环氧涂层处理的Mg-3Al-1Zn镁合金在齿轮油中的腐蚀疲劳性能
来源期刊:中国有色金属学报(英文版)2014年第11期
论文作者:贺秀丽 卫英慧 侯利锋 闫志峰 郭春丽 韩鹏举
文章页码:3429 - 3440
关键词:镁合金;齿轮油;环氧涂层;腐蚀疲劳;疲劳极限;疲劳裂纹萌生
Key words:magnesium alloy; gear oil; epoxy coating; corrosion fatigue; fatigue limit; fatigue crack initiation
摘 要:研究环氧涂层处理后的Mg-3Al-1Zn合金在齿轮油环境下的腐蚀疲劳行为。采用扫描电子显微镜(SEM)观察疲劳试验后试样的腐蚀形貌及疲劳断口特征,并通过能谱仪(EDS)分析试样在齿轮油中的腐蚀产物成分。分析环氧涂层处理前、后2种试样的腐蚀疲劳性能和疲劳裂纹萌生机制。结果表明:经环氧涂层处理后,试样在齿轮油中的腐蚀疲劳极限高于未处理试样的。这是由于环氧涂层可以将镁合金与周围腐蚀环境良好地隔离。环氧涂层的力学性能比镁合金的差,这是疲劳裂纹优先从环氧涂层萌生的重要原因。另外,齿轮油的润滑作用可以使环氧涂层产生剥落现象。
Abstract: The corrosion fatigue behavior of epoxy-coated Mg-3Al-1Zn alloy in gear oil was investigated. The corrosion and the fracture surfaces after fatigue test were analyzed by scanning electron microscopy (SEM) and the corrosion compositions were measured by energy-dispersive spectrometry (EDS). The fatigue properties and the crack initiation mechanisms of the specimens before and after epoxy coating treatment were discussed. The results indicate that the fatigue limit after epoxy coating treatment in gear oil is higher than that of the uncoated specimens. The epoxy coating is an excellent way to prevent direct contact between the Mg-3Al-1Zn alloy and surrounding environments. The mechanical properties of the epoxy coating layer are lower than that of magnesium alloy, which is the main reason for the fatigue crack initiation on the epoxy coating layer. In addition, the gear oil lubrication could lead to the flaking off of the epoxy-coated layer.
Trans. Nonferrous Met. Soc. China 24(2014) 3429-3440
Xiu-li HE1, Ying-hui WEI1,2, Li-feng HOU1, Zhi-feng YAN1, Chun-li GUO1, Peng-ju HAN1
1. College of Materials Science and Engineering, Taiyuan University of Technology, Taiyuan 030024, China
2. Department of Chemistry, Luliang College, Lishi 033000, China
Received 10 December 2013; accepted 23 May 2014
Abstract: The corrosion fatigue behavior of epoxy-coated Mg-3Al-1Zn alloy in gear oil was investigated. The corrosion and the fracture surfaces after fatigue test were analyzed by scanning electron microscopy (SEM) and the corrosion compositions were measured by energy-dispersive spectrometry (EDS). The fatigue properties and the crack initiation mechanisms of the specimens before and after epoxy coating treatment were discussed. The results indicate that the fatigue limit after epoxy coating treatment in gear oil is higher than that of the uncoated specimens. The epoxy coating is an excellent way to prevent direct contact between the Mg-3Al-1Zn alloy and surrounding environments. The mechanical properties of the epoxy coating layer are lower than that of magnesium alloy, which is the main reason for the fatigue crack initiation on the epoxy coating layer. In addition, the gear oil lubrication could lead to the flaking off of the epoxy-coated layer.
Key words: magnesium alloy; gear oil; epoxy coating; corrosion fatigue; fatigue limit; fatigue crack initiation
1 Introduction
Magnesium alloys, which possess high specific strength, good stiffness and excellent cutting performance [1], are extensively used in the aerospace and automotive industries [2,3]. Besides dynamic loading, as a fuel, the oil is a main service environment for these pillar industries. As such, investigating the fatigue behavior of magnesium alloys in oil environments is an important endeavor.
Many studies on the corrosion fatigue properties of magnesium alloys in common environments, such as different humidity [4-12], sprayed or aqueous NaCl solutions [13-17], have been conducted. And they demonstrated that magnesium alloys are sensitive to the environments, which have significant effects on the corrosion fatigue properties. In addition, there are many other factors affecting the corrosion fatigue properties of magnesium alloys, such as loading frequency and ageing heat treatment [18,19], forming state of the alloys [20-22], and microstructure [23]. By contrast, studies on the corrosion fatigue properties of magnesium alloys in oil environments are few [24].
Attempts for improving the corrosion fatigue properties of magnesium alloys have been conducted, like chemical conversion coating [25,26], anodizing [27,28], diamond-like carbon coatings [29], electroless Ni-plating and electrolytically-plated methods [30,31]. They indicate that compared with that in air or under low humidity, the corrosion fatigue limits of the specimens after treatment are still degraded, and no approach is considered to be perfect.
A common characteristic of the aforementioned approaches is that the coatings can good corrosion resistance, which is a basic requirement for coatings. Considering this characteristic, epoxy coating is worthy of further research. Aside from its undisputable corrosion resistance [32,33], epoxy resin presents several other advantages, including excellent bonding strength to metal and nonmetal materials surfaces, and stability to alkali and most other solvents. As such, epoxy coating has attracted increasing attention for its applicability in electronic and industrial products [34,35]. In this work, the application of magnesium alloys in the aerospace and automotive industries was considered, and the corrosion fatigue behavior of epoxy-coated Mg-3Al-1Zn alloy in an oil environment was investigated using an axial tensile fatigue test machine.
2 Experimental
2.1 Materials and uncoated specimens
An extruded Mg-3Al-1Zn alloy was used as the substrate material in this work. The coating material consisted of diglycidyl ether of bisphenol A (DGEBA) epoxy resin (E-51), curing agent amine (triethylene tetramine, TETA), and acetone (AR) as diluent. They were purchased from Bluestar New Chemical Materials Co. Ltd. in China.
Prior to epoxy coating, the specimen was labeled as uncoated with a minimum gauge diameter of 6 mm and a gauge length of 40 mm. Figure 1 shows the configuration of the round bar specimen. The surface of the specimen was polished with 800 to 1500 grit emery papers to obtain a smooth surface (roughness 0.093 μm). The specimen surface was rinsed with distilled water and wiped with acetone before testing.

Fig. 1 Shape and dimension of specimen for fatigue test
2.2 Epoxy coating process
Specimens prior to epoxy coating were also polished with 800 to 1500 grit emery papers. The coating process was performed after sample pretreatment according to ASTM D2651-01 standard. The epoxy coating liquid included 50% E-51, 6% TETA, and 5% acetone. This liquid was stirred well for about 15 min at room temperature in a holder. The preprocessed specimens were dipped into the solution for 3 min. And then, they were lifted and rotated manually until the mixed solution showed no liquidity. The epoxy was cured at room temperature for about two weeks, and all specimens were processed at once. All specimens obtained after epoxy coating were labeled as coated.
