DOI:10.19476/j.ysxb.1004.0609.2019.01.21
不锈钢硫酸洗涤废酸中金属离子的草酸络合沉淀行为
张健1, 2,朱兆武1, 2,王丽娜1, .2,易爱飞1, 2,齐涛1, 2
(1. 中国科学院 过程工程研究所 湿法冶金清洁生产技术国家工程实验室,北京 100190;
2. 中国科学院 绿色过程与工程重点实验室,北京 100190)
摘 要:不锈钢表面处理常采用稀硫酸溶液预洗涤,洗涤过程产生大量含铁及其他微量元素的废酸。实际生产中废酸多采用中和方法进行处理,试剂耗量大,产生大量废渣,污染环境。针对典型不锈钢硫酸预洗涤过程中产生的废酸,研究废酸体系中草酸络合沉淀Fe(II)、常见微量金属Cr(III)、Ni(II)以及Mn(II)的行为,并探讨沉淀去除金属离子后废酸的循环利用。结果表明:随着废酸溶液中酸度的增高,金属沉淀率降低,但在起始H2SO4浓度<200 g/L、x(H2C2O4):x(Fe(II))摩尔比为1:1下,Fe(II)的沉淀率仍达80%以上;金属离子的沉淀顺序为:Ni(II)>Fe(II)>Mn(II)>>Cr(III);金属离子沉淀后废酸溶液中酸度得到有效恢复,经x(H2C2O4):x(Fe(II))>0.75:1处理后的废酸对不锈钢表面氧化层具有较好的洗涤效果,实现了不锈钢洗涤废酸的再生循环利用。
关键词:不锈钢洗涤;硫酸废酸;草酸;沉淀法;循环利用
文章编号:1004-0609(2019)-01-0179-08 中图分类号:TQ09 文献标志码:A
不锈钢材具有优异的力学性能、抗腐蚀性能以及可加工性能,已广泛用于生活器具、机械、建筑、化工、军工等领域[1]。近年来,不锈钢材的年消费量逐年递增,我国不锈钢产量也迅速增长,年增长率为4.17%,2017年上半年我国不锈钢粗钢产量达1225.6万t[2]。随着不锈钢需求量的迅速增长,不锈钢行业所带来的环保压力也大大加重。不锈钢生产需要酸洗进行表面处理,酸洗主要分为冷轧热退火酸洗线和冷退火酸洗线,包括多个工艺段[3-4]。其中常常需要预酸洗工艺,该工艺多采用180~220 g/L的硫酸去除不锈钢表面的氧化层[5-6]。不锈钢预酸洗产生的废硫酸溶液中含有大量的Fe,主要以Fe(II)形式存在,通常还含有其他微量金属元素,如Cr(III)、Ni(II)、Mn(II)等。该类废酸产生量大(1.15 m3/t)、污染严重、难处理[6]。
随着环保力度加大,开发清洁、高效的不锈钢废酸处理技术势在必行。近年来,不锈钢洗涤废酸综合利用以及废酸循环再利用成为研究的热点[7-9]。代表性的研究大致可分为三类[7-11]:1) 酸回收循环利用技术,主要包括扩散渗析法、双极膜电渗析法、蒸发法、树脂吸附法等;2) 金属离子回收技术,典型工艺为选择性沉淀技术;3) 酸和金属离子综合回收技术,包括热解法、纳滤-结晶法等。其中,部分工艺已经得到工业化应用。但是,大多数工艺存在流程复杂、技术不成熟、成本高等问题,尚未得到大规模的生产应用[12]。目前,不锈钢生产企业针对硫酸体系废酸主要采用中和沉淀法[13-15],该方法采用石灰或石灰石直接中和处理,试剂耗量大、成本高,而且产生大量的废渣,该类废渣处理难度大、污染严重,废酸无法回用,严重制约了不锈钢行业的发展。
本文作者针对典型不锈钢硫酸洗涤工艺产生的废酸,提出草酸沉淀去除废酸中金属离子的方法,研究了草酸络合沉淀除去大量的Fe(II)离子以及其他金属离子的行为,探讨了金属离子沉淀后酸液的循环利用,以及金属离子沉淀产物的高附加值利用,确定了硫酸洗涤废液资源综合利用的新方法。
1 实验
1.1 模拟不锈钢硫酸洗涤废液的配制
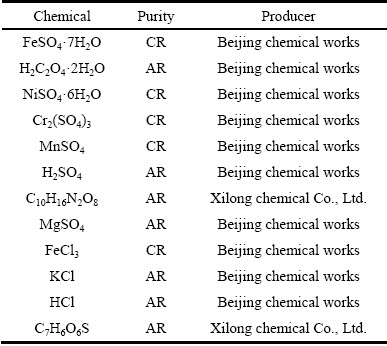
