4, 5 and 7 gene copy numbers were cultured at 30 °C. The samples of 30 h and 78 h were collected by centrifugation and immediately extracted their total RNA with E.Z.N.A.TM Yeast RNA Kit and then reversely transcribed into cDNA by using Reverse Transcription Kit. Finally, the mRNA expression levels for different transformants were detected by real-time quantitative PCR. In the second experimental group, three different fermentation strategies denoted as strategies A, B and C were used for β-mannanase expression, first 4 strains with 1, 3, 4 and 7 gene copy numbers were cultured at 30 °C for 24 h, then in strategy A, the temperature was maintained at 30 °C throughout the whole cultivation, in strategies B and C, the temperature was decreased from 30 °C to 26 °C and 22 °C, respectively.
2.9 High cell-density fermentation
The recombinant P. pastoris strain was cultured in 5 L fermenter having 2 L initial working volume (BTF-A5L, BIOTOP Process & Equipment Inc.) using a fed-batch method. The basal media for fermentation contained: 10 g/L yeast extract, 20 g/L tryptone, 20 g/L glucose, 5.7 g/L (NH4)2HPO3,4 g/L MgSO42H2O, 4 g/L K2SO4,0.2 g/L CaSO4, 4.35 mL/L PTM1 trace salts solution, 1 g/L edible defoamer. For fed-batch cultivation, the feed media contained 500 g/L of glucose and 12 mL/L of PTM1. The recombinant strain clone was inoculated into 10 mL of YPD medium and cultured at 30 °C for 24 h. The culture was further inoculated to 200 mL YPD medium and cultured at 30 °C till the OD600 reached 10–12 and then it was added into the fermenter. During fermentation, the temperature was maintained at 30 °C in the first 24 h, then the temperature was reduced to 26 °C for the accumulation of recombinant β-mannanase; the dissolved oxygen (DO) was controlled above 20% by adjusting the stirring speed and aeration; the feed media was fed to ensure that the concentration of glucose was 0.2%–0.5%; the 25% ammonia was added to ensure that the pH value within the desired range (5.0–7.0). The culture was timely collected and centrifuged at 10000 g for 5 min for the determination the change of β-mannanase activity, cell wet weight and glucose concentration.
2.10 β-mannanase assay and other analyses
β-mannanase activity was determined as described by WANG et al [38]. One unit of β-mannanase activity was defined as the amount of liquid enzyme or solid enzyme catalyze the hydrolysis of locust bean gum (G0753, Sigma) to yield 1 μmol of mannose per minute. The cell concentration was calculated by optical density at 600 nm (OD600) with a UV/Vis spectrophotometer (Epoch, BioTek, USA). The concentration of total protein in culture supernatant was determined by the method of Bradford. The measurement of cell viability was performed by methylene blue dye method [26]. The protease in culture supernatant was determined according to the manufacturer’s protocol (DPPIV-GloTM Protease Assay Kit, Promega).
3 Results and discussion
3.1 Analysis of copy number by qPCR
To reveal the relationship between the number of inserted gene copies and the recombinant protein expression level, a method based on qPCR was developed. The method takes advantage of the fact that Pichia pastoris genome contains only one copy of GAPDH gene [37]. Two standard plasmids for building qPCR standard curve were constructed. The positive transformants were identified through simple colony PCR, gel electrophoresis and DNA sequencing. The correct plasmid was named as T-GAP. Standard curves were established by using serially diluted T-GAP and T-man standard plasmid. Both of them kept quite linear relation respectively with R2=0.998 and 0.999. The melting curve of the GAP and GFP gene had only a single peak, indicating that the PCR products were not non-specific products.
The amplified DNA fragment of colony PCR (Figure 1(a)) was dyed with Super GelRedTM (US Everbright INC.) marked by DNA Marker III (Tiangen Biotech.). The β-mannanase gene copy number of screened strains increased with elevated concentration of zeocin (Figure 1(b)) except one sample 2000-1, this result indicates that zeocin selection method can initially screen the strains with different gene copy numbers. The result also showed that low gene copy 2000-1 clone was recovered at high zeocin concentration. This may be due to false positive, and the strain has tolerance for high antibiotic concentration but express little or no recombinant protein [39].