2.3 Fatigue test
Fatigue tests were performed on a PLG-200D electromagnetic resonance high frequency tension- compression fatigue machine using a sinusoidal waveform with a stress ratio (R) of 0.1 and a frequency range of 99.0-102 Hz at room temperature. Gear oil (API GL-4 SAE 75W-90), which can be used to simulate a transmission oil environment, was used as the experimental environment. During the fatigue test, the gauge part of the specimen immersed in the gear oil was kept constantly. The fatigue test was also performed in air (35% relative humidity, (25±1) °C) for comparison. The fatigue tests were continued until either specimen failure occurred or the fatigue life exceeded 1.0×107 cycles without evident damage.
2.4 Tension test
Tension testing of the uncoated specimen (AZ31 magnesium alloy substrate), the coated specimen, and the pure epoxy coating specimen was conducted on an electronic universal tensile testing machine (CMT5205), respectively. The tensile speed was 0.5 mm/min.
2.5 Analysis and characterization methods
The adhesion between the epoxy coating and the substrate was determined using a cross-cut test according to ISO 2409. A surface roughness tester (TR240) was used to examine the surface roughness of the uncoated and the coated specimen prior to the corrosion fatigue test.
A scanning electron microscope (SEM, VEGA3SBH) was applied to observing the interface and the epoxy coating morphology of the coated specimen, the fracture surfaces and the corrosion morphologies of the specimens after the fatigue test. The corrosion products were removed by the immersion of specimens in boiling chromic acid (20% CrO3 + 1% AgNO3) for about 5 min. Specimens were then washed with deionized water and dried thoroughly.
An energy-dispersive spectrometer (EDS, OXFORD/ZNCA150) was used to analyze the compositions of the fracture surfaces and the corrosion products.
3 Results
3.1 Characteristics of epoxy coating
Figure 2 shows the morphology of the coated specimen surface. It indicated that there were no cracks and other defects, and it was dense, uniform and smooth. The cross-section of the epoxy coating layer was uniform and featured a mean thickness of (148±2) μm (Fig. 3). The uneven interface was attributed to the preprocessing procedures, which have a positive effect on the bonding of the epoxy coating to the substrate.
A cross-cut test was used to identify the adhesion strength between the substrate and the epoxy coating in accordance with ISO2409 (Fig. 4). The edges of the cut areas were completely smooth and none of the lattice squares were detached, which demonstrated the strong adhesion between the substrate and the epoxy coating. Adhesion between the alloy and the epoxy coating reached the ‘0’ classification, the highest possible level.
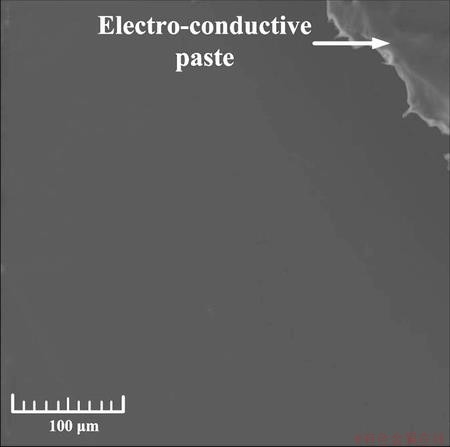
Fig. 2 SEM image showing morphology of coated specimen surface
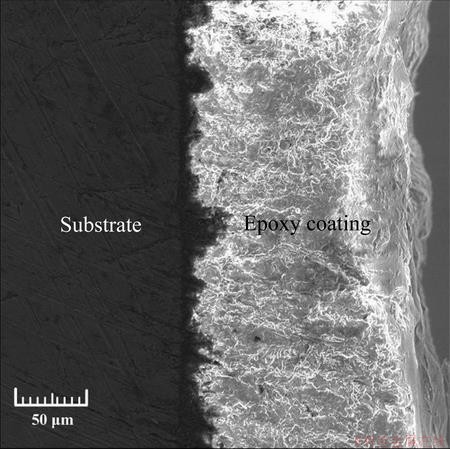
Fig. 3 SEM image showing cross-sectional observation of coated specimen

Fig. 4 Appearance of coated specimen after cross-cut test
A surface roughness parameter, Ra (the outline arithmetic average deviation) was tested. The surface roughnesses were 0.093 and 0.085 μm for the uncoated and the coated specimens, respectively. This was a slight difference. Thus, the effect of roughness on the fatigue properties of the magnesium alloy was negligible in the present experiment.
Figure 5 shows the tension curves of the pure epoxy coating specimen, the uncoated specimen (the Mg-3Al- 1Zn alloy substrate), and the coated specimen. And the tension property was increased in that order. The tensile strength, the yield strength and the elongation of the pure epoxy coating specimen were 22.19 MPa, 18.45 MPa and 2.26%, respectively. And they were 275.00 MPa, 183.50 MPa and 22.70% for the uncoated specimen, and 276.76 MPa, 193.37 MPa and 20.18% for the coated specimen, respectively.
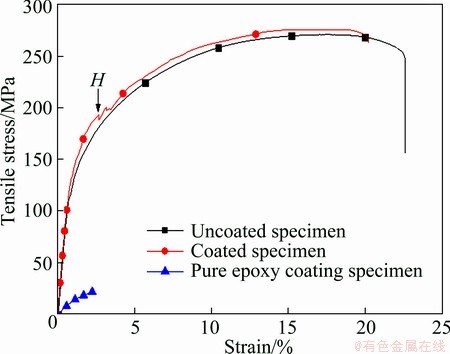
Fig. 5 Tension curves of pure epoxy coating, uncoated specimen (substrate Mg-3Al-1Zn alloy) and coated specimen
The first inflection point H of the coated specimen was the cracking point of the epoxy coating layer, which was observed during the tension. The tensile stress at this point was calculated as the yield strength of the coated specimen. This proved that the cracking of the coated specimen was caused by the incompatible deformation of the epoxy coating and the substrate.
3.2 Stress-cycle number (S-N) curves
The S-N curves between the maximum applied stress and the number of cycles to fatigue failure in air and gear oil are shown in Fig. 6, and they were processed by taking double logarithms (base 10) according to ISO 12107: 2012. Other related parameters are listed in Table 1. The correlation coefficient R2 is a useful parameter evaluating the quality of fitted curves. Its value is closer to 1, showing that the fit is better. The residual sum of squares (RSS) is the sum of squares of the difference between the actual value and the average, which was calculated according to the results of regression analysis. The smaller the RRS value, the better the fit.