根据文献[5, 16]报道的不锈钢预酸洗硫酸废液的组成以及某企业硫酸洗涤工段产生的废液成分配制了模拟废酸料液,其组成为:Fe2+,70 g/L;H2SO4,120 g/L(酸度影响除外)。同时添加了0.5 g/L的Cr(III)、Mn(II)、Ni(II)离子作为示踪元素。料液配制过程中使用的化学试剂见表1,溶液采用去离子水配制。
表1 配制模拟废酸料液使用的主要试剂
Table 1 Main chemical reagents for preparation of waste acid solutions
1.2 实验步骤
取50 mL模拟废酸料液置于200 mL锥形瓶中,按照设定的x(H2C2O4):x(Fe)加入所需量的H2C2O4·2H2O,将锥形瓶放在恒温水浴振荡器上振荡(温度波动范围±1 ℃)30 min使沉淀达到平衡,体系产生黄色沉淀,真空过滤。固体在100 ℃下干燥10 h。
1.3 溶液中金属离子浓度分析
取适量的溶液样品用去离子水稀释到合适的倍数,在电感耦合等离子发射光谱(ICP-OES,美国Pekin-Elmer公司,Optima 5300V)上分析溶液中各金属离子的质量浓度。
1.4 沉淀固体样品分析
干燥后的沉淀固体样品分别采用X射线荧光光谱仪(XRF,PANalytical B.V公司,AXIOS-MAX)以及X射线衍射仪(XRD,PANalytical B.V公司,X′Pert PRO MPD)测定固体沉淀的组成以及晶体结构。
1.5 溶液中自由酸度测定
根据文献[17-18],采用EDTA-Mg溶液作掩蔽剂,通过固定pH点滴定方法测定酸溶液中的自由酸度。具体步骤如下:首先配制0.2 mol/L EDTA-Na溶液(pH=7.0),然后加入MgSO4使其浓度为0.1 mol/L。采用200 g/L H2SO4溶液以及NaOH溶液调节溶液的pH=6.5得到EDTA-Mg溶液备用。
取20 mL配制好的EDTA-Mg溶液,加入0.5 mL样品,用0.1 mol/L标准NaOH溶液滴定,直到溶液的pH=6.5时,滴定结束。根据消耗的标准NaOH溶液的体积计算样品溶液中的H+浓度,得到的浓度即为溶液的自由酸度。
1.6 溶液中草酸的测定
根据文献[19],采用比色法测定沉铁后溶液中残留的H2C2O4。利用Fe3+的磺基水杨酸络合物吸光度随H2C2O4量的增加而降低,测得溶液中的H2C2O4含量。具体步骤如下:首先分别配制0.5 g/L的FeCl3溶液、pH = 2.1的HCl-KCl缓冲液、0.5%磺基水杨酸溶液和2 g/L的Na2C2O4标准溶液。然后在50 mL容量瓶中,先后加入4 mL 0.5 g/L的FeCl3溶液,40 mL pH = 2.1的HCl-KCl缓冲液,2.4 mL 0.5%磺基水杨酸溶液,再分别加入0、0.2、0.4、0.8、1.6 mL 2g/L的Na2C2O4标准溶液,用去离子水定容,显色20 min后在紫外分光光度计(北京莱伯泰科仪器有限公司,UV9100)上于波长510 nm处测定吸光度。绘制H2C2O4浓度与吸光度的标准曲线,拟合出两者的线性关系。再取1 mL待测样品,按上述方法,在相同条件下测定吸光度,根据H2C2O4质量浓度和吸光度的线性关系计算H2C2O4质量浓度。
2 结果与讨论
2.1 强硫酸介质中草酸沉淀Fe、Ni、Mn和Cr
在模拟废酸料液中按不同x(H2C2O4):x(Fe)(少量其他金属没有考虑,下同)加入二水合草酸,室温下搅拌后溶液中产生大量的黄色沉淀,金属沉淀率(p)按式(1)计算,得到的各金属沉淀率见图1。
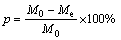 (1)
(1)
式中:M0为金属离子的起始质量浓度;Me为金属离子在沉淀平衡残液中的质量浓度。