Figure 1 Identification of expression vectors by colony PCR (a) and relation between the recombinant gene dosage and the zeocin resistance (b) (The x-axis represents the zeocin selection concentration. At each concentration test, two samples were named as ‘-1’ and ‘-2’. The β-mannanase gene copy number was determined by qPCR)
3.2 Growth profiles of Pichia pastoris strains have different copies of β-mannanase gene
Recombinant Pichia pastoris strains M1, M3, M4, M5 and M7 harboring 1, 3, 4, 5, 7 copies of β-mannanase gene, respectively, were compared for their growth characteristics (Figure 2). In the initial 12 h, no significant change in growth profiles could be observed for all strains (M1, M3, M4, M5, and M7). However, when cell grew into the log phase, specific growth rates increased with the decrease of man gene copy number. From Figure 2, we also found that the final cell concentration increased with the increase of gene copy number, final cell concentration of strain M1 was the highest which achieved 9×108 cells/mL, but strain M7 just had 6×108 cells/mL. The results indicate that exogenous gene with high copy number has a significant negative effect on cell growth. Increased copy number of foreign gene can result in the alteration of normal metabolism in Pichia pastoris [40]. This may be because of the lower ability using glucose in the high gene copy number strains, which leads to inadequate supply of energy and carbon source. MALHOTRA et al [41] reported that high gene copy number strains decelerate their cell growth rates due to the strong oxidative stress.
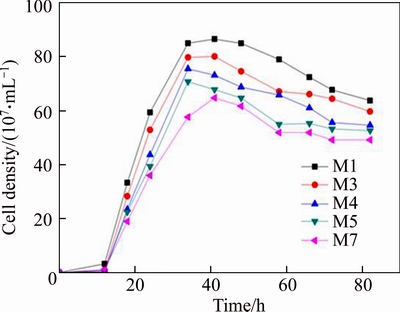
Figure 2 Growth curve of different gene copy number strains (To facilitate comparative analysis, all strains had same initial inoculum)
In order to further understand the physiological changes, the glucose consumption rates in different gene copy number strains were detected (Figure 3). The rate of glucose consumption rates in low gene copy groups were faster than which in high gene copy groups, the results confirmed our previous forecast that the high gene copy strains result in the lower ability using glucose.
3.3 Effect of gene dosage on β-mannanase protein expression levels
The production of β-mannanase in shake-flask cultures with YPD medium for Pichia pastoris strains with 1, 3, 4, 5 and 7 copies were detected (Figure 4). The β-mannanase activity remarkably increased with the increasing gene dosage from one copy strain to four copy strain, the highest β-mannanase activity of the testing broth increased from 50 U/mL in one copy strain M1 to 202 U/mL in four copy strain M4. Heterologous protein expression increased with increasing gene dosage have been demonstrated in many previous reports. TENG et al [13] found that multiple gene copy number enhances plectasin secretion in the yeast Pichia pastoris, and NORD N et al [16] also reported that increasing gene dosage greatly enhances recombinant expression of aquaporins in Pichia pastoris. There maybe two reasons for the increase of protein expression. First, the transcription of the exogenous protein was enhanced with the increase of gene copy number to increase the synthesis of exogenous protein. VASSILEVA et al [24] proved that HbsAg mRNA levels increased with the increase of gene copy number. Second, the increase of gene copy number could result in some changes in the metabolic pathway. When the inserted gene copy number was more than four, no significant increase was detected in the β-mannanase activity, the β-mannanase activity levels kept around 200 U/mL for strain M5 and M7. The increase of gene dosage not always enhance the β-mannanase activity when the gene copy number has already increased to some level. The reason for this was likely to be the bottleneck of the foreign protein production might shift from gene dosage to other limiting factors such as protein modification, secretion or protein enzymatic degradation [40, 41]. Furthermore, we also found that the β-mannanase activity levels decreased for all strains during the late phase of fermentation. This could be due to the target protein degradation caused by the accumulation of extracellular protease in dead phase [42].
N et al [16] also reported that increasing gene dosage greatly enhances recombinant expression of aquaporins in Pichia pastoris. There maybe two reasons for the increase of protein expression. First, the transcription of the exogenous protein was enhanced with the increase of gene copy number to increase the synthesis of exogenous protein. VASSILEVA et al [24] proved that HbsAg mRNA levels increased with the increase of gene copy number. Second, the increase of gene copy number could result in some changes in the metabolic pathway. When the inserted gene copy number was more than four, no significant increase was detected in the β-mannanase activity, the β-mannanase activity levels kept around 200 U/mL for strain M5 and M7. The increase of gene dosage not always enhance the β-mannanase activity when the gene copy number has already increased to some level. The reason for this was likely to be the bottleneck of the foreign protein production might shift from gene dosage to other limiting factors such as protein modification, secretion or protein enzymatic degradation [40, 41]. Furthermore, we also found that the β-mannanase activity levels decreased for all strains during the late phase of fermentation. This could be due to the target protein degradation caused by the accumulation of extracellular protease in dead phase [42].

