Article ID: 1003-6326(2005)06-1380-08
Non-flammable electrolytes based on trimethyl phosphate solvent for lithium-ion batteries
HU Chuan-yue(胡传跃)1, 2, LI Xin-hai(李新海)2
(1. School of Metallurgical Science and Engineering, Central South University,
Changsha 410083, China;
2. Department of Chemistry and Material Science,
Hunan Institute of Humanities, Science and Technology, Loudi 417000, China)
Abstract: The properties of trimethyl phosphate(TMP)-based nonflammable electrolytes with LiPF6 as solute were investigated using graphite anode and LiCoO2 cathode. The effect of TMP on non-flammability of electrolytes was also evaluated. It is found that the TMP reduction decomposition on graphite electrode at the potential of 1.3V (vs Li/Li+) is suppressed with ethylene carbonate(EC), dimethyl carbonate(DMC) and ethylmethyl carbonate(EMC) cosolvents and vinylene carbonate(VC) additives. The results show that the non-flammable electrolyte of 1mol/L LiPF6 61%(EC1.5-DMC1.0-EMC1.0)-39% TMP has good electrochemical properties. The discharge capacities of half-cells after 20 cycles are 254.8mA·h/g for Li/graphite and 144.1mA·h/g for Li/LiCoO2. The graphite/LiCoO2 prismatic lithium-ion cell delivers a discharge capacity of 131mA·h/g at first cycle. With an addition of 4%VC to this non-flammable electrolyte, a discharge capacity of 134mA·h/g at first cycle and a capacity ratio of 84.3% after 50 cycles are obtained for prismatic lithium-ion batteries. Furthermore, a nail penetration test demonstrates that the safety of prismatic lithium-ion batteries is dramatically improved by using TMP-containing non-flammable electrolytes.
Key words: graphite; LiCoO2; nonflammable electrolyte; trimethyl phosphate; lithium-ion battery CLC
number: O646; TM912 Document code: A
1 INTRODUCTION
Lithium-ion rechargeable batteries contain electrolyte solutions composed of Li salts and organic solvents, typically organic carbonates. Almost all these solutions are flammable and may be ignited when hot solvents expelled from the cells comes in contact with oxygen under abusive conditions such as overcharge[1-3]. In extreme case, abused lithium-ion batteries have been reported to combust and explode[4-6].
Some separators have been used to exhibit shutdown performance to protect the batteries against abuse[7]. A few additives are found to be effective in preventing overcharge[8, 9]. One way to reduce electrolyte flammability is to replace liquid electrolytes with solid electrolytes. Many polymer and polymer-gel electrolytes have been investigated and are expected to improve flame resistance[10]. However, the improved flame retardancy was accompanied by degraded battery performance including significantly lower conductivity. Especially at low temperatures electrolytes are not practical for many application where high power and low temperature performance are required.
The combustion of organic solvents is largely vapor phase oxidations, involving active H, OH, and O radicals[11]. Compounds that can trap these active radicals and produce less active radicals result in flame inhibition. When compounds contain more than one element that can retard burning, the non-flammability would be better.
Initial investigations on flame retarding additives for lithium-ion batteries focused on organo phosphorous compounds, in particular, on trialkylphosphates such as trimethyl phosphate(TMP)[11-14] and triethyl phosphate(TEP)[13], fluorinated alkyl phosphates such as tris(2, 2, 2-trifluoroethyl) phosphate(TFP)[14], bis(2, 2, 2-trifluoroethyl)methyl phosphate(BMP)[15, 16], and cyclophosphazenes such as hexamethoxycyclophosphazene(HMPN)[13]. However, none of these materials is ideal enough.Trialkylphosphates-containingcells have problems associated with their reduction on the battery anode. HMPN has good electrochemical stability towards the electrodes but high concentration required for reduction in flammability results in capacity loss during the cell cycling[13].