由图1可以看出,随着H2C2O4加入量的增加,草酸与Fe的摩尔比增大,Fe、Ni、Mn的沉淀率均增大。当加入的H2C2O4和Fe的摩尔比从0.5:1提高到1:1时,Fe、Ni、Mn沉淀率迅速增大,Fe的从53.04%增大到90.73%、Ni的从63.63%增大到95.19%、Mn的从37.24%增大到69.05%。继续加入H2C2O4,提高草酸和Fe的摩尔比,除Cr外三种金属的沉淀率增加缓慢。Cr的沉淀率较低,而且随H2C2O4加入量的增大变化不明显。金属沉淀的顺序为:Ni(II)>Fe(II)>Mn(II)>>Cr(III)。
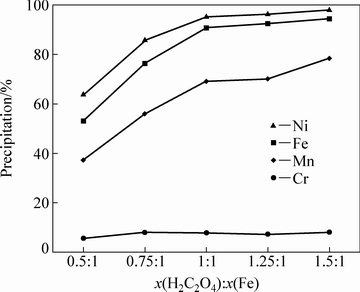
图1 草酸加入量对金属离子沉淀率的影响
Fig. 1 Effect of oxalic acid addition on metal precipitation
二价金属离子,包括Fe(II)、Ni(II)、Mn(II)与C2O42-配位形成难溶的草酸盐结晶,随着结晶数的增加,形成的草酸盐溶解度降低,PARCKTER[20]在pH=6~7,测得金属离子和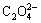 形成晶体结晶数的顺序为:Mn(II)<Fe(II)<Ni(II)。因此,溶解度顺序为:NiC2O4<FeC2O4<MnC2O4,这个顺序与本研究在高浓度H2SO4溶液中测得的沉淀率顺序一致。H2C2O4与二价金属离子通过络合反应(如式(2),(3)所示,M表示金属元素)形成不溶的沉淀结晶。
形成晶体结晶数的顺序为:Mn(II)<Fe(II)<Ni(II)。因此,溶解度顺序为:NiC2O4<FeC2O4<MnC2O4,这个顺序与本研究在高浓度H2SO4溶液中测得的沉淀率顺序一致。H2C2O4与二价金属离子通过络合反应(如式(2),(3)所示,M表示金属元素)形成不溶的沉淀结晶。
H2C2O4=2H++C2O42- (2)
M2++C2O42-=MC2O4 (3)
随着H2C2O4的加入,H2C2O4根浓度增大,金属的沉淀率升高。金属沉淀后对溶液中剩余H2C2O4的质量浓度进行了计算(草酸加入量减去金属沉淀的消耗量)和实际测定,如表2所列。可以看出,测定的值与理论计算值非常接近。二价金属与H2C2O4形成1:1摩尔比的络合物沉淀,当H2C2O4加入量小于沉淀反应所需要的理论用量时,H2C2O4基本与二价金属离子(Fe,Ni,Mn)形成沉淀,剩余H2C2O4的浓度很低。因此,控制H2C2O4的加入量在理论用量以内,可以大大减少残液中H2C2O4的剩余量。
随着金属草酸盐的沉淀,溶液中的自由酸度升高。按总的式(4)计算,不同H2C2O4加入量时溶液的自由酸测定值和计算值见图2。
表2 金属离子沉淀过程中H2C2O4剩余量
Table 2 Remaining oxalic acid in solution after metal precipitation
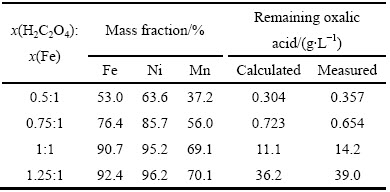
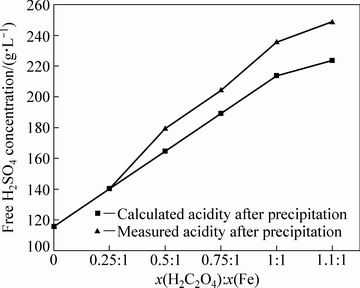
图2 溶液中自由酸度随H2C2O4加入量的变化
Fig. 2 Variation of free acid concentration with oxalic acid addition
H2C2O4+MSO4=MC2O4+H2SO4 (4)
实测的自由H2SO4浓度和计算的值基本相符,金属离子沉淀去除后体系的酸度得到很好的恢复,可以返回不锈钢的洗涤段循环使用。
由图1可以看出,Cr的沉淀很少(<10%),而且随H2C2O4加入量变化不大。研究表明[21],H2C2O4与Cr(III)在较高pH(>2)下形成可溶性配合物,低pH下Cr(III)不与H2C2O4发生反应。废酸中的酸度较高,因此,在废酸体系加入H2C2O4对Cr(III)没有去除效果,废酸中的Cr可以在循环积累达到较高浓度后进行分流处理。实验中少量Cr的沉淀可能是由于沉淀物表面吸附引起的,但实际结果还需进一步研究证实。