Figure 3 Glucose consumption rates in different copy number strains

Figure 4 Time-course activities of β-mannanase in Pichia pastoris strains with different gene copy numbers in shaking flask culture (Strain M1, M3, M4, M5, M7 respectively harbor 1, 3, 4, 5 and 7 copies β-mannanase gene)
The total protein concentration curves of different strains showed similarity with the trend of enzyme activities (Figures 5 and 6). The total protein content initially increased and then reached a bottleneck with the increase of gene copy number. β-mannanase activities were decreased for all strains during the late phase of fermentation (Figure 4), but total protein contents still increased slowly (Figure 5). This may be due to the cell death during the late phase of fermentation.
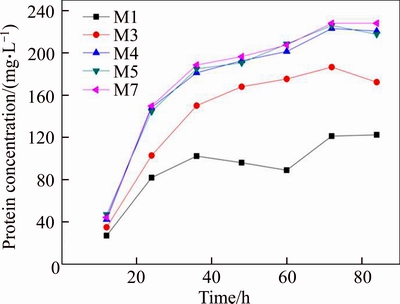
Figure 5 Effect of gene dosage on concentration of total protein in culture supernatant (Protein concentration in culture supernatant was analyzed with method of Bradford)
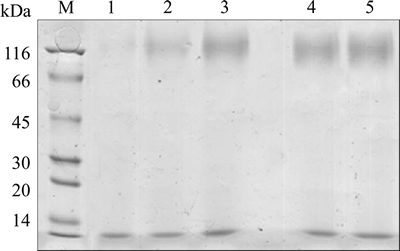
Figure 6 SDS-PAGE electrophoresis of 10 μL culture supernatant samples at 60 h (1 to 5: supernatant samples respectively taken from strains with 1, 3, 4, 5, 7 gene copy numbers. M: Protein Marker II)
3.4 Effect of gene dosage on β-mannanase mRNA expression levels
Increasing exogenous gene copy number would enhance the level of transcription of the exogenous protein [15]. To verify that the levels of β-mannanase-specific mRNA promoted with the increase of β-mannanase gene copy number, the cell samples of culture time 30 h and 72 h were chosen for mRNA detection with real-time qPCR method. The data (Figure 7) clearly showed that β-mannanase mRNA levels closely parallel gene dosage. Both β-mannanase mRNA levels at 30 h (log phase) and 72 h (decline phase) raised with the increase of gene dosage. While the inserted gene copy number was more than four, β-mannanase mRNA levels still increased with the increase of gene dosage, while the expression of β-mannanase was not increased. Also, in this case, the bottleneck of the foreign protein production might shift from gene dosage to other limiting factors such as protein secretion or protein degradation [40, 41]. The data (Figure 7) also showed that the mRNA expression levels in decline phase were similar to that of log phase, while β-mannanase activities were not accumulated in decline phase, this was probably due to the protein degradation or the limiting factors of translation and posttranslational modification.
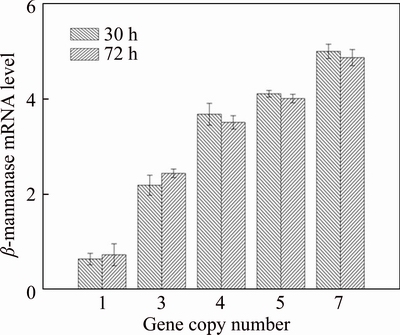
Figure 7 Transcription levels of β-mannanase in strains with different gene copy numbers at 30 h and 72 h
3.5 Effect of temperature on β-mannanase expression by Pichia pastoris strains with different gene dosages
Lowering the culture temperature is another commonly used strategy for high level expression of heterologous protein. Heterologous protein secretion could be enhanced by reducing the protein synthesis rate so as to fit its secretion rate [45]. We set the fermentation temperature from 30 °C to 26 °C and 22 °C to study the strategy could improve the β-mannanase production. To study the effect of fermentation temperature on producing β-mannanase by recombinant Pichia pastoris fermentation, strains harboring different copy number of β-mannanase gene were cultured in lower temperature condition. Results (Figure 8) showed that either low copy or high copy gene number strains increased β-mannanase activities at lower culture temperatures. The β-mannanase activity increased steadily until the end of the fermentation, which revealed that the secretion rate fit the mannanase synthesis rate and the reduction of protein degradation under lower temperatures. As a result, at 22 °C, maximum β-mannanase activities of strain M1, M3, M4 and M7 increased by 40%, 54%, 68% and 83%, respectively compared to culture at 30 °C.The results also showed that lower temperature was more effective to promote β-mannanase expression in high gene copy strains than in low gene copy strains. This may be due to the high gene copy strains suffer from more protein folding and secretion stress, and low culture temperature can reduce protein synthesis rate and folding burden [34].