Table 1 Statistic parameters of S-N curves

Fig. 6 S-N curves of uncoated and coated specimens in different environments
It can be seen from the figure that the fatigue properties of all specimens were improved after the epoxy coating treatment (Fig. 6). The horizontal arrow on the point in each curve in the figure indicated that the specimen was not fractured at 1.0×107 cycles, and the experiment could continue if it was necessary. The fatigue limits are usually estimated at 1.0×107 cycles (for non-ferrous metals). The fatigue limits of the uncoated specimens were 163.89 MPa in air and 158.12 MPa in gear oil, respectively. The respective fatigue limits of the coated specimens in air and gear oil were 175.95 MPa and 178.51 MPa. Obviously, it was given rise to the application of the epoxy coating.
3.3 Corrosion morphology observation
Figure 7(a) shows the corrosion morphologies of the uncoated specimen surface after the fatigue test in gear oil. Apart from some machining traces, there were some substances like chewing gum pasting on the specimen. The elliptical area was amplified and shown in Fig. 7(b). And the composition (marked by the white solid dot) was analyzed by EDS (Fig. 7(c)). The C element was believed to originate from the ambient environment (gear oil) and the electro-conductive paste. The latter was used to fix the specimen tested at the object stage during SEM observation. The O element came from the gear oil and ambient air. The Mg and Al elements were the main components of the Mg-3Al-1Zn magnesium alloy. Changes in the morphology of the specimen surface and the significant reduction in the contents of Mg and Al elements indicated the reactions between the gear oil and magnesium alloy. Substances such as “chewing gum” were believed to be the corrosion products.
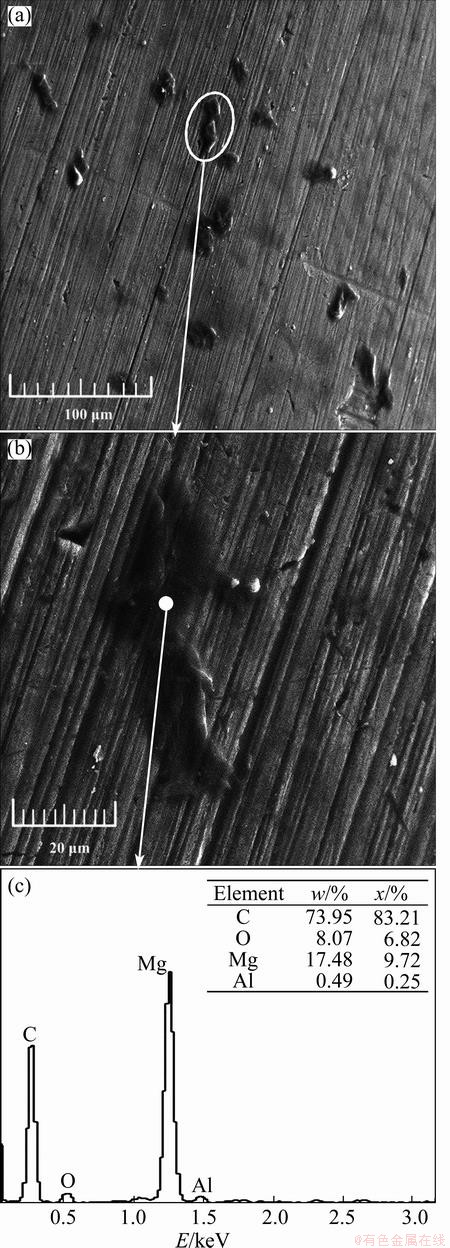
Fig. 7 Corrosion morphologies of uncoated specimen surface after fatigue test in gear oil and corresponding EDS result
The morphologies of the uncoated specimen surface were observed following the removal of corrosion products (Fig. 8(a)). The remaining corrosion products can still be observed. The circle part was magnified in Fig. 8(b). The composition (the white solid dot) was tested (Fig. 8(c)). The results showed that the elemental components of specimens after corrosion product removal differed from those before removal (Fig. 7(c)). The Mg, Al and Zn elements were the main components of the Mg-3Al-1Zn alloy. The C element was from the electro-conductive paste and gear oil. However, the O content of specimens after corrosion product removal was similar to that before corrosion product removal. This finding proved that the corrosion products containing oxygen were not so easy to be removed from the substrate, and the O element was mainly from the oxygen-containing compounds and ambient air. Considering that the content of C element decreased after the removal of the corrosion products, it can be speculated that the gear oil on the specimen surface was removed to greater extent. After the removal of the corrosion products, more surface of the magnesium alloy was exposed, leading to the observed increase in the contents of Mg, Al, and Zn elements. Strips on the specimen surface in Figs. 8(a) and (b) were machining traces.

Fig. 8 Morphologies and EDS result of uncoated specimen surface
Figure 9(a) shows the corrosion morphologies of the coated specimen surface obtained after the fatigue test in gear oil. There were some granular materials. The EDS was used to analyze the compositions of the corrosion morphologies (as the rectangular mark indicated in Fig. 9(a)), as shown in Fig. 9(b). In addition to the gear oil and the electro-conductive paste, some C elements were also from the epoxy coating layer. The sources of the O element were not changed and also from the gear oil and ambient air. The occurrence of Au element was because of the metal spraying process before SEM and EDS test. It was used to improve the conductivity of the epoxy coating for achieving better images. Those granular materials were from the gold dust. It is worth noting that there was no element from the magnesium alloy substrate, which implied that no corrosion reaction happened on the coated specimen surface.
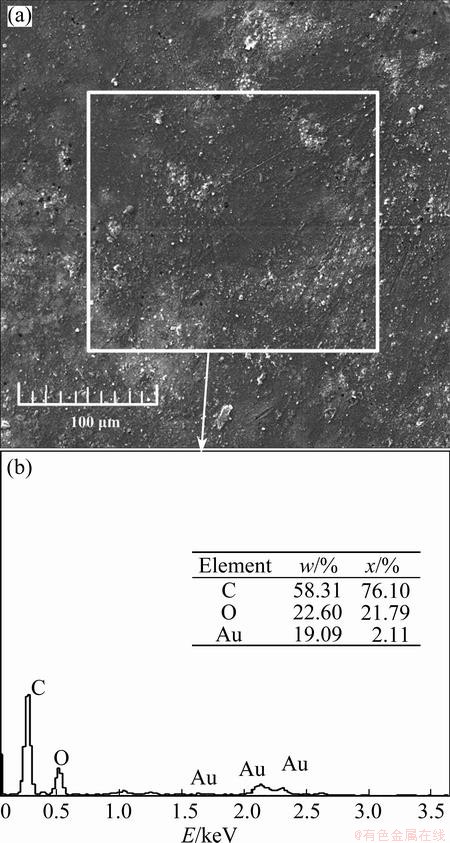
Fig. 9 Corrosion morphology and EDS result of coated specimen surface after fatigue test in gear oil
3.4 Fracture observation
The fracture surface of the coated specimen was observed as shown in Fig. 10(a), which was tested at the stress level of 190 MPa in air. Several circular flaws (as indicated by the arrow) were distributed on the epoxy coating layer. Secondary cracks originating from the layer-substrate interface were also observed. Stress appeared to concentrate on the circular flaws, leading to nucleation of fatigue cracks. Minor cracks eventually merged into a main crack. Figure 10(b) shows a magnified portion of one circular flaw. The inner wall of this flaw was smooth and contained no other particles. The circular flaws were pores originating from the formation of epoxy coating layer in air which had inadequate time to discharge completely.