The question thus arises as how to improve the cycling performance of nonflammable electrolytes. The reduction decomposition of TMP solvent on graphite surface was very similar to propylene carbonate(PC) solvent decomposition. It is significant to refer to the available ways of restraining PC solvent decomposition by adding the functional additives to the electrolytes[17]. It was found that vinylene carbonate(VC) was an effective additive to improve the electrochemical stability of electrolyte by forming a good solid electrolyte interphase (SEI) film on the anode[18-22]. In this paper, the authors mainly characterize the electrochemical stability of 1mol/L LiPF6 TMP-based nonflammable electrolytes by adding cosolvents EC, DMC, EMC and VC additives.
2 EXPERIMENTAL
2.1 Electrolyte preparation
TMP solvent (Shanghai Chemical Reagent Station, with purity higher than 99%) was used after being dehydrated by molecule sieve 0.3nm. No impurity peak was detected using gas chromatography. Vinylene carbonate(VC) (battery grade), ethylene carbonate(EC) (battery grade), dimethyl carbonate(DMC) (battery grade), and ethylmethyl carbonate (EMC) (battery grade) solvents, and LiPF6 solute (battery grade, made in Zhangjiagang Guotai-Huarong New Chemical Materials Co., LTD) were used to prepare electrolyte. The preparation of electrolytes was performed in an argon glove box. The water content in the resultant electrolytes was detected to be less than 20×10-6 by Karl-Fischer titration (Zibo Zhonghui Apparatus Co. LTD, SC-3 Microanalysis Water Apparatus).
2.2 Evaluation of non-flammable electrolyte
Non-flammability of electrolyte was evaluated by analyzing the self-extinguishing time(SET) of electrolyte versus the liquid mass as described previously by Xu et al[15, 16]. A micropipet was used to transfer 100μL electrolyte, with mass of about 125mg. The self-extinguishing time(SET) was obtained by igniting the preweighed electrolyte solutions soaked in a glassy filter ball-wick (about 0.5cm in diameter), followed by recording the time that it took for the flame extinguish and by normalizing the flame burning time against the electrolyte mass. For each sample, the experiment was repeated ten times to provide an average value and standard deviation.
2.3 Electrochemical measurement
The effect of flame retardant on the cell performance was tested on coin cells or prismatic lithium-ion batteries. The composition of cathode was 89% LiCoO2, 4% conductive carbon black, and 7% PVDF binder. The composition of the anode was 92% graphite and 8% PVDF binder. The polymer separator of 20μm thickness was used. The coin cells were prepared in an argon glove box (Nanjing University Experiment Apparatus Factory, China). The designed capacity of prismatic lithium-ion batteries was about 700mA·h. All batteries were tested on a lithium-ion recharge battery testing device (BS9300, Guangzhou Kinte Industrial Co., LTD) at room temperature.
The charge/discharge cycle was galvanostatically carried out at current density of 0.2mA/cm2. The cutoff potentials were set to 0-1.5V for Li/graphite half-cell, and 4.2-2.8V for Li/LiCoO2 half-cell and graphite/LiCoO2 prismatic lithium-ion battery.
The AC impedance method was used to determine the impedance of graphite electrode in Li/graphite half-cell. The frequency was set from 10kHz to 0.01Hz and amplitude was 5mV. The measurement device was CHI 660 Electrochemistry Station (CH Instruments, Inc.)
Reduction stability of the TMP-based electrolyte was investigated by slow scanning cyclic voltammetry. A graphite sheet electrode (1.4cm in diameter) served as working electrode, the lithium electrode served as counter electrode and reference electrode. The potential was initially set to open circuit potential, then scanned to 0V and reversed to 1.5V at scanning rate of 0.04mV/s.
The conductivity measurements were performed with a Metrohm 712 conductivity meter. The cell constant is 1cm-1. The temperature was controlled within about ±0.1℃ at room temperature.