另外,如果废酸中含有Fe(III),同样会与H2C2O4形成可溶的配合物,可以用铁粉将Fe(III)还原为Fe(II)进行沉淀除去。
2.2 硫酸浓度对Fe、Ni、Mn和Cr除去效果的影响
不锈钢洗涤工艺不同,产生的废酸酸度也不同[16],在金属离子浓度不变的条件下,加入理论所需量的H2C2O4,测定了金属离子在不同酸度下的沉淀效果(见图3)。由图3看出,随起始硫酸浓度的增大,Fe、Ni、Mn三种金属离子的沉淀率逐渐减小,当起始H2SO4质量浓度小于200 g/L时沉淀率下降缓慢,尤其是Fe和Ni分别从起始酸度为50 g/L的89.38%、96.07%下降到起始酸度为200 g/L的80.47%、89.14%,下降幅度约为10%。但是,当起始H2SO4质量浓度提高到300 g/L时,金属离子的沉淀率有较大幅度的下降,Fe、Ni和Mn的沉淀率分别只有46.65%、65.19%和5.56%,因此在很高酸度下,H2C2O4沉淀除去二价金属离子的效果将大大减弱。
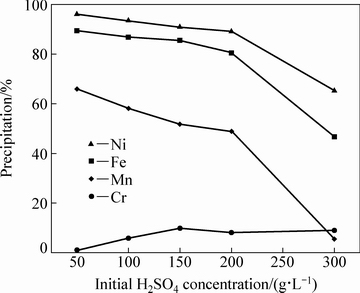
图3 起始H2SO4质量浓度对金属离子沉淀率的影响
Fig. 3 Effect of initial H2SO4 concentration on metal precipitation (x(H2C2O4):x(Fe)=1:1)
H2C2O4是一种弱酸,随着溶液中H2SO4浓度增大,式(2)中平衡向左移动,抑制了H2C2O4的水解,因此增大了草酸盐的溶解度。表3所示为不同起始H2SO4浓度下沉淀平衡后溶液中的平衡H2SO4浓度,并测定了金属离子的浓度。根据溶液中铁离子的质量浓度,推测了不同H2SO4浓度下FeC2O4的溶解度(见图4)。李唤民等[22]测定了FeC2O4在不同稀H2SO4溶液中的溶解度,15 ℃和25 ℃下的测定值与本实验在常温下(20 ℃)得到的值示于图4(其中,FeC2O4浓度由溶液中Fe浓度计算得到)。由图4可以看出,在可比较的H2SO4浓度范围内(150~200 g/L),实验得到的FeC2O4溶解度略低于文献报道的结果,但非常相近。在平衡H2SO4浓度小于300 g/L时,FeC2O4溶解度较小,Fe(II)具有较大的沉淀率。
表3 沉淀平衡后H2SO4质量浓度与金属离子的浓度
Table 3 Equilibrium concentrations of H2SO4 and metal ions after precipitation
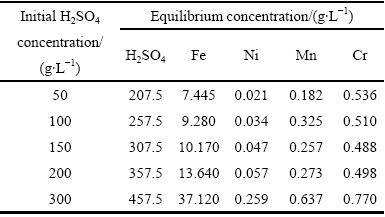
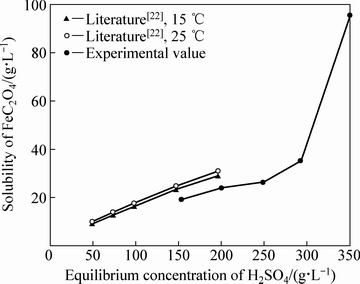
图4 FeC2O4在不同稀H2SO4浓度溶液中的溶解度
Fig. 4 Solubility of ferrous oxalate in diluted sulphuric acid solutions (values obtained in this work and reported in literature)
Cr(III)的沉淀率在测定的H2SO4浓度范围内仍较小,但随酸度增高有略有增大的趋势。考虑到Cr(III)在高酸度下不与H2C2O4发生络合反应,沉淀率的增加可能是因为高 硫酸根浓度下形成的Cr2(SO4)3更易被FeC2O4沉淀吸附,但实际结果需要进一步研究。
硫酸根浓度下形成的Cr2(SO4)3更易被FeC2O4沉淀吸附,但实际结果需要进一步研究。
2.3 温度对Fe、Ni、Mn和Cr除去效果的影响