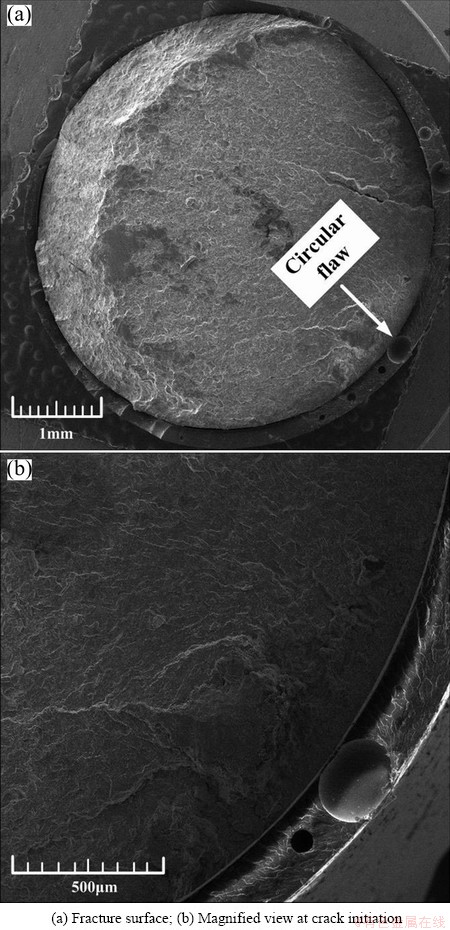
Fig. 10 SEM images of coated specimen tested at 190 MPa in air
Figure 11(a) shows the fracture surface of the coated specimen tested at the stress level of 195 MPa in gear oil. A radial pattern similar to that in air was observed. Figure 11(b) reveals that the crack initiation region (indicated by an ellipse in Fig. 11(a)) is relatively flat. The epoxy coating layer near the crack initiation surface disappeared. This phenomenon was caused by three main reasons. First, epoxy coating can be dissolved in organic solvents (gear oil) [36], which made the specimen surface roughness and the coating layer thinner and thinner. Thus, the crack initiation sources increased and the actual bearing area decreased; they were both dangerous for the fatigue property of the specimen. Second, once the epoxy coating surface cracked, the adhesion between the epoxy coating and the substrate decreased upon penetration of the gear oil, which is due to the lubricating property of the gear oil. Third, along with the vibration under the cyclic fatigue loading, the epoxy coating layer flaked away. No pores were found on the epoxy coating layer, similarly to observations on the fracture surface of the coated specimens in air. Such finding, however, did not imply that fatigue cracks were not initiated from these pores since pores had been stripped off the alloy as the epoxy coating layer flaked off. Figure 11(b) shows traces of some fuzzy shaped substances (the red solid dot) that were believed to have formed because of gear oil viscosity. These substances were found to be the gear oil adsorbates or corrosion products and analyzed by EDS (Fig. 11(c)). It included elements from the gear oil (C) and the substrate (Mg and Al). The O element was sourced from the gear oil, the oxides, and ambient air, which indicated that the adsorption of gear oil on the magnesium alloy was physical and chemical in nature. Reductions in Mg and Al contents compared with those observed in the substrate indicated the occurrence of corrosion reactions. The role of these fuzzy shaped substances will be discussed in the succeeding sections.
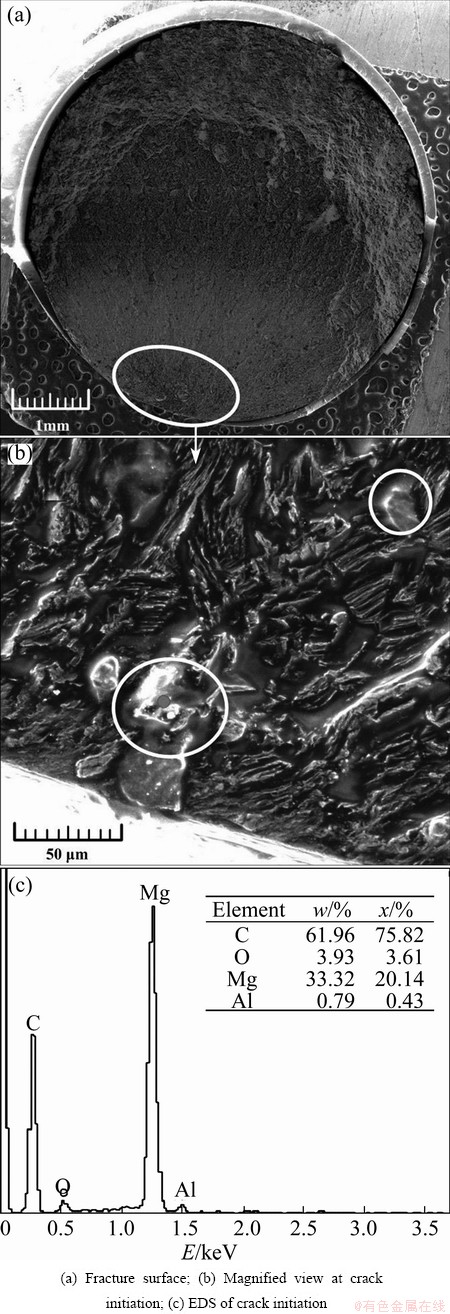
Fig. 11 SEM images of coated specimen tested at 195 MPa in gear oil
4 Discussion
The reduction rate (Rr) or the increase rate (Ri) of fatigue limits under different environments was calculated qualitatively using the equation given by
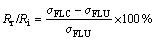 (1)
(1)
where σFLC and σFLU are the fatigue limits of the coated and the uncoated specimens, respectively. The results are listed in Table 2.
The interval of the two maximum applied stress levels corresponding to 1.0×107 cycles was 10 MPa, and an error of ±5 MPa was allowed.
4.1 Effect of epoxy coating on fatigue limit
S-N curves of the coated specimens were located above those of the uncoated specimens regardless of the environment (Section 3.2). The fatigue properties of the coated specimens were better than those of the uncoated specimens in air or gear oil.
Table 2 Comparison of parameters of fatigue limits (FL) of uncoated and coated specimens in different environments
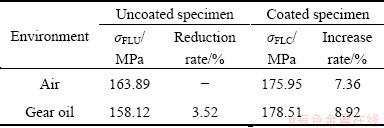
According to Eq. (1) and Table 2, the fatigue limit of the coated specimens increased by approximately 7.36% compared with the uncoated specimens in air; an increase of about 12.90% was observed in gear oil.
The fatigue limit of the coated specimens in gear oil was not only higher than that of the uncoated specimens in gear oil (as mentioned in the last paragraph), but also higher than that of the uncoated specimens in air and yielded an increase of about 8.92%. While differences observed are small. This method shows an advantage over the other [26-32].