2.4 Safety of prismatic lithium-ion battery
The effect of non-flammable electrolyte on the safety of prismatic lithium-ion batteries was evaluated by the method of nail penetration experiment with a rate of 20mm/s. The diameter of nail was 5 or 2mm. The surface temperature measurements were carried out through two thermocouples. The two thermocouples were distributed as follows: one at the flank and the other at the facade of the prismatic can. The higher temperature tested by the two thermocouples is considered the surface temperature of battery. The thermocouple was bunched to the surface of prismatic lithium-ion battery and enveloped with adiabetics asbestine.
3 RESULTS AND DISCUSSION
3.1 Cyclic voltammetric study
From the viewpoint of safety, it is promising to use TMP as the only solvent that certainly exhibits nonflammability. To get an exact electrochemical picture of the TMP solvent based electrolyte, the slow scan cyclic voltammetric behavior was analyzed, as shown in Fig.1. It is found that the electrolyte with TMP solvent is suffered from reduction decomposition on graphite electrode at potential of about 1.3V (Li/Li+). Considering the good reduction stability of LiPF6 solute, the excessive electrolyte decomposition may only attribute to the poor reduction stability of the TMP solvent. As the potential further scans negatively to 1.1V, the gas bubble is observed on the graphite electrode surface, which indicates the gas generation along with TMP solvent decomposition. The cathodic peaks exist in the potential range from 0.85 to 0.40V corresponding to the cointercalation of TMP solvent due to the failure of solid electrolyte interphase(SEI) film formation, similar with the reduction decomposition of TMP solvent on the natural graphite electrode[11]. No anodic peak is observed, suggesting that lithium intercalation fails in the charging process.
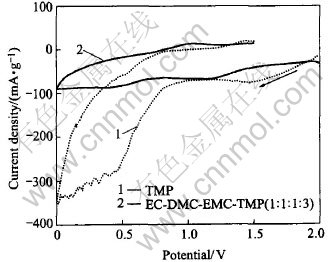
Fig.1 Slow cylic voltammograms of graphite electrodes in 0.5mol/L LiPF6 electrolytes with TMP solvent and EC-DMC-EMC-TMP (1∶1∶1∶3) mixture solvents
On the other hand, when the electrolyte with EC-DMC-EMC-TMP (1∶1∶1∶3, mass ratio) is used as mixture cosolvents, the reduction decomposition and cointercalation of TMP solvent obviously decrease. The result shows that the reduction decomposition of TMP solvent may be suppressed by using EC-DMC-EMC as cosolvents.
3.2 Influence of TMP on non-flammability of electrolytes
It has been well known that the use of cosolvents may dramatically reduce the TMP-based electrolyte decomposition[11]. Therefore, in order to suppress the reduction decomposition of the TMP solvent, the common C-H-O solvents were considered the candidates of cosolvents for TMP solvent. It is found that the nonflammability of mixed electrolytes is strongly influenced by the type of C-H-O solvent. The lowest TMP contents needed to maintain the nonflammability of some binary mixed electrolytes with 1mol/L LiPF6 as solute are listed in Table 1. The lowest TMP content needed to maintain the non-flammability of electrolytes increases in the following order: EC〈PC〈GBL〈DMC〈DEC〈EMC.
Table 1 Lowest TMP content needed to maintain non-flammability of 1mol/L LiPF6/cosolvent and TMP binary mixed electrolyte(mole fraction, %)

Previous work shows that longer self-extinguishing times (tSET) are consistent with greater flammability of the liquid solution[11, 14 ]. We define an electrolyte with 67%-reduced flammability as a flame retarded electrolyte, and that with 90%-reduced flammability as a non-flammable electrolyte. The relative values of 1-tSET/tSET0, where SET0 is the SET of the solution without TMP as presented in Fig.2. For the presented solutions of ECn-DMC1.0-EMC1.0, tSET0≈35s. The ECn-DMC1.0-EMC1.0 cosolvent means that the mass ratio of EC∶DMC∶EMC was n∶1∶1. Additions of TMP to solutions of ECn-DMC1.0-EMC1.0 provide gradual loss of electrolyte flammability. The electrolytes became non-flammable when adding 44%, 39%, 35% and 29% of TMP to ECn-DMC1.0-EMC1.0 solutions with n=1.0, 1.5, 2.0 and 2.5. These results suggest that the higher the EC content of ECn-DMC1.0-EMC1.0 is, the less the TMP content needed to convert the electrolyte to non-flammable.