在加入理论用量的H2C2O4实验条件下,测定了不同温度下金属在模拟废酸料液中的沉淀率(如图5所示),当温度从30 ℃提高至40 ℃时,Fe、Ni和Mn的沉淀率略有升高,分别从80.47%、89.14%和48.93%升高至86.57%、95.36%和64.42%。再继续提高温度至60 ℃时,各金属的沉淀率基本不变。温度对金属草酸盐沉淀的影响比较复杂,可能主要受两方面因素影响,一是H2C2O4的解离度;二是金属草酸盐的溶解度。随温度升高,H2C2O4解离度增大[23-24],有利于金属草酸盐沉淀,而金属草酸盐溶解度随温度升高而增大[22],又抑制了金属的沉淀。因此,在30 ℃到40 ℃之间,第一方面的因素可能起了主导作用,使金属沉淀率略有增加,而继续升高温度,两方面的因素相互抵消,金属沉淀率不再有明显的变化。Cr(III)的沉淀率在30~60 ℃的温度范围内基本为8%左右。
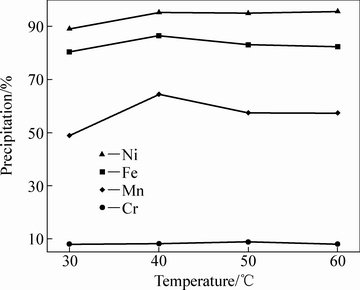
图5 温度对金属离子沉淀率的影响
Fig. 5 Effect of temperature on the metal precipitation
2.4 沉淀物分析
在模拟废酸料液中,用x(H2C2O4) : x(Fe(II))=1:1 在室温下沉淀,得到的黄色固体过滤烘干(见图6),XRF测定固体中金属的组成(见表4),XRD测定固体样品的晶体结构(见图7)。
从表4的结果可知,沉淀固体中主要金属离子是Fe离子,这是因为模拟料液中金属离子主要为Fe(II)。由于Ni的沉淀率较高,在沉淀固体中测得了一定量的Ni,Mn的沉淀率较低,固体中含量较少。由于Cr的沉淀率太小,在固体中没有测到Cr的量。根据金属的沉淀率对固体金属组成进行了计算也列于表4,测得的组成与计算得到的组成基本一致。

图6 沉淀固体干燥后的样品
Fig. 6 Precipitate sample after drying
表4 沉淀固体组成XRF分析结果
Table 4 Composition analysis of precipitate sample by XRF
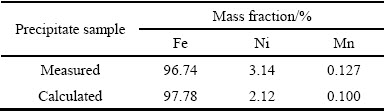
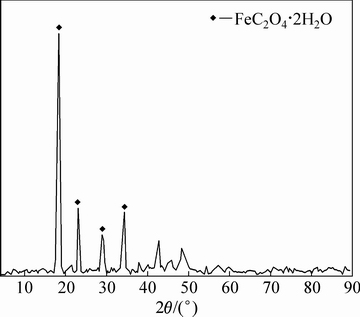
图7 沉淀固体样品的XRD谱
Fig. 7 XRD pattern of precipitate sample
根据图7的XRD衍射结果,得到的结晶固体主要为FeC2O4·2H2O。尽管不锈钢成分不同,洗涤液中金属离子的组成会有变化,但一般Fe离子浓度较高, 沉淀得到的FeC2O4纯度较高,有可能作为制备高附加值铁产品的原料,如LiFePO4、Fe2O3颜料等。FeC2O4加热可生产氧化铁,而其中的微量金属Ni、Mn、Cr等又是微合金必须的元素,因此,得到的沉淀加热分解后可以作为微合金的原料加以回收利用,从而提高处理过程的产品附加值,同时消除废渣的环境污染。
2.5 处理后酸溶液的循环利用
不锈钢硫酸预洗涤主要目的是除去表层的氧化 层[4-5],洗酸中的酸度是决定洗涤效果的主要因素,自由酸度越高,洗涤效果越好。洗涤过程酸洗液不断循环利用,金属离子在洗液中不断积累,自由酸逐渐被金属离子消耗,酸洗效果下降。通常200 g/L的洗酸在消耗掉40%~50%时,金属离子接近饱和,洗酸基本失去效果,需要进行处理[15]。经过草酸处理,金属离子形成草酸盐沉淀,自由酸得到释放(如式(4)所示),因此废酸溶液处理后酸度可以得到很好的恢复,从而恢复溶液的洗涤效果,使其能够循环利用。为验证处理后的废酸循环利用的洗涤效果,分别取200 mL未处理的某企业硫酸洗涤工段产生的硫酸废液(废酸溶液组成:Fe(II),71 g/L;H2SO4,125 g/L;Cr(III),0.7 g/L)。用x(H2C2O4):x(Fe(II))分别为0.5:1、0.75:1和1:1处理后的硫酸溶液洗涤不锈钢片样品,样品编号依次为S-0、S-1、S-2和S-3,洗涤效果如图8所示。