The quality of the coating was crucial for the property of the coated specimen. First, the epoxy coating surface was dense, uniform and smooth, and no defects such as cracks were observed (section 3.1). Second, the bonding interface status is a key factor, which determines the service quality of the coating. In this study, it was physical bonding between the substrate and the epoxy coating layer. The interface was uneven, which strengthened the friction and favored the development of adhesive forces. The static adhesion (i.e., without external stress) between the substrate and the epoxy coating was tested in section 3.1, and the highest level of adhesion was achieved according to ISO 2409. The dynamic adhesion, on the other hand, was determined by the fatigue test. Figure 12 shows the appearance of the coated specimens under fatigue limits in different environments. No loosening or separation was observed. Dynamic adhesion between the epoxy coating and the substrate was good even under fatigue limits. Third, the coating surface roughness is an important parameter to consider especially in structures under cyclic loading (fatigue stress). Rougher surfaces generally promote stress concentration and corrosion reactions. The roughness has an important influence on fatigue crack nucleation [37] and corrosion property [38,39] of a material. The roughnesses of the coated and uncoated specimens were tested, and similar results were found (Section 3.1). Thus, the effect of roughness on the fatigue property and corrosion resistance can be neglected in this study. This result revealed another advantage of the epoxy coating over anodizing [27,28].

Fig. 12 Appearance of coated specimen under corrosion fatigue limit in air (a) and in gear oil (b)
Improvements in the fatigue property of the coated specimens were attributed to the epoxy coating following the exclusion of binding force and surface roughness effects. The epoxy coating layer can strengthen the substrate [40,41], which was mainly realized by the surface friction between the epoxy coating and the substrate. And the thickness of epoxy coating layer was about 148 μm, which increased the bearing area of the specimen. These were the main reasons for the enhancement of the fatigue limit of the coated specimens in air.
Moreover, the epoxy coating layer has good corrosion resistance [32,33] and separates the substrate from ambient environments (air and gear oil) during the fatigue test, thereby lessening the impact of detrimental factors, such as water molecules, oxygen, and gear oil, on the alloys. For instance, no elements from the substrate were observed in the corrosion morphology of the coated specimens after fatigue tests in gear oil (Fig. 9). This observation implied that the epoxy coating layer protected the substrate, inhibited gear oil penetration, and extended the fatigue lives of the coated specimens. These benefits showed the fatigue limit improvement of the coated specimens in gear oil.
The fatigue limit of coated specimens was consistently higher than those of the uncoated specimens in air and in gear oil (Fig. 6). The epoxy coating not only has a powerful surface friction with substrate but also has an excellent corrosion resistance. The coating is dense and sound, preventing direct contact between the Mg-3Al-1Zn alloy and the surrounding environments. Epoxy coating was considered an alternative to protect the substrate from ambient environments.
4.2 Effects of gear oil on fatigue limit
For the uncoated specimens, the fatigue limit in gear oil was reduced slightly, about 3.52%, compared with that in air. This was consistent with the results of AZ91D magnesium alloy in gear oil [24]. It was due to the gear oil adsorption: physical adsorption and chemical adsorption [42]. According to the morphology of the uncoated specimens in gear oil after fatigue test (Figs. 7 and 8), the corrosion products (mainly produced by chemical adsorption) always existed before and after the corrosion product removal. The chemical adsorbate, just like the chewing gum, pasting on the specimen, made the specimen surface rough. Stress concentration easily occurred at the root of the chemical adsorbate under fatigue loading, thus providing necessary conditions for fatigue crack initiation and the specimen fracture in gear oil. However, the reduction rate was considerably small because the corrosive effect of gear oil on the magnesium alloy was relatively small [43].
There was an intersection on the S-N curves of the uncoated specimens in air and gear oil (Fig. 6). The fatigue lives in gear oil were higher than those in air at high fatigue loading stress. And the former was lower than the latter at low fatigue loading stress. This was mainly because of the differences of the service environments and service time. In general, without the effect of environment, the fatigue lives were longer (the corresponding service time was longer) with the fatigue loading stress decreasing.
The service time was relatively short at high fatigue load stresses in gear oil; here, the amount of the gear oil adsorbate was minimal. The roughness of the specimen surface did not noticeably change, and no stress concentration on the specimen surface was observed. Moreover, the gear oil environment produced minimal corrosive effect and can protect the magnesium alloy from the water molecules in air. From this perspective, the oil adsorption film formed on the specimen surface and also played a protective role. Thus, the fatigue lives of the uncoated specimens in gear oil were higher than those in air at high fatigue loading stress. However, the amount of the gear oil adsorbate increased at low fatigue loading stress, and the specimen surface was no longer smooth. More opportunities for stress concentration, which enhanced the specimen failure, were presented at the root of the gear oil adsorbate. Therefore, fatigue lives in gear oil were lower than those in air at low fatigue loading stress.
On the other hand, due to the gear oil adsorption, there were some fuzzy shaped substances (the gear oil adsorbate). A small amount of the gear oil could prevent the propagation of the fatigue cracks because of the gear oil viscosity [44,45]. However, as the service time went on, the amount of gear oil increased. The gear oil lubrication [46,47] was more dominant than the gear oil viscosity. Thus, to some extent, it accelerated the fatigue crack propagation. This observation also explains the lower fatigue lives of the uncoated specimens in gear oil than those in air at low fatigue loading stress.
In addition, the reduction of fatigue property of the uncoated specimens could be explained by ratcheting effect (That is, there is an increasing cyclic plastic strain accumulation due to the asymmetrical cyclic stress function on a structure). The ratcheting strain was accumulated during the fatigue process continually, which was increased with the increase of the loading stress. The results were in accordance with those described in Refs. [48-51], which concentrated on the low-cycle fatigue life of AZ31 and AZ91. This conduced to explain the reduction in the corrosion fatigue property of AZ31 magnesium alloy. Under a certain loading stress, when the cumulative strain reached a critical value which the specimen could not afford, a rupture would take place. For specimens in the gear oil, the critical cumulative strain of the specimen which could afford was reduced compared with that in air. And thus the maximum applied stress (fatigue limit) of the specimen which could sustain at 1.0×107 cycles was degraded.
For the coated specimen, the fatigue limit in gear oil was slightly higher (1.45%) than that in air. This was caused by the following reasons. First, the epoxy coating layer enhanced the substrate. Second, there was no corrosion reaction between the gear oil and the epoxy coating. Third, the adsorption between the gear oil and the epoxy coating was only uniform physical adsorption. It has no influence on the roughness and the fatigue property of the coated specimen. Moreover, the epoxy coating layer was dense and sound, thereby preventing the chemical adsorption between the gear oil and the magnesium alloy substrate. This can be proved by the morphology of the coated specimens in gear oil after fatigue test (Fig. 9). There were no elements in the substrate. As the aforementioned, the fatigue limits of the coated specimens in air and gear oil were nearly identical. The epoxy coating shortened the gap of fatigue properties between two service environments: gear oil and air.