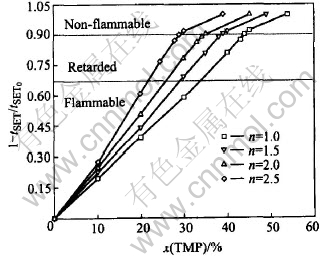
Fig.2 Relative flame retardancy of 1mol/L LiPF6 (1-y%)(ECn-DMC1.0-EMC1.0)- y%TMP electrolyte determined by self-extinguish time(SET)
So far, the physical and chemical mechanisms of flame retarding are as follows. The low boiling phosphorus compounds, such as trialkyl phosphate, were found to act as gas phase flame retardant. This was because a few phosphorus radical species, which was detected by mass spectroscopy, had been verified to scavenge the hydrogen radicals[11].
3.3 Electrochemical performance of non-flammable electrolytes
Fig.3 shows the charge/discharge curves at first cycle of Li/graphite half-cells with 1mol/L LiPF6 (1-y%)(ECn-DMC1.0-EMC1.0)-y% TMP non-flammable electrolytes. It is found that the performances of Li/graphite half-cells were greatly influenced by TMP additive. For example, discharge capacity and coulomb efficiency of Li/graphite half-cells with (n=1.0, y=0.0) electrolyte were much higher when compared with the Li/graphite half-cells with non-flammable electrolyte. It is due to the decomposition of non-flammable electrolyte on graphite electrode.
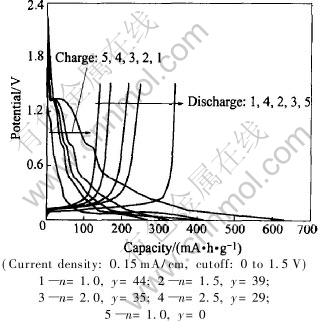
Fig.3 Charge/discharge curves at first cycle of Li/graphite half-cells with 1mol/L LiPF6 (1-y%)(ECn-DMC1.0-EMC1.0)-y%TMP non-flammable electrolytes
The reduction decomposition of TMP solvent occurred according to cathodic peaks at 1.3V (vs Li/Li+) and 0.85V (vs Li/Li+) in the first charge curves. The cathodic peaks turn to be smaller with the increase of EC content and decrease of TMP content in non-flammable electrolytes, suggesting that the reduction decomposition of TMP solvent is gradually suppressed. Furthermore, it is found that the discharge capacity increases with the mole fraction of TMP changing from 44% to 35%. However, a steep decrease in discharge capacity is observed at TMP content of 29%. It could be explained that the high adhesive EC could increase the resistance of Li+ movement in electrolyte and 39.4% EC decreased the electrolyte conductivity.
Fig.4 shows the cycling performances of the above Li/graphite half-cells. Though the Li/graphite half-cells with electrolyte containing 39% or 35%TMP has small initial discharge capacities, the stable discharge capacities after 20 cycles of these cells were almost the same, with the value of 260mA·h/g. The discharge capacities rapidly decreases with cycle when the electrolytes containing 44% or 29% TMP content. With a TMP content of 44%, the discharge capacity is also found to increase during the first 5 cycles though the absolute value was small.
