
Effects of F-Si sealer on electrochemical characteristics of 15%Al-85%Zn alloy thermal spray coating
Seong-Jong KIM, Seung-Jun LEE
Division of Marine System Engineering, Mokpo Maritime University, Mokpo City, Jeonnam 530-729, Korea
Received 27 January 2011; accepted 26 August 2011
Abstract: Most hulls of the ships are protected with paintings, sacrificial anode, and impressed current cathodic protection methods against corrosion problems. However, these conventional methods are not very effective because the rudder of ships stern are exposed to very severe corrosive environment such as tides, speeds of ships, cavitations and erosion corrosion. The environmental factors such as cavitation and corrosion will cause damage for materials with the shock wave by the creation and destruction of bubble. To solve these problems, the cavitation and electrochemical experiments are executed for thermal spray coating with Al-Zn alloy wire material. Thereafter, and sealed specimens with F-Si sealer on Al-Zn alloy coated specimen are executed to improve electrochemical and anti-cavitation characteristics in sea water. The application of fluorine silicon sealing after spray coating of 15%Al-85%Zn seems to be appropriate not only in static environment but also in dynamic environment.
Key words: cavitation; Al-Zn alloy wire material; thermal spray coating; corrosion
1 Introduction
Cavitation is the damage of material with the formation of air bubbles due to the low pressure parts created when liquid rotates at high speed, and often occurs in centrifugal pump, hydraulic turbine, and marine propellers. In particular, it has serious adverse effects on the life of machinery by causing mechanical and electrochemical damages to the metal materials exposed to corrosive environment [1]. The cavitation damages were studied by photographing and examining the air bubble generation patterns in test solutions with cavitation equipment using a piezoelectric element. It was reported that the diameters of pits generated on the specimen surface by cavitation erosion were greater than those of air bubbles [2]. The effects of the flow velocity and environmental solution on cavitation damages and restrictions were studied, and it was found that the faster the flow velocity is, the more delayed the incubation period under the cavitation erosion [3]. LIMA et al [4] studied the fracture toughness and cavitation resistance of thermal spray coating through indentation, and found that correlations between these two variables could be established, and higher fracture toughness was associated with better cavitation characteristics. A study on damages to metal surface in the incubation period of cavitation erosion revealed three types of typical pits: complete pit that is generated from micro jets by fluid current, incomplete pit generated by shock wave from micro jet, and thermal pit that is generated in hot compressed air bubbles at the moment of extinction. Recently, spray systems of thermal spray coating are used to apply protective coating to metal surface which melts spray wires with electric arc and fumes low-temperature jet air to form a coating on the metal surface. To find coating material that has better corrosion resistance and cavitation characteristics than painting, the thermal spray coating with Al-Zn alloy wires was performed. Furthermore, fluorine silicon sealer was additionally applied over the coating layer, and the electrochemical and cavitation characteristics were compared between these two conditions.
2 Experimental
In this experiment, high tensile steel with compositions of 0.1617% C, 0.013% Si, 0.659% Mn, 0.0146% P, 0.0076% S, balance Fe, was used. The tensile strength, yield strength, elongation were 463 MPa, 312 MPa and 23%, respectively. Al-Zn wire was used for thermal spray coating and fluorine silicone sealing was conducted. For coating, KMS-300 arc spray was used to coat the steel in the thickness of 400 μm or higher with Al-Zn alloy, under the conditions of spray transfer speed of 10 cm/s, gas pressure of 49-58.8 N and wire feed speed of 12 m/min. The scale of the specimen surface was removed through sand blasting before coating and the sealer was spread with the brush at room temperature for several times. To observe the surface, the cross- section of coating layer was microscopically analyzed and the sample was evaluated so as to investigate the corrosion mode of the surface. Furthermore, Vickers hardness testing machine (Model: HMV-112, Akashi, Japan) was used to measure the hardness of the coating surface under of load 980 N for 10 s. The hardness test was performed for several times, and the average value was used. A Ag/AgCl chloride electrode was used as reference electrode. A platinum electrode was used as counter electrode. The multi-channel potentio/ galvanostat WMPG-1000 was used for the corrosion test. All electrochemical tests were conducted in natural seawater. The natural potential measurement test was performed for 24 h to measure the potential behavior. Anodic and cathodic polarization trends were tested from the open circuit potential of +3.0 V to -2.0 V at the scanning rate of 2 mV/s. For potentiostatic experiment to compare corrosion resistance, the changes in current density for 1 h at a constant potential and those after 1 h were compared under various applied potential conditions. For Tafel analysis, the corrosion potential and corrosion current density were obtained by polarizing ± 0.25 V at the scanning rate of 1mV based on the open circuit potential. Cavitation test was performed using an ultrasonic vibration generator. The amplitude was set to be 10 μm by static amplitude automatic control. An electronic balance that can measure down to 10-4 g was used to measure mass before and after test.