Hence, the effect of gear oil was more significant on the fatigue properties of the uncoated specimens than on those of the coated specimens. And the influence of gear oil on the magnesium alloy can be neglected following the epoxy coating.
4.3 Effects of gear oil and epoxy coating on crack initiation mechanisms
For the uncoated specimens, the fatigue cracks are all initiated on or near the specimen surface in air and gear oil [13-15,24,52-55]. Stress concentration generally forms at or near the specimen surface area. Furthermore, the effect of the gear oil on the magnesium alloy is limited and insufficient to change the crack initiation mechanism.
However, for the coated specimens, it is imperative to identify the position of the fatigue crack nucleation. According to the analysis on the tension properties of the pure epoxy coating specimen, the coated specimen and the uncoated specimen (the AZ31 magnesium alloy substrate) in section 3.1; the tensile stress of the pure epoxy coating specimen was the minimum, 22.19 MPa. The first inflection point H (Fig. 5) of the coated specimen was the cracking point (the yield strength point) of the epoxy coating layer. This demonstrated that when the deformation of the epoxy coating and the substrate became incompatible, the crack occurred on the epoxy coating layer. Similarly, for the fatigue test of the coated specimens, the conditions for the specimen failure were found to be as follows: the one is when the stress loading on the substrate or the epoxy coating layer is greater than the tensile strength; the other is when the deformations of the two materials (the epoxy coating and the substrate) are incompatible.
The dynamic adhesion between the epoxy coating and the substrate can be evaluated by the following equation:
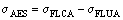 (2)
(2)
where σAES is the dynamic adhesion between the epoxy coating and the substrate under fatigue loading; σFLCA and σFLUA are the fatigue limits of the coated and the uncoated specimens in air, respectively (see Table 2).
According to Eq. (2), the adhesion σAES between the epoxy coating and the substrate was 12.06 MPa. That is, for the coated specimens in air, when the stress loading on the coated specimen was 175.95 MPa (under the fatigue limit), the stress loading on the epoxy coating layer was 12.06 MPa. The tension curve of the pure epoxy coating specimen indicated that the epoxy coating was in the elastic stage (Fig. 5). By contrast, even at 175.95 MPa, the substrate remained in the elastic stage. Thus, the deformation of the two materials matched well at this stress level, and the whole specimen (the coated specimen) was in the elastic stage.
With the fatigue loading stress increasing, the stress loading on the substrate or the epoxy coating layer was increased as well. As can be seen from Fig. 5, the tension properties were decreased in the following order: the coated specimen, the uncoated specimen (the AZ31 magnesium alloy substrate), and the pure epoxy coating specimen. Hence, no matter from the deformation or from the loading stress point of view, the fatigue crack was nucleated from the epoxy coating layer. Actually, in the case of material continuous damage, this process cannot be changed but can be speeded up.
The pores existing in the epoxy coating layer weakened the quality and increased the chances of fatigue crack initiation from the epoxy coating. In other words, in air, originally, the fatigue crack was initiated in these pores, which caused the stress concentration easily under the cyclic loading stress. Then, the fatigue crack prolonged to the substrate rapidly due to the strong adhesion, and propagated until the specimen failure.
Differing from that of the coated specimens in air (Fig. 10), for the coated specimens, the epoxy coating layer on the edge of the fracture flaked off in gear oil (Fig. 11). In fact, the fatigue crack was also initiated in the pores like that analyzed above in air. Next, the ambient gear oil seeped into the interface and penetrated along the depth direction, thereby weakening the adhesion between the epoxy coating and the substrate under the gear oil lubrication. This observation is consistent with the results in Fig. 11, wherein the epoxy coating was separated from the substrate. Then, the substrate was exposed to the gear oil environment. The fatigue crack was initiated near the substrate surface anew, and propagated until the specimen fractured. Therefore, it can be speculated that the fatigue crack initiation of the coated specimens can be divided into two steps in gear oil. The first step was the crack nucleated from the pores on the epoxy coating layer. The second step was similar like those of the uncoated specimens.
5 Conclusions
1) Fatigue limits in air and gear oil were improved after epoxy coating treatment. In addition to the role of the surface friction, epoxy coating is also an excellent way to prevent direct contact between the Mg-3Al-1Zn alloy and its surrounding environments.
2) The negligible effect of the gear oil on the fatigue properties of the coated specimens is attributed to two main reasons: the poor corrosive gear oil and the good corrosion protection of the epoxy coating layer.
3) The deformation abilities of the epoxy coating and the AZ31 magnesium alloy were consistent with findings in the tension test in the elastic stage. Fatigue failure occurred when the deformations of the two materials differed. The stress experienced by the epoxy coating layer and the deformation produced at this stress during fatigue tests always reached the maximum that the epoxy coating layer can bear firstly, the fatigue crack always initiated from the epoxy coating layer.
4) Once the crack generated, the ambient gear oil penetrated into the interface and the substrate. The adhesion between the epoxy coating and the substrate was impaired under the interaction of the lubrication of the gear oil and the cyclic fatigue loading, and the epoxy coating layer near the fatigue crack initiation area flaked off. A new crack initiation source then formed near the substrate surface quickly and the crack propagated until the specimen fractured.
References
[1] PAN F S, ZHANG J, WANG J F, YANG M B, HAN E H, CHEN R S. Key R&D activities for development of new types of wrought magnesium alloys in China [J]. Transactions of Nonferrous Metals Society of China, 2010, 20: 1249-1258.
[2] YAN Zhi-feng, ZHANG Hong-xia, WANG Wen-xian, WANG Kai, PEI Fei-fei. Temperature evolution and fatigue life evaluation of AZ31B magnesium alloy based on infrared thermography [J]. Transactions of Nonferrous Metals Society of China, 2013, 23(7): 1942-1948.
[3] MORITA S, OHNO N, TAMAI F, KAWAKAMI Y. Fatigue properties of rolled AZ31B magnesium alloy plate [J]. Transactions of Nonferrous Metals Society of China, 2010, 20(S): s523-s526.
[4] ZENG R C, HAN E H, KE W. Fatigue and corrosion fatigue of magnesium alloys [C]//Materials Science Forum 2005. USA: Trans Tech Publications Inc. 2005: 721-724.
[5] UNIGOVSKI Y, ELIEZER A, ABRAMOV E, SNIR E, GUTMAN E M. Corrosion fatigue of extruded magnesium alloys [J]. Materials Science and Engineering A, 2003, 360(1): 132-139.
[6] BHUIYAN M S, MUTOH Y, MURAI T, IWAKAMI S. Corrosion fatigue behavior of extruded magnesium alloy AZ61 under three different corrosive environments [J]. International Journal of Fatigue, 2008, 30(10): 1756-1765.
[7] MUTOH Y, BHUIYAN M S, SAJURI Z. High cycle fatigue behavior of magnesium alloys under corrosive environment [J]. Key Engineering Materials, 2008, 378: 131-146.