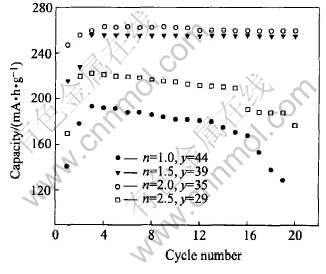
Fig.4 Cycling performances of Li/graphite half-cells as illustrated in Fig.3
To understand the suppress of ECn-DMC1.0-EMC1.0 cosolvents on the reduction decomposition of TMP solvent, the impedances of graphite electrode of the above Li/graphite half-cells were evaluated as shown in Fig.5. The electrolyte impedance of Li/graphite half-cells is in the following order: (n=1.0, y=44)>(n=1.5, y=39)>(n=2.5, y=29)>(n=2.0, y=35), while the SEI film impedance is in the order as: (n=1.5, y=39)>(n=2.0, y=35)>(n=1.0, y=44)>(n=2.5, y=29). A long line was observed at high frequency in impedance spectroscopy in non-flammable electrolyte containing 44%TMP, indicative of the failure of SEI film formation due to TMP reduction decomposition. It was because that the decomposition resultants of TMP were composed of H3PO4, CH4 and CH2CH2[12]. H3PO4 was easy to solve in organic electrolyte, while CH4 and CH3CH3 were gas. At a TMP content of 39%, the SEI film was successfully formed due to having a half-circle at high frequency. Especially at TMP content of 35% or 29%, a good SEI film was formed, which was due to the increase of EC content in electrolyte. The decomposition resultants of EC were (CH2OCO2Li)2 and CH2CH2. (CH2OCO2Li)2 was
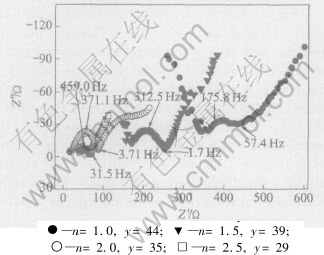
Fig.5 Impedances of Li/graphite half-graphite cells after 5 cycles with 1mol/L LiPF6 (1-y%)(ECn-DMC1.0-EMC1.0)-y%TMP non-flammable electrolyte
difficult to solve in organic electrolyte and deposited on the surface of graphite electrode. That is to say, (CH2OCO2Li)2 was helpful to form good SEI film on graphite electrode.
Table 2 summarizes the physical and electrochemical properties of four kinds of non-flammable electrolytes used in Li/graphite half-cells. The low Coulomb efficiency of Li/graphite cells at first cycle may mainly come from the conductivity and decomposition of non-flammable electrolyte on graphite electrode. Though the reduction stability of non-flammable TMP solvent on graphite electrode may be considerably improved by mixing EC, DMC and EMC cosolvents, the TMP decomposition is still a main factor aggravating the cell performance. In order to realize good cell performance, TMP content should be limited between 39% and 35% for (1-y%)(ECn-DMC1.0-EMC1.0 )-y% TMP non-flammable electrolytes.
Next, the cycling behavior of Li/LiCoO2 half-cells with 1mol/L LiPF6 (1-y%)(ECn-DMC1.0-EMC1.0)-y% TMP non-flammable electrolytes was examined. Fig.6 shows the charge/discharge
Table 2 Physical and electrochemical properties of four kinds of non-flammable electrolytes with 1mol/L LiPF6 solute for Li/graphite half-cells
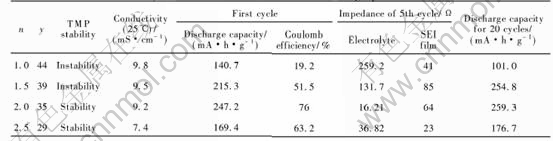
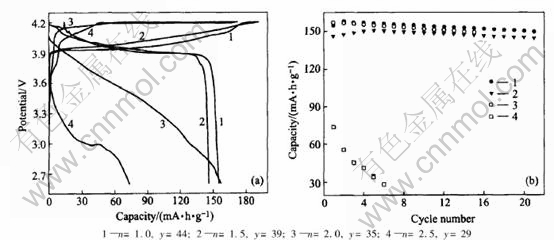
Fig.6 Charge/discharge curves at first cycle cycling performance of Li/LiCoO2 half-cellswith 1mol/L LiPF6 (1-y%)(ECn-DMC1.0-EMC1.0)-y%TMP non-flammable electrolytes
curves at first cycle and the cycling performances. Two good discharge curves with the discharge plateau of about 3.9V (vs. Li/Li+) at first cycle are observed when the Li/LiCoO2 half-cells used non-flammable electrolytes with 56% (EC1.0-DMC1.0-EMC1.0)-44%TMP and 61% (EC1.5-DMC1.0-EMC1.0)-39%TMP as mixture solvents.