There seem to be several mechanisms behind the effect of culture temperature on β-mannanase expression, such as protein synthesis and secretion rate, protein folding burden, protein degradation, cell viability, mRNA level and so on. To investigate the effects of temperature on protein degradation, cell viability, β-mannanase mRNA level, we took sample of strain M4 at culture time of 80 h to detect.
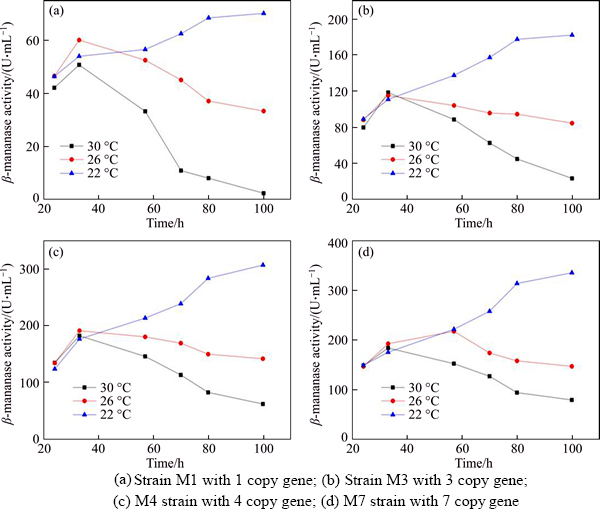
Figure 8 Fermentation profiles of 4 strains with different gene dosages in response to different culture temperature:
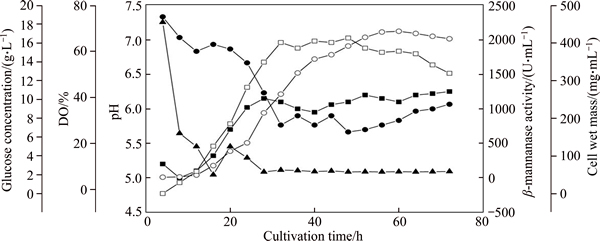
Figure 9 Time course of glucose concentration-sustained fed-batch fermentation in 5 L fermenter at 30 °C (first 24 h) and 26 °C (after 24 h) (pH was controlled around 6.0 by feeding aqueous ammonia. Glucose concentration (▲); dissolved oxygen (●); pH (■); β-mannanase activity (○); wet cell weight (□))
Table 3 showed the results. First, β-mannanase mRNA level reduced with the reduction of culture temperature, which verifies that as the culture temperature reduction could reduce the protein synthesis rate to fit its secretion rate. Second, the final cell concentrations were similar in the different incubation temperatures, which shows that the culture temperature (above 22 °C) has no effect on the final cell concentration. The last, cell viability improved and protease activity reduced as the culture temperature decreased, which led to β-mannanase accumulation. The maximum β-mannanase activity under 30 °C reached 82.2 U/mL. Setting the temperature to 26 °C and 22 °C resulted in a 1.8-fold (149.6 U/mL) and 3.5-fold (283.7 U/mL) increase in the maximum β-mannanase activity compared to that at 30 °C.
Table 3 β-mannanase activity, cell concentration, cell viability, protease activity and mRNA level in response to culture temperature

3.6 High cell-density fermentation
The recombinant Pichia pastoris strain with 4 copy β-mannanase gene was used in 5 L scale fed-batch fermentation to achieve higher level β-mannanase production, which had a highest β-mannanase activity at flask scale. The fermentation has a large amount of heat release in the fermentation course so that the heat cooling system of fermenter could not decrease the temperature to 22 °C. Therefore, the fermentation temperature after 24 h was set to 26 °C instead of 22 °C (the optimum temperature in flask-shaking tests). The parameters in the fermenting yield of recombinant β-mannanase such as glucose concentration, dissolved oxygen, cell wet weight and pH were studied. Fermentation of the recombinant Pichia pastoris strain with 4 copy β-mannanase gene was conducted using a fed-batch mode based on controlling the glucose concentration, dissolved oxygen and pH. The culture reached a cell wet weight of 405.0 mg/mL when 48 h after inoculation. After 60 h of inoculation, the recombinant β-mannanase activity reached 2124 U/mL. The expression level was significantly higher than that of many other recombinant β-mannanase expressed in Pichia pastoris, such as the β-mannanase gene from Bispora sp. (500 U/mL, in 3.7 L fermenter) [47] and A.niger (1612 U/mL, in 5 L fermenter) [48].