[8] NAN Z Y, ISHIHARA S, GOSHIMA T. Corrosion fatigue behavior of extruded magnesium alloy AZ31 in sodium chloride solution [J]. International Journal of Fatigue, 2008, 30(7): 1181-1188.
[9] BHUIYAN M S, MUTOH Y. Two stage S-N curve in corrosion fatigue of extruded magnesium alloy AZ31 [J]. Songklanakarin Journal of Science and Technology, 2009, 31(5): 463-470.
[10] CHAMOS A N, PANTELAKIS Sp G, SPILIADIS V. Fatigue behaviour of bare and pre-corroded magnesium alloy AZ31 [J]. Materials and Design, 2010, 31(9): 4130-4137.
[11] BHUIYAN M S, MUTOH Y, MURAI T. Corrosion fatigue behavior of extruded magnesium alloy AZ80-T5 in a 5% NaCl environment [J]. Engineering Fracture Mechanics, 2010, 77(10): 1567-1576.
[12] ZENG R C, HAN E H, KE W. Effect of temperature and relative humidity on fatigue crack propagation behavior of AZ61 magnesium alloy [C]//Materials Science Forum 2007. USA: TTP. 2007: 409-412.
[13] ZENG Rong-chang, HAN En-hou, KE Wei, LIU Lu, XU Yong-bo. Corrosion fatigue of as-extruded AM60 magnesium alloy [J]. Chinese Journal of Materials Research, 2005, 19(1): 1-7. (in Chinese)
[14] ZENG Rong-chang, HAN En-hou, KE Wei, LIU Lu, XU Yong-bo. Mechanism of corrosion fatigue for as-extruded magnesium alloy AZ80 [J]. Chinese Journal of Materials Research, 2004, 18(6): 561-567. (in Chinese)
[15] LIU Ma-bao, LEI Jun, GAO Yu-xia, ZHANG Ying-jie, QI Yan-jie, SUO Jian-qin. Corrosion fatigue of die-cast magnesium alloy [J]. Journal of Aeronautical Materials, 2007, 17(1): 76-80. (in Chinese)
[16] ZHOU Hua-mao, WANG Jian-qiu, ZHANG Bo, HAN En-hou, ZANG Qi-shan. Acoustic emission signal analysis for rolled AZ31B magnesium alloy during corrosion fatigue process [J]. Journal of Chinese Society for Corrosion and Protection, 2009, 29(2): 81-87. (in Chinese)
[17] ZHOU H M, WANG J Q, ZANG Q S, HAN E H. Study on the effect of Cl- concentration on the corrosion fatigue damage in a rolled AZ31B magnesium alloy by acoustic emission [J].Key Engineering Materials, 2007, 353: 327-330.
[18] ZENG R C, KE W, HAN E H. Influence of load frequency and ageing heat treatment on fatigue crack propagation rate of as-extruded AZ61 alloy [J]. International Journal of Fatigue, 2009, 31(3): 463-467.
[19] ZENG R C, XU Y B, KE W, HAN E H. Fatigue crack propagation behavior of an as-extruded magnesium alloy AZ80 [J].Materials Science and Engineering A, 2009, 509(1): 1-7.
[20] KHAN S A, BHUIYAN M S, MIYASHITA Y, MYTOHC Y, KOIKED T. Corrosion fatigue behavior of die-cast and shot-blasted AM60 magnesium alloy [J]. Materials Science and Engineering A, 2011, 528(4): 1961-1966.
[21] ISHIHARA S, NAN Z Y, NAAMITO T, GOSHIMA T, SUNADA S. On electrochemical polarization curve and corrosion fatigue resistance of the AZ31 magnesium alloy [J]. Key Engineering Materials, 2010, 452: 321-324.
[22] ELIEZER A, GUTMAN E M, ABRAMOV E, UNIGOVSKI Y. Corrosion fatigue of die-cast and extruded magnesium alloys [J]. Journal of Light Metals, 2001, 1(3): 179-186.
[23] ZENG R C, HAN E H, KE W, DIETZEL W, KAINER K U, ATRENS A. Influence of microstructure on tensile properties and fatigue crack growth in extruded magnesium alloy AM60 [J].International Journal of Fatigue,2010, 32(2): 411-419.
[24] ELIEZER A, MEDLINSKY O, HADDAD J, BEN H G. Corrosion fatigue behavior of magnesium alloys under oil environments [J]. Materials Science and Engineering A, 2008, 477(1): 129-136.
[25] BHUIYAN M S, MUTOH Y. Corrosion fatigue behavior of conversion coated and painted AZ61 magnesium alloy [J]. International Journal of Fatigue, 2011, 33(2): 1548-1556.
[26] BHUIYAN M S, OSTUK Y, MUTOH Y, MURAI T, IWAKAMI S. Corrosion fatigue behavior of conversion coated AZ61 magnesium alloy [J]. Materials Science and Engineering A, 2010, 527(18): 4978-4984.
[27] KHAN S A, MIYASHITA Y, MUTOH Y, KOIKE T. Fatigue behavior of anodized AM60 magnesium alloy under humid environment [J]. Materials Science and Engineering A, 2008, 498: 377-383.
[28] KHAN S A, MIYASHITA Y, MUTOH Y, KOIKE T. Effect of anodized layer thickness on fatigue behavior of magnesium alloy [J]. Materials Science and Engineering A, 2008, 474(1): 261-269.
[29] UEMATSU Y, KAKIUCHI T, TERANTANI T, HARADA Y, TOKAJI K. Improvement of corrosion fatigue strength of magnesium alloy by multilayer diamond-like carbon coatings [J]. Surface and Coatings Technology, 2011, 205(8): 2778-2784.
[30] ISHIHARA S, NAMITO T, NOTOYA H, OKADA A. The corrosion fatigue resistance of an electrolytically-plated magnesium alloy [J]. International Journal of Fatigue, 2010, 32(8): 1299-1305.
[31] ISHIHARA S, NOTOYA H, OKADA A, NAN Z Y, GOSHIMA T. Effect of electroless-Ni-plating on corrosion fatigue behavior of magnesium alloy [J]. Surface and Coatings Technology, 2008, 202(10): 2085-2092.
[32] BAJAT J B,  Protective properties of epoxy coatings electrodeposited on steel electrochemically modified by Zn–Ni alloys [J]. Progress in Organic Coatings, 2004, 49(3): 183-196.
Protective properties of epoxy coatings electrodeposited on steel electrochemically modified by Zn–Ni alloys [J]. Progress in Organic Coatings, 2004, 49(3): 183-196.
[33] HU J M, ZHANG J T, ZHANG J Q, CAO C N. Corrosion electrochemical characteristics of red iron oxide pigmented epoxy coatings on aluminum alloys [J]. Corrosion Science, 2005, 47(11): 2607-2618.