根据图8的不锈钢表面情况,对比各不锈钢片表面氧化层残留情况可知,未处理的废酸溶液洗涤效果较差,用草酸络合沉淀法处理后的废酸循环利用洗涤效果明显增强,且随H2C2O4与Fe摩尔比的增加,废酸循环洗涤效果逐渐变好,同时不锈钢表面未留下明显的破坏痕迹。根据实验结果观察,以x(H2C2O4):x(Fe(II))为0.75:1处理的废酸洗涤后的不锈钢片已没有明显的氧化层,洗涤效果能够达到实际生产的需求,因此在废酸处理过程中,H2C2O4的用量可低于金属沉淀的理论用量,减少H2C2O4在溶液中的残余量。

图8 不同不锈钢片废酸处理后的洗涤效果对比
Fig. 8 Comparison of different stainless steel washing effect
3 结论
1) H2SO4起始浓度<200 g/L的不锈钢酸洗液中加入H2C2O4可以有效去除Fe(II)、Ni(II)和Mn(II),当H2C2O4加入量接近金属沉淀理论用量时,Fe(II)的沉淀率>80%,金属离子的沉淀顺序为:Ni(II)>Fe(II)>Mn(II)>>Cr(III)。
2) 沉淀后得到的固体产物主要成分为FeC2O4·2H2O,结晶度和纯度较高,具有深加工利用的潜能。
3) 废酸溶液中金属离子沉淀去除后酸度得到有效恢复,处理后的酸溶液可以循环利用,实现了废酸的清洁化循环利用。
4) 废酸处理时,H2C2O4为金属沉淀所需用量的0.75倍时,可以较好地恢复洗涤效果,废酸处理后H2C2O4残余量少,不影响不锈钢的洗涤效果,也不会对不锈钢表面造成新的破坏。
致谢:感谢中国科学院过程工程研究所人才计划提供的资金支持,河南金汇不锈钢产业集团提供的实验样品。
REFERENCES
[1] 肖纪美. 不锈钢的金属学问题[M]. 北京: 冶金工业出版社, 2006: 1.
XIAO Ji-mei. Metallograpy problems of stainless steel[M]. Beijing: Metallurgical Industry Press, 2006: 1.
[2] 中国特钢企业协会不锈钢分会. 2017年1-6月中国不锈钢粗钢生产、进出口、表观消费量的统计数据[Z]. 2017.
Stainless Steel Council of China Special Steel Enterprises Association. Statistic of the production, import, export and apparent consumption of crude stainless steel in China during January to June, 2017 [Z]. 2017.
[3] 陈治国, 简小龙, 吴 栋. 不锈钢冷轧含酸、含铬废水处理重金属污泥回收技术研究[J]. 工业水处理, 2013, 33(6): 93-95.
CHEN Zhi-guo, JIAN Xiao-long, WU Dong. Research on the recycling of sludge containing heavy metal for the treatment of stainless steel cold-rolling wastewater with acid and chromium[J]. Industrial Water Treatment, 2013, 33(6): 93-95.
[4] 师培俭. 不锈钢超滤膜在处理太钢冷轧乳化液废水中的应用[J]. 山西冶金, 2014, 37(5): 59-60.