4 Conclusions
High β-mannanase gene copy number transformants were obtained by elevated the zeocin resistance of culture. The copy number of β-mannanase gene in genome was determined by qPCR method. From the results, increasing the concentration of zeocin to obtain high copy number transformants of Pichia pastoris for high-level β-mannanase gene expression, the expression of β-mannanase increased up to 4 copies and decreased thenceforward. Moreover, lowering the culture temperature can improve the fermentation activity of recombinant β-mannanase especially in high gene copy strains. A fed-batch strategy based on controlling the glucose concentration was developed for the high-level expression of β-mananase in 5 L fermenters. The highest recombinant β-mananase activity level in 5 L fermenters cultures was 2124 U/mL. The results in this report could be effectively used for the expression of other heterologous protein with Pichia pastoris.
References
[1] DHAWAN S, KAUR J. Microbial mannanases: An overview of production and applications [J]. Critical Reviews in Biotechnology, 2007, 27(4): 197–216.
[2] TUNTRAKOOL P, KEAWSOMPONG S. Kinetic properties analysis of beta-mannanase from Klebsiella oxytoca KUB-CW2-3 expressed in Escherichia coli [J]. Protein Expression and Purification, 2018, 146: 23–26.
[3] EOM G T, OH J Y, PARK J H, JEGAL J, SONG J K. Secretory production of enzymatically active endo-β-1, 4-mannanase from Bacillus subtilis by ABC exporter in Escherichia coli [J]. Process Biochemistry, 2016, 51(8): 999–1005.
[4] HATADA Y, TAKEDA N, HIRASAWA K, OHTA Y, USAMI R, YOSHIDA Y, GRANT W D, ITO S, HORIKOSHI K. Sequence of the gene for a high-alkaline mannanase from an alkaliphilic Bacillus sp. strain JAMB-750, its expression in Bacillus subtilis and characterization of the recombinant enzyme [J]. Extremophiles, 2005, 9(6): 497–500.
[5] SONG Ya-feng, FU Gang, DONG Hui-na, LI Jian-jun, DU Yu-guang, ZHANG Da-wei. High-efficiency secretion of β-mannanase in Bacillus subtilis through protein synthesis and secretion optimization [J]. Journal of Agricultural and Food Chemistry, 2017, 65(12): 2540–2548.
[6] PRIMA A, HARA K Y, DJOHAN A C, KASHIWAGI N, KAHAR P, ISHII J, NAKAYAMA H, OKAZAKI F, PRASETYA B, KONDO A, YOPI, OGINO C. Glutathione production from mannan-based bioresource by mannanase/ mannosidase expressing Saccharomyces cerevisiae [J]. Bioresource Technology, 2017, 245(12): 1400–1406.
[7] LI Yan-xiao, YI Ping, LIU Jun, YAN Qiao-juan, JIANG Zheng-qiang. High-level expression of an engineered β-mannanase (mRmMan5A) in Pichia pastoris for manno-oligosaccharide production using steam explosion pretreated palm kernel cake [J]. Bioresource Technology, 2018, 256: 30–37.
[8] LUO Zhang-cai, MIAO Jing, LI Guo-ying, DU Yao, YU Xiao-bin. A recombinant highly thermostable β-mannanase (ReTMan26) from thermophilic bacillus subtilis (TBS2) expressed in Pichia pastoris and its pH and temperature stability [J]. Applied Biochemistry and Biotechnology, 2017, 182(4): 1259–1275.
[9] YANG Hong, SHI Peng-jun, LU Hai-qiang, WANG Hui-min, LUO Hui-ying, HUANG Huo-qing, YANG Pei-long, YAO Bin. A thermophilic β-mannanase from Neosartorya fischeri P1 with broad pH stability and significant hydrolysis ability of various mannan polymers [J]. Food Chemistry, 2015, 173: 283–289.
[10] YU Shi, LI Zhe-zhe, WANG Ya-ping, CHEN Wan-ping, FU Lin, TANG Wei, CHEN Chen, LIU Yunyun, ZHANG Xue, MA Li-xin. High-level expression and characterization of a thermophilic β-mannanase from Aspergillus niger in Pichia pastoris [J]. Biotechnology Letters, 2015, 37(9): 1853–1859.
[11] HSU Y, KOIZUMI H, OTAGIRI M, MORIYA S, ARIOKA M. Trp residue at subsite-5 plays a critical role in the substrate binding of two protistan GH26 β-mannanases from a termite hindgut [J]. Applied microbiology and Biotechnology, 2018: 1–11.
[12] JIAO Liang-cheng, ZHOU Qing-hua, SU Zhi-xin, LI Xu, YAN Yun-jun. High-level extracellular production of Rhizopus oryzae lipase in Pichia pastoris via a strategy combining optimization of gene-copy number with co-expression of ERAD-related proteins [J]. Protein Expression and Purification, 2018, 147: 1–12.