3 Results and discussion
Figure 1 presents the morphologies of the surface and cross-section of the specimen coated with 15%Al-85%Zn alloy and sealed with F-Si sealer. A splat shape was found in the surface microstructure, which seems to be due to the fact that high-speed molten metal particles collided with the surface of base metal which simultaneously crushed and coagulated. Furthermore, voids and inclusions of oxides were observed around the center which was a laminated layer of coatings. The cause of this spray layer is the peculiarity of the splat lamination structure. Coarse large voids are formed by the lamination of incomplete splats, and the molten particles melted by the hot flames during spray collide with the base metal at high speed and spread out in sheet form or in radial direction. Then, small particles are separated from the thin, unstable tips, and subsequent molten particles collide with the coagulated particles at these splat tips, forming voids with the median size of small particles. When there are many such voids, corrosion resistance tends to decrease due to porous coating. To solve this problem, sealing, painting, heating, or complex techniques such as shot peening are used [5]. Overall, the shapes were formed similarly regardless of the existence of sealing. The surface roughness (Ra) of the coated specimen was 12.38 μm and that of the coated and sealed (sealed specimen after coating) specimen was 13.54 μm. Other studies found that sealing after spray coating tended to decrease the roughness, but the result of this study was different [6-7]. From the observation of the cross-section, voids were observed sporadically in the case of spray coating only. However, for the sealed specimen after coating, voids in the coating layer decreased due to the penetration of sealer. It is believed that this would improve the corrosion resistance. An observation of the interface between the base metal and the coating layer found that the sealed specimen after coating was well-attached. It is predicted that different electrochemical characteristics will appear depending on the interface between these voids and the coating layer of base metal. Voids observed in the spray coating of zinc or aluminum are known to account for 5%-10%. Even if the base metal was exposed to seawater environment due to defects caused by voids or oxide layer, the base metal electrochemically exhibits high potential and acts as the cathode whereas the spray coating layer exhibits a lower potential and works as anode. Thus, it is expected that if micro cells are formed, the spray coating layer will be able to resist corrosion because the spray coating layer exhibits the characteristics of sacrificial anode. However, for the defects of this coating layer, we can inhibit the generation of hydrogen in the coating that is in contact with the solution. The maximum corrosion resistance is affected by electrically insulating cathode and anode through sealing or painting. Furthermore, it seems that there are many voids inside the coating layer because of irregular coagulation. The hardness measurement for the spray coating specimen with 15%Al-85%Zn was HV41.8, but it was HV48.4 for the sealed specimen after coating. The high hardness by sealing seems to be the removal of voids by sealing.
Figure 2 shows the potential measurements in seawater with or without sealing for 24 h. The spray coated specimen showed the potential of -1.02 V at first and steadily shifted to active direction until around 13 000 s. After that, it maintained a constant potential followed by active directional at around 53 000 s, which seems to be due to the penetration of seawater through the voids in the spray coating layer. The sealed specimen after coating showed the potential of -0.98 V during the initial stage of immersion, and maintained a constant potential until the end of the experiment. The reason is because sealing fills the voids in the coating layer, thus inhibiting the penetration of chlorine ions on the specimen surface into the coating. Overall, the coating only specimen showed the variations of potential while the sealed specimen after coating showed a constant value from the beginning to the end of the test. Because the spray coating is much more active than the base metal, it acts as protective coating by contacting with the base metal by environmental blocking effect. If the base metal is exposed due to damaged coating, the life-span of various spray coating types is determined by the potential difference at the formation of galvanic cell and the gradient of anodic polarization at Tafel analysis.