SHI Pei-jian. Stainless steel ultrafiltration membrane used in cold rolling emulsion wastewater treatment[J]. Shanxi Metallurgy, 2014, 37(05): 59-60.
[5] 张 颖, 李慎松. 国外不锈钢酸洗技术[J]. 金属制品, 2012, 38(1): 21-29.
ZHANG Ying, LI Shen-song. Overseas stainless steel pickling technology[J]. Metal Products, 2012, 38(1): 21-29.
[6] 张 颖. 国内不锈钢酸洗技术[J]. 金属制品, 2011, 37(5): 37-41.
ZHANG Ying. Domestic stainless steel pickling technology[J]. Metal Products, 2011, 37(5): 37-41.
[7] GHARE N Y, WANI K S. A review on methods of recovery of acid(s) from spent pickle liquor of steel industry[J]. Environmental Engineering Science, 2013, 55(2): 253-266.
[8] ROGENER F, SARTOR M, BAN A, BUCHLOH D, REICHARDT T. Metal recovery from spent stainless steel pickling solutions[J]. Resources, Conservation and Recycling, 2012, 60: 72-77.
[9] 谢明森, 高远飞, 张晓丽. 不锈钢酸洗废液中金属的综合利用[J]. 广州化工, 2015, 43(9): 109-111.
XIE Ming-sen, GAO Yuan-fei, ZHANG Xiao-li. Comprehensive utilization of the metals in waste stainless steel pickling liquor[J]. Guangzhou Chemical Industry, 2015, 43(9): 109-111.
[10] 高 亮. 不锈钢酸洗废水处理中的污泥减排技术[J]. 中国给水排水, 2009, 25(10): 83-86.
GAO Liang. Sludge reduction technology in stainless steel acid cleaning wastewater treatment[J]. China Water & Wastewater, 2009, 25(10): 83-86.
[11] REGEL-ROSOCKA M A. Review on methods of regeneration of spent pickling solutions from steel processing[J]. Journal of Hazardous Materials, 2010, 177(1/3): 57-69.
[12] 王绍文, 邹元龙, 杨晓莉. 冶金工业废水处理技术及工程实例[M]. 北京: 化学工业出版社, 2009.
WANG Shao-wen, ZOU Yuan-long, YANG Xiao-li. Wastewater treatment technologies in metallurgical industries and typical practices[M]. Beijing: Chemical Industry Press, 2009.
[13] 李圭白, 张 杰. 水质工程学[M]. 北京: 中国建筑工业出版社, 2005.
LI Gui-bai, ZHANG Jie. Water quality engineering[M]. Beijing: China Construction Industry Press, 2005.
[14] 何 慧, 赵俊学, 马红周, 李小明. 不锈钢酸洗废水处理技术分析[J]. 甘肃冶金, 2009, 31(5): 42-46.
HE Hui, ZHAO Jun-xue, MA Hong-zhou, LI Xiao-ming. Technology analysis on treatment of stainless steel pickling waste liquors[J]. Gansu Metallurgy, 2009, 31(5): 42-46.
[15] 阎 震. 石灰中和法在不锈钢酸洗液处理中的优化设计[J]. 给水排水, 2013, 39(4): 70-72.
YAN Zhen. Optimal design of the neutralization method with lime in the treatment of acidic solution discharged from stainless steel pickling process[J]. Water & Wastewater Engineering, 2013, 39(4): 70-72.
[16] 晁 兵, 陆东方. 钢材的酸洗处理[J]. 涂装与电镀, 2009, 3: 19-21.
CHAO Bing, LU Dong-fang. The acid dipping treatment for steel materials[J]. Painting & Electroplating, 2009, 3: 19-21.
[17] ROLIA E, DUTRIZAC J E. The determination of free acid in zinc processing solutions[J]. Canadian Metallurgical Quarterly, 1984, 23(2): 159-167.
[18] ZHU Z, BIAN Z, LONG Z. Determination of free acid in rare earth solution by a fixed pH method[J]. Analytical Methods, 2010, 2(1): 82-85.
[19] 段立珍, 汪建飞, 赵建荣. 比色法测定菠菜中草酸含量的条件研究[J]. 安徽农业科学, 2007, 35(3): 632-633.
DUAN Li-zhen, WANG Jian-fei, ZHAO Jian-rong. Study on the determination of oxalate content in spinach with coloration method[J]. Agricultural Sciences, 2007, 35(3): 632-633, 643.