Table 3 lists the electrochemical properties of non-flammable electrolytes for Li/LiCoO2 half-cells. The results show that the cycling performances of Li/LiCoO2 half-cells are independent of the TMP content and only slight declined in discharge capacity after 20 cycles except the (n=2.5, y=29) electrolyte. This suggests that the TMP solvent has high oxidation potential of above 4.2V. In order to realize good cell performance, TMP content should be limited between 44% and 39% for (1-y%)(ECn-DMC1.0-EMC1.0 )-y% TMP non-flammable electrolyte.
As described above, the non-flammable electrolyte with n=1.5 and y=39 demonstrates the best total electrochemical properties. However, the TMP solvent of this non-flammable electrolyte is still instable. The reduction decomposition of TMP solvent was very similar with that of PC solvent on graphite electrode. Therefore, it seems to be applicable to refer to the available achievements on how to suppress PC decomposition. So far, the functional additives of electrolyte have been found to be effective. Furthermore, the lithium electrode of half-cells may also be an important reason of the reduction decomposition of non-flammable electrolytes.
Therefore, the cycling behavior of grahite/LiCoO2 lithium-ion cells with 1mol/L LiPF6 61%(EC1.5-DMC1.0-EMC1.0)-39%TMP-x%VC non-flammable electrolytes were evaluated. The quantity of graphite anode was designed to be excess to that of LiCoO2 cathode with a capacity ratio of graphite to LiCoO2 of 1.15∶1 in capacity. It is found that the addition of 4%VC has no effects on the non-flammability of electrolyte because the VC additives are almost exhausted out and used to form SEI film on graphite at the first charge process.
Fig.7 show the charge/discharge curves at first cycle and the cycling performances of graphite/LiCoO2 prismatic batteries. When the battery with 61%(EC1.5-DMC1.0-EMC1.0)-39% TMP electrolyte, a discharge capacity of 131mA·h/g and coulombic efficiency of 78.8% are obtained at first cycle. A capacity ratio of 80.6% is retained after 50 cycles. Addition of functional additive of 4%VC, a higher discharge capacity of 134mA·h/g and promising coulombic efficiency of 80% at first cycle are obtained. A capacity ratio of 84.3% was retained after 50 cycles. These results show that addition of 4%VC has an important role in improving the electrochemical performances of graphite/LiCoO2 batteries with 1mol/L LiPF6 61%(EC1.5-DMC1.0-EMC1.0)-39%TMP non-flammable electrolytes. It could be explained that the electro-chemical polymerization reaction of VC occurred and the resultant of polymer deposited on graphite electrode to form SEI film[19]. A good film can suppress the decomposition of electrolyte.
Another attempt was made to evaluate the safety of the charged grahite/LiCoO2 prismatic lithium-ion batteries with 1mol/L LiPF6 61%(EC1.5-DMC1.0-EMC1.0)-39%TMP-4%VC non-flammable electrolyte. Table 4 shows the resultsof nail penetration experiments. The highest
Table 3 Electrochemical properties of non-flammable electrolytes with 1mol/L LiPF6 solute for Li/LiCoO2 half-cells
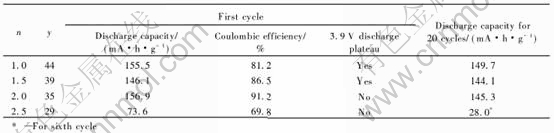
Table 4 Nail penetration for grahite/LiCoO2 prismatic lithium-ion battery with 1mol/L LiPF6 solute
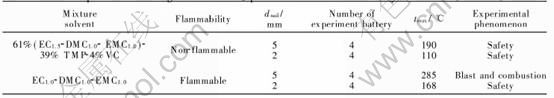
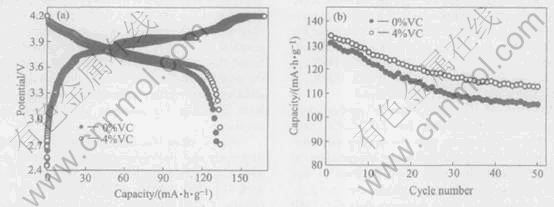
Fig.7 Charge/discharge curves at first cycle(a) and cycling performance(b) of graphite/LiCoO2 lithium-ion batteries of 1mol/L LiPF6/61%(EC1.5-DMC1.0-EMC1.0)-39%TMP-xVC electrolytes
face temperature of all batteries with non-flammable electrolyte is much lower than that of the battery with flammable electrolyte. That is to say, the safety characterization of lithium-ion batteries is dramatically improved by using the non-flammable electrolyte.