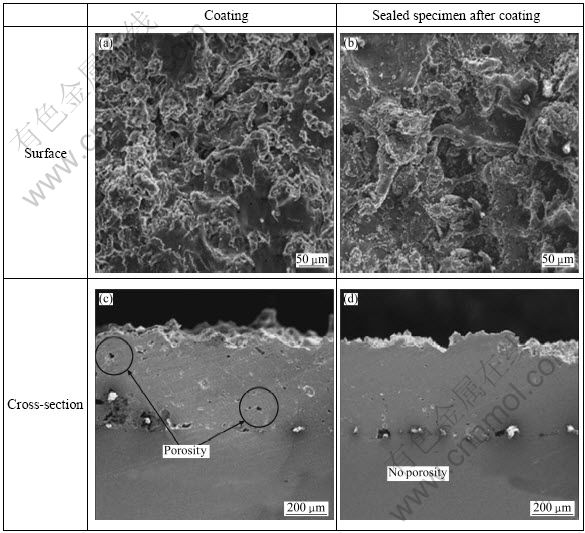
Fig. 1 Surface and cross-section morphologies of thermal spray coating and sealing
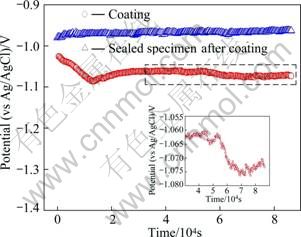
Fig. 2 Potential measurement for thermal spray coating and sealing in sea water (The insert is the enlarged part for the scaled specimen)
Figure 3 depicts the polarization trends of the spray coating specimen and the sealed specimen after coating. The anodic polarization curves exhibited almost similar behaviors in general, and mostly steady increase of current density was observed with increasing potential. This coating material did not show passivation trend in which current density decreased as potential increased. The sealed specimen exhibited good characteristics: the current density of the sealed specimen was low in the entire range from the beginning to the end of the experiment. The cathodic polarization trend of both specimens was concentration polarization by the reduction of dissolved oxygen and activation polarization by the hydrogen gas. The closer to the activation polarization range, the lower the difference of current density at the same potential condition. However, it is expected that the sealed specimen after coating will show better electrochemical behaviors by exhibiting low current density at all potentials applied. Also, regarding the polarization curves for Tafel analysis, the anodic polarization curve showed steady increase of current density with increasing potential, which indicated similar values. Regarding the cathodic polarization curves, the sealed specimen after coating exhibited considerably lower current densities than in the case of anodic polarization. The Tafel analysis revealed that the corrosion current densities of the coating only specimen and the sealed specimen after coating were 2.0×10-6 A/cm2 and 9.9×10-7 A/cm2, respectively. Because the value of the sealed specimen is much lower, its corrosion rate will slow down.
Figure 4 presents the time—current density curves and the current density after 3 600 s in potentiostatic experiment at various applied potentials. At the applied potential of 3 V, the sealed specimen after coating exhibited low current density at first, and its current density increased at about 900 s. Then, it maintained a constant potential from around 2 000 s till the end of the experiment, and exhibited lower current density than the coating only specimen. At the applied potential of -1 V, the sealed specimen after coating tended to show slowly increasing current density from the early stage of immersion whereas the current density of the coating only specimen rapidly decreased during the early stage of immersion, but rapidly increased as the chlorine ions in seawater penetrated into the voids in the coating layer. Then it stabilized from around 1 500 s to the end of the experiment. At -3 V, both specimens showed generally constant values of high density current, but the current density of the sealed specimen after coating was a little lower. From the comparison of current densities after potentiostatic experiment for 3 600 s at various potentials, the lowest current density was observed at -1 V which was close to the open circuit potential regardless of the existence of sealer. From the potentiostatic experiment with anodic and cathodic polarization at -1 V, the higher the potential was, the higher the current density was. Overall, the sealed specimen after coating showed lower current density at all potentials, implying its excellent corrosion resistance.