[20] PARKTER A. The precipitaiton of transition metal oxalate powders from aqueous solution: Crystal numbers and final sizes[J]. Kristall und Technik, 1976, 11: 1131-1138.
[21] 田 荟, 付丽红. 草酸与铬配位影响因素[J]. 皮革科学与工程, 2010, 20(3): 23-27.
TIAN Hui, FU Li-hong. Study on influence factors on the coordination reaction between oxalic acid and chromium complex[J]. Leather Science and Engineering, 2010, 20(3): 23-27.
[22] 李唤民, 沈志亚. 草酸亚铁在稀硫酸中溶解度的测定[J]. 化学世界, 1965, 12: 552-553.
LI Yu-min, SHEN Zhi-ya. Determination of the solubility of ferrous oxalate in diluted sulfuric acid solutions[J]. Chemical World, 1965, 12: 552-553.
[23] PINCHING G D, BATES R G. Second dissociation constant of oxalic acid from 0 ℃ to 50 ℃, and the pH of certain oxalate buffer solutions[J]. Journal of Research National Bureau of Standard, 1948, 40: 405-416.
[24] KETTLER R M, PALMER D A, WESOLOWSKI D J. Dissociation quotients of oxalic acid in aqueous sodium chloride media to 175 ℃[J]. Journal of Solution Chemistry, 1991, 20(9): 905-927.
Behaviors of metal complex precipitation with oxalic acid in waste sulfuric acid solutions from stainless steel washing processes
ZHANG Jian1, 2, ZHU Zhao-wu1, 2, WANG Li-na1, 2, YI Ai-fei1, 2, QI Tao1, 2
(1. National Engineering Laboratory for Hydrometallurgical Cleaner Production Technology, Institute of Process Engineering, Chinese Academy of Sciences, Beijing 100910, China;
2. Key Laboratory of Green Process and Engineering, Chinese Academy of Sciences, Beijing 100910, China)
Abstract: Sulfuric acid solution was commonly used for the surface pre-washing in the process of stainless steel treatment to remove the metal oxide layer. Large amounts of waste acid solutions containing high concentrations of iron and low concentrations of other metals were discharged in the process. Neutralization method was widely used in industrial practices, resulting in high reagent consumption, large amounts of solid waste discharge and serious environmental pollution. With respect to the typical waste sulfuric acid solutions, the complex precipitation behaviors of Fe(II) and some commonly found trace metals, such as Cr(III)、Ni(II) and Mn(II), with oxalic acid were investigated. The recycling of acid solutions after metal removal was also discussed. The results show that the metal precipitation decreases with the increase of the acid concentration. As the initial acid concentration is less than 200 g/L, the precipitation of Fe(II) still reaches more than 80%. The metal precipitation order is: Ni(II)>Fe(II)>Mn(II)>>Cr(III). After metal removal by precipitation, the acid concentration in the solution increases, restoring the pre-washing effectiveness. The waste acid solutions are treated using H2C2O4:Fe (II) molar ratio higher than 0.75:1, good pre-washing effectiveness can be achieved. Therefore, the waste acid can be well recycled after metal precipitation using oxalic acid.
Key words: stainless steel washing; waste sulfuric acid; oxalic acid; precipitation; recycling
Foundation item: Projects(21506233, 21606241, 51402303) supported by the National Natural Science Foundation of China; Project(QYZDJ-SSW-JSC021) supported by Key Research Program of Frontier Sciences of Chinese Academy of Sciences; Project(KFJ-SW-STS-148) supported by Science and Technology Service Network Initiative, China; Project(201509053) supported by Nonprofit Industry Research Subject of Environmental Protection, China
Received date: 2017-11-21; Accepted date: 2018-01-24
Corresponding author: ZHU Zhao-wu; Tel: +86-10-82244847; E-mail: zhwzhu@ipe.ac.cn
(编辑 王超)
基金项目:国家自然科学基金资助项目(21506233,21606241,51402303);中国科学院前沿科学重点研究项目(QYZDJ-SSW-JSC021);中国科学院科技服务网络计划(STS计划)(KFJ-SW-STS-148);环保公益性行业科研专项(201509053)
收稿日期:2017-11-21;修订日期:2018-01-24
通信作者:朱兆武,研究员,博士;电话:010-82244847;E-mail:zhwzhu@ipe.ac.cn