[13] TENG Da, XI Di, ZHANG Jun, WANG Xiu-min, MAO Ruo-yu, ZHANG Yong, WANG Jian-hua. Multiple copies of the target gene enhances plectasin secretion in Pichia pastoris X-33 [J]. Process Biochemistry, 2015, 50(4): 553–560.
[14] C MARA E, LANDES N, ALBIOL J, GASSER B, MATTANOVICH D, FERRER P. Increased dosage of AOX1 promoter-regulated expression cassettes leads to transcription attenuation of the methanol metabolism in Pichia pastoris [J]. Scientific Reports, 2017, 7: 44302.
MARA E, LANDES N, ALBIOL J, GASSER B, MATTANOVICH D, FERRER P. Increased dosage of AOX1 promoter-regulated expression cassettes leads to transcription attenuation of the methanol metabolism in Pichia pastoris [J]. Scientific Reports, 2017, 7: 44302.
[15] YANG Hu, ZHAI Chao, YU Xian-hong, LI Zhe-zhe, TANG Wei, LIU Yun-yun, MA Xiao-jian, ZHONG Xing, LI Guo-long, WU Di, MA Li-xin. High-level expression of Proteinase K from Tritirachium album Limber in Pichia pastoris using multi-copy expression strains [J]. Protein Expression and Purification, 2016, 122: 38–44.
[16] NORD N K, AGEMARK M, DANIELSON J
N K, AGEMARK M, DANIELSON J  H, ALEXANDERSSON E, KJELLBOM P, JOHANSON U. Increasing gene dosage greatly enhances recombinant expression of aquaporins in Pichia pastoris [J]. BMC Biotechnology, 2011, 11(1): 47.
H, ALEXANDERSSON E, KJELLBOM P, JOHANSON U. Increasing gene dosage greatly enhances recombinant expression of aquaporins in Pichia pastoris [J]. BMC Biotechnology, 2011, 11(1): 47.
[17] COS O, SERRANO A, MONTESINOS J L, MONTESIONS J L, FERRER P, CREGG J M, VALERO F. Combined effect of the methanol utilization (Mut) phenotype and gene dosage on recombinant protein production in Pichia pastoris fed-batch cultures [J]. Journal of Biotechnology, 2005, 116(4): 321–335.
[18] HOHENBLUM H, GASSER B, MAURER M, BORTH N, MATTANOVICH D. Effects of gene dosage, promoters, and substrates on unfolded protein stress of recombinant Pichia pastoris [J]. Biotechnology and Bioengineering, 2004, 85(4): 367–375.
[19] ZHU Tai-cheng, GUO Mei-jin, SUN Chen, QIAN Jiang- chao, ZHUANG Ying-ping, CHU Ju, ZHANG Si-liang. A systematical investigation on the genetic stability of multi-copy Pichia pastoris strains [J]. Biotechnology Letters, 2009, 31(5): 679–684.
[20] INAN M, ARYASOMAYAJULA D, SINHA J, MEAGHER M M. Enhancement of protein secretion in Pichia pastoris by overexpression of protein disulfide isomerase [J]. Biotechnology and Bioengineering, 2006, 93(4): 771–778.
[21] INAN M, FANDERS S A, ZHANG Wen-hui, HOTEZ P J, ZHAN Bin, MEAGHER M M. Saturation of the secretory pathway by overexpression of a hookworm (Necator americanus) Protein (Na-ASP1) [C]// Pichia Protocols. Totowa: Humana Press, 2007: 65–75.
[22] ZHAN Rong-rong, MU Wan-meng, JIANG Bo, ZHOU Liu-ming, ZHANG Tao. Efficient secretion of inulin fructotransferase in Pichia pastoris using the formaldehyde dehydrogenase 1 promoter [J]. Journal of Industrial Microbiology & Biotechnology, 2014, 41(12): 1783–1791.
[23] SCORER C A, CLARE J J, MCCOMBIE W R, ROMANOS M A. SREEKRISHNA K. Rapid selection using G418 of high copy number transformants of Pichia pastoris for high–level foreign gene expression [J]. Nature Biotechnology, 1994, 12(2): 181–184.
[24] VASSILEVA A, CHUGH D A, SWAMINATHAN S, KHANNA N. Expression of hepatitis B surface antigen in the methylotrophic yeast Pichia pastoris using the GAP promoter [J]. Journal of Biotechnology, 2001, 88(1): 21–35.
[25] CREGG J M. Pichia protocols [M]. Totowa: Humana Press, 2007.