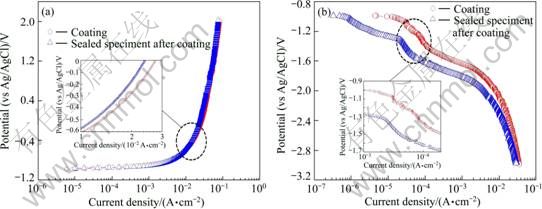
Fig. 3 Anodic (a) and cathodic (b) polarization curves for thermal spray coating and sealing in seawater (The inserts are the enlarged part)
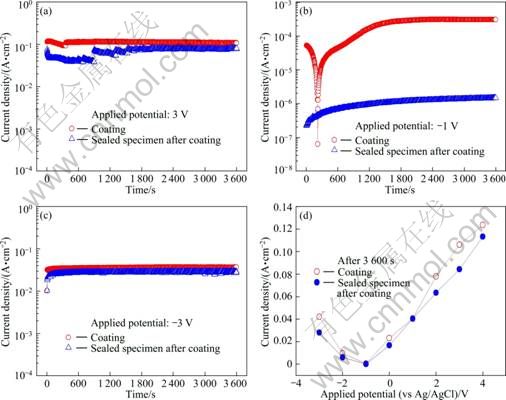
Fig. 4 Comparison of current density after 3600 s and time—current density curves in potentiostatic experiment at different applied potentials
Figure 5 shows the surface morphologies of the specimens after potentiostatic experiment. At -1 V which is close to the open circuit potential, the coating only specimen showed very partial corrosion in the coating layer. However, in the case of the sealed specimen after coating, the sealer coating remained without corrosion. At -2 V, the coating layer of the sealed specimen after coating was more severely corroded than at -1 V but it still remained. At 3 V, the coating of the sealed specimen after coating remained very partially. At 4 V, the surface was greatly damaged due to harsh active dissolution reaction. The sealed specimen showed a little less damage but the difference was not large.
Figure 6 shows the mass loss and cavitation rate during cavitation test. The measurements of mass loss found that the coating only specimen showed higher mass loss than the sealed specimen after coating at all sections, and they tended to increase constantly over time. On the other hand, the sealed specimen after coating showed relatively slower mass loss. At 300 min, the mass loss of the sealed specimen after coating was 61% that of the coating only specimen, which indicates it improved resistance to cavitation. For both specimens, the greatest cavitation rate was observed at 20 min. After the beginning of cavitation test, it slowly decreased. Initially, the coating only specimen indicated twice as large cavitation rate as the sealed specimen after coating, but the difference of the cavitation rate tended to decrease after that. At all conditions, the coating only specimen showed higher cavitation rate than the sealed specimen after coating. This result is in contrast to the result of the previous study in which the sealing of 85%Al-14%Zn-1%Zr caused lower hardness and worsened cavitation characteristics by chemical reaction between the coating layer and the sealing material [8].

Fig. 5 Surface morphologies after 3 600 s in potentiostatic experiment
Figure 7 presents the surface morphologies of the spray coating specimen and the sealed specimen after coating with cavitation test time in seawater. At 20 min, both specimens showed almost similar trends. This result seems to be in conflict with the large difference in the cavitation rate (Fig. 6(b)). However, although the cavitation rate is the mass loss (g) in unit time, and appears large even at a small mass loss, it is difficult to determine the difference under microscope. At 90 min, the coating only specimen showed conspicuous surface damage and some parts of the base metal were exposed, but the sealed specimen after coating still retained coating layer and the base metal was not exposed yet. At 300 min, both the specimens exposed the base metal, although they showed similar trends, the differences in dispersion and depth of the damaged parts between the two specimens could be ascertained by naked eye.
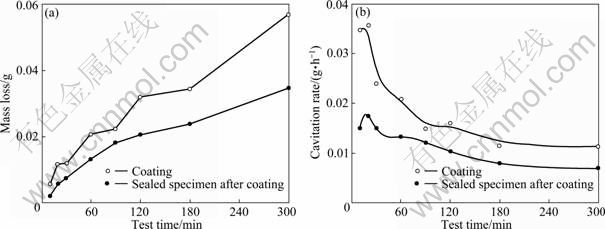
Fig. 6 Mass loss (a) and cavitation rate (b) after cavitation test for thermal spray coating and sealing in sea water
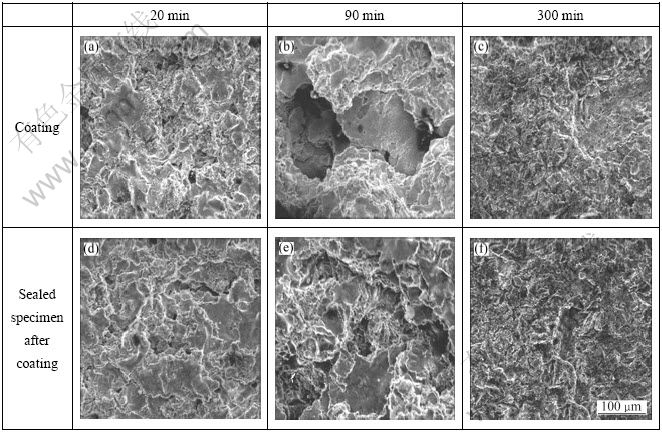
Fig. 7 Surface morphologies with cavitation test time for thermal spray coating and sealing in sea water
4 Conclusions
1) From the result of various electrochemical experiments with spray coating specimen and spray sealed specimen after coating using 15%Al-85%Zn spray wires, the sealed specimen after coating shows lower current density at varying potentials and lower corrosion current density from the Tafel analysis result. Therefore, it is concluded that the corrosion rate of the sealed specimen after coating is lower. Furthermore, the improvement effects by sealing can be confirmed from the potentiostatic experiment at various potentials.