[34] BRUSCIOTTI F, SNIHIROVA D V, XUE H B, MONTEMOR M F, LAMAKA S V, FERREIRA G S. Hybrid epoxy-silane coatings for improved corrosion protection of Mg alloy [J]. Corrosion Science, 2013, 67: 82-90.
[35] ZHANG S Y, DING Y F, LI S J, LUO X W, ZHOU W F. Effect of polymeric structure on the corrosion protection of epoxy coatings [J]. Corrosion Science, 2002, 44(4): 861-869.
[36] ZENG Rong-chang, HAN En-hou. Corrosion and protection of materials [M]. Beijing: Chemical Industry Press, 2006. (in Chinese)
[37] PROUDHON H, FOUVRY S,  J Y. A fretting crack initiation prediction taking into account the surface roughness and the crack nucleation process volume [J]. International Journal of Fatigue, 2005, 27(5): 569-579.
J Y. A fretting crack initiation prediction taking into account the surface roughness and the crack nucleation process volume [J]. International Journal of Fatigue, 2005, 27(5): 569-579.
[38] KHUN N W, FRANKEL G S. Effects of surface roughness, texture and polymer degradation on cathodic delamination of epoxy coated steel samples [J]. Corrosion Science, 2013, 67: 152-160.
[39] LEE S M, LEE W G, KIM Y H, JANG H, Surface roughness and the corrosion resistance of 21Cr ferritic stainless steel [J]. Corrosion Science, 2012, 63: 404-409.
[40] KINGSTON J G R, HAND R J, Strengthening mechanisms of epoxy based coatings on glass [J]. Society of Glass Technology, 2000, 41(1): 1-5.
[41] LEE H K, KIM B R, HA S K. Numerical evaluation of shear strengthening performance of CFRP sheets/strips and sprayed epoxy coating repair systems [J]. Composites B: Engineering, 2008, 39(5): 851-862.
[42] BIRESAW G. Adsorption of amphiphiles at an oil-water vs an oil-metal interface [J]. Journal of the American Oil Chemists’ Society, 2005, 82(4): 285-292.
[43] NEFEDOV Y U. Corrosion resistance of oil piping [J]. Zashch Metal, 1988, 24(4): 634-636.
[44] ROUNDS F G. Effects of base oil viscosity and type on bearing ball fatigue [J]. ASLE Transactions, 1962, 5(1): 172-182.
[45] SHANAHAN M E R, SCHULTZ J. A kinetic effect in the environmental stress cracking of polyethylene due to liquid viscosity [J]. Journal of Polymer Science: Polymer Physics Edition, 1976, 14(9): 1567-1573.
[46] KELLY J F, COTTERELL M G. Minimal lubrication machining of aluminium alloys [J]. Journal of Materials Processing Technology, 2002, 120(1): 327-334.
[47] GUO J, WANG L P, LIANG J, XUE Q J, YAN F Y. Tribological behavior of plasma electrolytic oxidation coating on magnesium alloy with oil lubrication at elevated temperatures [J]. Journal of Alloys and Compounds, 2009, 481(1): 903-909.
[48] LIN Y C, LIU Z H, CHEN X M, CHEN J. Uniaxial ratcheting and fatigue failure behaviors of hot-rolled AZ31B magnesium alloy under asymmetrical cyclic stress-controlled loadings [J]. Materials Science and Engineering A, 2013, 573: 234-244.
[49] LIN Y C, LIU Z H, CHEN X M, CHEN J. Stress-based fatigue life prediction models for AZ31B magnesium alloy under single-step and multi-step asymmetric stress-controlled cyclic loadings [J]. Computational Materials Science, 2013, 73: 128-138.
[50] CHEN X M, LIN Y C, CHEN J. Low-cycle fatigue behaviors of hot-rolled AZ91 magnesium alloy under asymmetrical stress-controlled cyclic loadings [J]. Journal of Alloys and Compounds, 2013, 579: 540-548.
[51] LIN Y C, CHEN X M, LIU Z H, CHEN J. Investigation of uniaxial low-cycle fatigue failure behavior of hot-rolled AZ91 magnesium alloy [J]. International Journal of Fatigue, 2013, 48: 122-132.
[52] NAN Z, ISHIHARA S, GOSHIMA T, NAKANISHI R. Fatigue behavior of AZ31 extruded magnesium alloy in laboratory air [J]. Transactions of the Japan Society of Mechanical Engineers A, 2004, 70(696): 1146-1152.
[53] YANG F, YIN S M, LI S X, ZHANG Z F. Crack initiation mechanism of extruded AZ31 magnesium alloy in the very high cycle fatigue regime [J]. Materials Science and Engineering A, 2008, 491(1): 131-136.
[54] HUPPMANN M, LENTZ M,  K, REIMERS W. Fatigue properties of the hot extruded magnesium alloy AZ31 [J]. Materials Science and Engineering A, 2010, 527(21): 5514-5521.
K, REIMERS W. Fatigue properties of the hot extruded magnesium alloy AZ31 [J]. Materials Science and Engineering A, 2010, 527(21): 5514-5521.
[55] OCHI Y, MASAKI K, HIRASAWA T, WU X R. High cycle fatigue property and micro crack propagation behavior in extruded AZ31 magnesium alloys [J]. Materials Transactions, 2006, 47(4): 989-994.
贺秀丽1,卫英慧1,2,侯利锋1,闫志峰1,郭春丽1,韩鹏举1
1. 太原理工大学 材料科学与工程学院,太原 030024;
2. 吕梁学院 化学系,离石 033000
摘 要:研究环氧涂层处理后的Mg-3Al-1Zn合金在齿轮油环境下的腐蚀疲劳行为。采用扫描电子显微镜(SEM)观察疲劳试验后试样的腐蚀形貌及疲劳断口特征,并通过能谱仪(EDS)分析试样在齿轮油中的腐蚀产物成分。分析环氧涂层处理前、后2种试样的腐蚀疲劳性能和疲劳裂纹萌生机制。结果表明:经环氧涂层处理后,试样在齿轮油中的腐蚀疲劳极限高于未处理试样的。这是由于环氧涂层可以将镁合金与周围腐蚀环境良好地隔离。环氧涂层的力学性能比镁合金的差,这是疲劳裂纹优先从环氧涂层萌生的重要原因。另外,齿轮油的润滑作用可以使环氧涂层产生剥落现象。
关键词:镁合金;齿轮油;环氧涂层;腐蚀疲劳;疲劳极限;疲劳裂纹萌生
(Edited by Xiang-qun LI)
Foundation item: Projects (51001079, 21201129, 51208333, 51374151) supported by the National Natural Science Foundation of China; Project (201101102002) supported by the Natural Science Foundation of Shanxi Province, China; Project (20100471586) supported by the China Postdoctoral Science Foundation; Project (20091402110010) supported by the Doctoral Fund of Ministry of Education of China
Corresponding author: Ying-hui WEI; Tel: +86-351-6018685; E-mail: weiyinghui@tyut.edu.cn
DOI: 10.1016/S1003-6326(14)63486-5