[26] SINHA J, PLANTZ B A, INAN M, MEAGHER M M. Causes of proteolytic degradation of secreted recombinant proteins produced in methylotrophic yeast Pichia pastoris: Case study with recombinant ovine interferon-τ [J]. Biotechnology and Bioengineering, 2005, 89(1): 102–112.
[27] DAMASCENO L M, HUANG C J, BATT C A. Protein secretion in Pichia pastoris and advances in protein production [J]. Applied Microbiology and Biotechnology, 2012, 93(1): 31–39.
[28] MATTANOVICH D, GASSER B, HOHENBLUM H, SAUER M. Stress in recombinant protein producing yeasts [J]. Journal of Biotechnology, 2004, 113(1): 121–135.
[29] PEI Xiao-lin, WANG Qiu-yan, MENG Li-jun, LI Jing, YANG Zheng-fen, YIN Xiao-pu, YANG Li-rong, CHEN Shao-yun, WU Jian-ping. Chaperones-assisted soluble expression and maturation of recombinant Co-type nitrile hydratase in Escherichia coli to avoid the need for a low induction temperature [J]. Journal of Biotechnology, 2015, 203: 9–16.
[30] OVERTON T W. Recombinant protein production in bacterial hosts [J]. Drug Discovery Today, 2014, 19(5): 590–601.
[31] CHEN Wen-bo, NIE Yao, XU Yan, XIAO Rong. Enhancement of extracellular pullulanase production from recombinant Escherichia coli by combined strategy involving auto-induction and temperature control [J]. Bioprocess and Biosystems Engineering, 2014, 37(4): 601–608.
[32] GAO Min-jie, ZHAN Xiao-bei, GAO Peng, ZHANG Xu, DONG Shi-juan, LI Zhen, SHI Zhong-ping, LIN Chi-chung. Improving performance and operational stability of porcine interferon-α production by Pichia pastoris with combinational induction strategy of low temperature and methanol/sorbitol co-feeding [J]. Applied Biochemistry and Biotechnology, 2015, 176(2): 493–504.
[33] TAO Hui, GUO Dao-yi, ZHANG Yu-chen, DENG Zi-xin, LIU Tian-gang. Metabolic engineering of microbes for branched-chain biodiesel production with low-temperature property [J]. Biotechnology for Biofuels, 2015, 8(1): 92.
[34] JAHIC M, WALLBERG F, BOLLOK M, GARCIA P, ENFORS S O. Temperature limited fed-batch technique for control of proteolysis in Pichia pastoris bioreactor cultures [J]. Microbial Cell Factories, 2003, 2(1): 6.
[35] SCHEIN C H, NOTEBORN M H M. Formation of soluble recombinant proteins in Escherichia coli is favored by lower growth temperature [J]. Nature Biotechnology, 1988, 6(3): 291–294.
[36] ZHAO Wei, ZHENG Jia, ZHOU Hong-bo. A thermotolerant and cold-active mannan endo-1, 4-β-mannosidase from Aspergillus niger CBS 513.88: Constitutive overexpression and high-density fermentation in Pichia pastoris [J]. Bioresource Technology, 2011, 102(16): 7538–7547.
[37] WATERHAM H R, DIGAN M E, KOUTZ P J, LAIR S V, GREGG J M. Isolation of the Pichia pastoris glyceraldehyde-3-phosphate dehydrogenase gene and regulation and use of its promoter [J]. Gene, 1997, 186(1): 37–44.
[38] WANG Meng-fan, YOU Sheng-ping, ZHANG Shuai-shuai, QI Wei, LIU Zhao-hui, WU Wei-na, SU Rong-xin, HE Zhi-min. Purification, characterization, and production of β-mannanase from Bacillus subtilis TJ-102 and its application in gluco-mannooligosaccharides preparation [J]. European Food Research and Technology, 2013, 237(3): 399–408.
[39] CEREGHINO J L, CREGG J M. Heterologous protein expression in the methylotrophic yeast Pichia pastoris [J]. FEMS Microbiology Reviews, 2000, 24(1): 45–66.
[40] ZHU Tai-cheng, GUO Mei-jin, ZHUANG Ying-ping, CHU Ju, ZHANG Si-liang. Understanding the effect of foreign gene dosage on the physiology of Pichia pastoris by transcriptional analysis of key genes [J]. Applied Microbiology and Biotechnology, 2011, 89(4): 1127–1135.
[41] MALHOTRA J D, KAUFMAN R J. Endoplasmic reticulum stress and oxidative stress: a vicious cycle or a double-edged sword? [J]. Antioxidants & Redox Signaling, 2007, 9(12): 2277–2294.