2) As the sealed specimen after coating shows higher hardness, the cavitation test finds that it has good cavitation resistance characteristics with lower mass loss and cavitation rate. Therefore, the application of fluorine silicon sealing after spray coating of 15%Al-85%Zn seems to be appropriate not only in static environment but also in dynamic environment.
References
[1] TALKS M G, MORETON G. Cavitation erosion fluid system [C]//ASME Symposium. 1981: 139.
[2] SUN Z, KANG X Q, WANG X H. Experimental system of cavitation erosion with water-jet [J]. Materials and Design, 2005, 26(1): 59-63.
[3] HWANG J H, LIM U J. Effect of flow velocity on erosion-corrosion damage under vibratory cavitation [J]. Journal of the Corrosion Science Society of Korea, 1996, 25(3): 317-326.
[4] LIMA M M, GODOYA C, MODENESIA P J, AVELAR-BATISTA J C. Coating fracture toughness determined by Vickers indentation: An important parameter in cavitation erosion resistance of WC–Co thermally sprayed coatings [J]. Surface and Coatings Technology, 2004, 177-178: 489-496.
[5] CHOI H C, KIM G H. Effects of shot peening on the intergranular and pitting corrosion behavior of AISI 316 stainless steel [J]. Journal of the Korean Institute of Metal & Material, 1997, 35(3): 338-345.
[6] KIM S J, LEE S J. Investigation on cavitation and electrochemical characteristics for Al-Zn thermal spray coating and sealing effect [C]// The Korean Society of Marine Engineering, 2009 Year Spring Conference. 2009: 427.
[7] JANG S K, KO S C, HAN M S, KIM S J. Characteristics evaluation with coating thickness in Al thermal spray coating for 304 stainless steel [J]. Interfinish, 2008, 2008: 533.
[8] KIM S J, HAN M S, LEE S J. Effects of sealing on cavitation behavior of Al-Zn-Zr thermal spray coating and sealing [C]//The Korean Institute of Surface Engineering, 2009 Year Autumn Conference. 2009: 245.
F-Si封闭剂对热喷涂15%Al-85%Zn合金涂层电化学行为的影响
Seong-Jong KIM, Seung-Jun LEE
Division of Marine System Engineering, Mokpo Maritime University, Mokpo City, Jeonnam 530-729, Korea
摘 要:大部分船体外壳通过喷漆、牺牲阳极和外加电流阴极保护方法来解决抗腐蚀问题。但是,这些传统的方法不是非常有效,因为船尾的船舵常暴露在严重的腐蚀环境中,例如潮汐、船行驶速度、气蚀和冲刷腐蚀。由于气泡的生成与破裂而产生的振荡波而导致的气蚀和冲刷腐蚀会对材料产生破坏。对热喷涂Al-Zn合金涂层进行了气蚀和电化学性能测试实验。然后,Al-Zn合金涂层表面涂刷F-Si封闭剂以提高Al-Zn涂层的耐海水电化学腐蚀和抗气蚀性能。结果表明,该封闭剂不但适用静态环境,而且适用于动态环境。
关键词:气蚀;Al-Zn合金线材料;热喷涂层;腐蚀
(Edited by LI Xiang-qun)
Corresponding author: Seong-Jong KIM; E-mail: ksj@mmu.ac.kr; corr-pro@mmu.ac.kr
DOI: 10.1016/S1003-6326(11)61126-6