4 CONCLUSIONS
1) The single TMP solvent has good oxidation stability and poor reduction stability. The reduction decomposition of TMP solvent occurs at 1.3V (vs Li/Li+).
2) The nonflammability of TMP electrolyte declines with mixing flammable cosolvents. To maintain the nonflammability of mixed electrolytes with the lowest TMP content, the EC cosolvents with high boiling point should be applied.
3) The reduction decomposition of TMP solvents on graphite electrode may be gradually suppressed as increase of n value of ECn-DMC1.0-EMC1.0 cosolvents. The lower the TMP content and the higher the EC content of nonflammable electrolyte is, the better the electrochemical performance of Li/graphite half-cells is. This is attributed to the formation of good SEI film on graphite due to the high EC content in these nonflammable electrolytes.
4) The higher the TMP content and the lower the EC content of nonflammable electrolyte is, the better the electrochemical performance of Li/LiCoO2 half-cells is. It may be due to the improvement of the conductivity of non-flammable electrolyte. The non-flammable electrolyte deliveres good oxidable stability.
5) From the electrochemical properties viewpoint, the best compositions of non-flammable electrolyte should be 1mol/L LiPF6 61%(EC1.5-DMC1.0-EMC1.0)-39%TMP. The discharge capacities of half-cells after 20 cycles are 254.8mA·h/g for Li/graphite half-cell and 144.1mA·h/g for Li/LiCoO2 half-cell. The graphite/LiCoO2 prismatic lithium-ion cell deliveres a discharge capacity of 131mA·h/g at first cycle. With an addition of 4%VC additive to this non-flammable electrolyte, a discharge capacity of 134mA·h/g at first cycle for graphite/LiCoO2 battery is obtained and a capacity ratio of 84.3% after 50 cycles is retained, suggesting that the electrochemical stability of non-flammable electrolyte can be improved by VC additive.
6) The nail penetration test shows that the safety of graphite/LiCoO2 prismatic lithium-ion batteries is dramatically improved by using TMP-containing non-flammable electrolytes.
REFERENCES
[1]Hong J S, Maleki H, Hallaj A, et al. Electro-chemical-calorimetri studies of lithium-ion cells [J]. J Electrochem Soc, 1998, 145(5): 1489-1492.
[2]Farrington M D. Proposed amendments to UN ST/SG/AC.10/11: transport of dangerous goods-lithium batteries [J]. J Power Sources, 1999, 80(1-2): 278-285.
[3]Pals C R, Newman J. Thermal modeling of the lithium/polymer battery in electrolyte [J]. J Electrochem Soc, 1995, 142(10): 3282-3288.
[4]Richard M N, Dahn J R. Accelerating rate calorimetry study on the stability of lithium intercalated [J]. J Electrochem Soc, 1999, 146(6): 2068-2077.
[5]Maleki H, Hallaj S A, Selman J R, et al. Thermal properties of lithium-ion battery and components [J]. J Electrochem Soc, 1999, 146(3): 947-952.