[42] INAN M, ARYASOMAYAJULA D, SINHA J, MEAGHER M M. Enhancement of protein secretion in Pichia pastoris by overexpression of protein disulfide isomerase [J]. Biotechnology and Bioengineering, 2006, 93(4): 771–778.
[43] DAMASCENO L M, ANDERSON K A, RITTER G, GREGG J M, OLD L J, BATT C A. Cooverexpression of chaperones for enhanced secretion of a single-chain antibody fragment in Pichia pastoris [J]. Applied Microbiology and Biotechnology, 2007, 74(2): 381–389.
[44] DALY R, HEARN M T W. Expression of heterologous proteins in Pichia pastoris: A useful experimental tool in protein engineering and production [J]. Journal of Molecular Recognition, 2005, 18(2): 119–138.
[45] GASSER B, SALOHEIMO M, RINAS U, DRAGOSITS M, ESCARLATA R C, BAUMANN K, GIULIANI M, PARRILLI E, BRANDUARDI P, LANG C, PORRO D, FERRER P, TUTINO M L, MATTANOVICH D, VILLAVERDE A. Protein folding and conformational stress in microbial cells producing recombinant proteins: A host comparative overview [J]. Microbial Cell Factories, 2008, 7(1): 11.
[46] WANG Ye, ZHENG Jia, LIN Fu-lai, ZHOU Hong-bo. Improved extracellular endo-1, 4-β-mannosidase activity of recombinant Pichia pastoris by optimizing signal peptide [J]. Journal of Central South University, 2015, 22: 2088–2095
[47] LUO Hui-ying, WANG Ya-ru, WANG Hui, YANG Jun, YANG Yu-hui, HUANG Huo-qing, YANG Pei-long, BAI Ying-guo, SHI Peng-jun, FAN Yun-liu, YAO Bin. A novel highly acidic β-mannanase from the acidophilic fungus Bispora sp. MEY-1: Gene cloning and overexpression in Pichia pastoris [J]. Applied Microbiology and Biotechnology, 2009, 82(3): 453–461.
[48] YU Shi, LI Zhe-zhe, WANG Ya-ping, CHEN Wan-ping, FU Lin, TANG Wei, CHEN Chen, LIU Yun-yun, ZHANG Xue, MA Li-xin. High-level expression and characterization of a thermophilic β-mannanase from Aspergillus niger in Pichia pastoris [J]. Biotechnology Letters, 2015, 37(9): 1853–1859.
(Edited by HE Yun-bin)
中文导读
基因剂量和培养温度对重组毕赤酵母生产β-甘露聚糖酶的影响
摘要:使用GAP启动子可以实现β-甘露聚糖酶基因在毕赤酵母中的组成型表达。为了提高β-甘露聚糖酶在毕赤酵母中的产量,本研究通过梯度抗生素浓度筛选法构建了携带多拷贝β-甘露聚糖酶的毕赤酵母菌株,并利用实时荧光定量PCR技术确定了各菌株的拷贝数。考察了不同基因拷贝数对β-甘露聚糖酶在毕赤酵母中表达的影响,同时研究了不同拷贝数下,培养温度对β-甘露聚糖酶分泌表达的影响。研究获得了β-甘露聚糖酶基因拷贝数为1、3、4、5、7的毕赤酵母重组菌株,结果显示β-甘露聚糖酶的mRNA水平随着拷贝数的增加而升高,β-甘露聚糖酶产量随着拷贝数的增加而提高,其中拷贝数为4时产酶水平最高,是单拷贝菌株的4.04倍,当拷贝数大于4时,酶的产量不再随着拷贝数的增加而增加。培养温度实验结果显示,较低的温度对重组菌株生产β-甘露聚糖酶有促进作用,当温度降低至22 °C时,携带4拷贝的重组菌株的酶活是30 °C时的3.5倍。为了探索上述实验结果在工业发酵上的应用潜力,我们进行了5 L反应器水平的发酵实验,4拷贝菌株在发酵72 h后,酶活达到2124 U/mL。
关键词:β-甘露聚糖酶;基因剂量;定量PCR;毕赤酵母
Foundation item: Project(31870115) supported by the National Natural Science Foundation of China; Project(2015JJ5006) supported by the Natural Science of Hunan Province & Changde City Joint Foundation, China; Projects(2015zzts268, ZY2015823) supported by the Fundamental Research Funds for the Central Universities, China
Received date: 2018-06-14; Accepted date: 2018-11-05
Corresponding author: ZHOU Hong-bo, PhD, Professor; Tel: +86-731-88877216; E-mail: zhouhb@csu.edu.cn; ORCID: 0000- 0001-6938-3650