[6]Pasquier A D, Disma F, Bowmer T, et al. Differential scanning calorimetry study of the reactivity of carbon anodes in plastic Li-ion batteries [J]. J Electrochem Soc, 1998, 145(2): 472-477.
[7]Laman F, Gee M, Denovan J. Impedance studies for separators rechargeable lithium batteries [J]. J Electrochem Soc, 1993, 140(4): L51-L53.
[8]Adachi M, Tanaka K, Sekai K. Aromatic compounds as redox shuttle additives for 4V class secondary lithium batteries [J]. J Electrochem Soc, 1999, 146(4): 1256-1261.
[9]Golovin M N, Wilkinson D P, Dudley J T, et al. Applications of metallocenes in rechargeable lithium batteries for overchargeable protection [J]. J Electrochem Soc, 1992, 139(1): 5-10.
[10]Dias F B, Veldhuis J B. Trends in polymer electrolytes for secondary lithium batteries [J]. J Power Sources, 2000, 88(2):169-191.
[11]Wang X M, Yasukawa E, Kasuya S. Nonflammable trimethyl phosphate solvent-containing electrolytes for lithium-ion batteries (Ⅰ): fundamental properties [J]. J Electrochem Soc, 2001, 148(10): A1058-A1065.
[12]Wang X M, Yasukawa E, Kasuya S. Nonflammable trimethyl phosphate solvent-containing electrolytes for lithium-ion batteries (Ⅱ): the use of anamorphous carbon anode [J]. J Electrochem Soc, 2001, 148(10): A1066-A1071.
[13]Xu K, Ding M S, Zhang S S, et al. An attempt to formulate nonflammable lithium ion electrolytes with alkyl phosphates and phosphazenes [J]. J Electrochem Soc, 2002, 149(5): A622-A626.
[14]Xu K, Zhang S, Allen J L, et al. Nonflammable electrolytes for Li-ion batteries based on a fluorinated phosphate [J]. J Electrochem Soc, 2002, 149(8): A1079-A1082.
[15]Xu K, Ding M S, Zhang S, et al. Evaluation of fluorinated alkyl phosphates as flame retardants in electrolytes for Li-ion batteries (Ⅰ): physical and electrochemical properties [J]. J Electrochem Soc, 2003, 150(2): A161-A169.
[16]Xu K, Zhang S, Allen J L, et al. Evaluation of fluorinated alkyl phosphates as flame retardants in electrolytes for Li-ion batteries (Ⅱ): performance in cell [J]. J Electrochem Soc, 2003, 150(2): A170-A175.
[17]Wrodnigg G H, Besenhard J O, Winter M. Ethylene sulfite as electrolyte additive for lithium-ion cells with graphite anodes [J]. J Electrochem Soc, 1999, 146(2): 470-472.
[18]Aurbach D, Gamolsky K, Markovsky B, et al. On the use of vinylene carbonate(VC) as an additive to electrolyte solutions for Li-ion batteries [J]. Electrchim Acta, 2002, 47(9): 1423-1439.
[19]Zhang X, Kostecki R, Richardson T J, et al. Electrochemmical and Infrared studies of reduction of organic carbonates [J]. J Electrochem Soc, 2001, 148(12): 1341- 1345.
[20]Broussely M, Herreyre S, Biensan P, et al. Aging mechanism in Li ion cells and calendar life predictions [J]. J Power Sources, 2001, 97-98: 13-21.
[21]Oesten R, Heider U, Schmidt M. Advanced electrolytes [J]. Solid State Ionics, 2002, 148(3-4):391-397.
[22]Han Y K, Lee S U, Ok J H, et al. Theoretical studies of the solvent decomposition by lithium atoms in lithium-ion battery electrolyte [J]. Chemical Physics Letters, 2002, 360(1): 359-366.
(Edited by LONG Huai-zhong)
Received date: 2005-04-24; Accepted date: 2005-07-18
Correspondence: HU Chuan-yue, PhD candidate; Tel: +86-738-8325065; E-mail: huchuanyue@vip.sina.com.cn