Trans. Nonferrous Met. Soc. China 25(2015) 1568-1574
Synthesis of high-capacity LiNi0.8Co0.1Mn0.1O2 cathode by transition metal acetates
Zheng-wei XIAO, Ying-jie ZHANG, Yi-fan WANG
Faculty of Metallurgical and Energy Engineering, Kunming University of Science and Technology, Kunming 650093, China
Received 25 August 2014; accepted 28 December 2014
Abstract: LiNi0.8Co0.1Mn0.1O2 cathode was synthesized using transition metal acetates under different synthesis conditions. Simultaneous thermogravimetric–differential scanning calorimetry–derivative thermogravimetric analysis was applied to investigating the mixture of transition metal acetates. X-ray powder diffraction and charge–discharge test were adopted to characterize the as-prepared LiNi0.8Co0.1Mn0.1O2. The mixture of transition metal acetates undergoes dehydration and decomposition during heating. All the examined LiNi0.8Co0.1Mn0.1O2 samples have a layered structure with 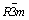 space group. LiNi0.8Co0.1Mn0.1O2 samples prepared with different lithium sources under different synthesis conditions exhibit very different charge–discharge performances. The sample synthesized via the procedure of sintering at 800 °C after heating lithium carbonate and transition metal acetates at 550 °C achieves a highest capacity of 200.8 mA·h/g and an average capacity of 188.1 mA·h/g in the first 20 cycles at 0.2C.
space group. LiNi0.8Co0.1Mn0.1O2 samples prepared with different lithium sources under different synthesis conditions exhibit very different charge–discharge performances. The sample synthesized via the procedure of sintering at 800 °C after heating lithium carbonate and transition metal acetates at 550 °C achieves a highest capacity of 200.8 mA·h/g and an average capacity of 188.1 mA·h/g in the first 20 cycles at 0.2C.
Key words: Ni-rich cathode material; transition metal acetate; lithium source; synthesis procedure
1 Introduction
Layered Ni-rich lithium oxide LiNi0.8Co0.1Mn0.1O2 is an attractive cathode material for lithium ion cells. It possesses desirable characteristics of relatively high capacity and rate capability and low cost in comparison with the most used LiCoO2 and LiNi0.8Co0.2O2 [1-4]. In the preparation of the mixed transition metal oxide LiNi1–y–zCoyMnzO2, a homogeneous mixing of Ni, Co and Mn is preferred so that a preparatory precursor synthesis is often carried out, and then a lithiation is done to produce the aimed product. The liquid phase co-precipitation and sol-gel method [5] are the most common ways to fulfill the purpose. In a typical aqueous phase method, sulfates or chlorides of the transition metals, Ni, Co and Mn, are used as the elemental sources and a carbonate or hydroxide is used to evenly precipitate the transition metals [6,7]. The deposited precursor is then mixed with a lithium source, such as LiOH·H2O, Li2CO3, LiNO3 and Li(CH3COO)·2H2O, and thermally treated at last to derive the desired material.
A liquid method is capable of making precursor particles with not only an even distribution of transition metals but a favored morphology and particle size when the experimental conditions are carefully controlled [6,7]. However, this method is troublesome due to the necessary but complicating aging, settling and repeated filtering and washing. The lengthy procedure reduces the production rate and efficiency and increases cost. Furthermore, a chemical analysis is required of each batch of precursor for the precise proportioning for final lithiation. A solid state method is suitable for mass production of cathode materials for lithium ion cells. Meanwhile, the lithium source can be mixed with transition metal sources in a strict stoichiometry at the beginning. However, in mixing starting materials, the transition metals cannot be distributed uniformly, which will affect adversely the mass transfer in the subsequent solid state reaction.
Herein, we introduce a strategy for addressing the above issues which at the same time keeps the advantages of the above methods to a great extent. The homogeneity for the transition metals can be achieved by a two-grinding procedure. First, the transition metal acetates are mixed and ground. The transition metals can be well distributed in the mixture for the transition metal acetates can be crushed more readily than their oxides or hydroxides. Then, a calcination is done to dehydrate and decompose the acetates. Due to the substantial gas evolution in decomposition, the product exhibits a loose and porous texture, which is beneficial to a second grinding process for a more even distribution of transition metals. A lithium source may be introduced in the first grinding or mixed with the calcined product in a precise stoichiometry for the fixed mass loss in the pretreatment. In this way, the lengthy and troublesome liquid method process is avoided, the even distribution of transition metals is achieved, and the proportioning issue is addressed.
2 Experimental
2.1 Simultaneous thermogravimetric–differential scanning calorimetry–derivative thermo- gravimetric analysis (TG-DSC-DTG)
The mixture of tetrahydrates Ni(CH3COO)2·4H2O, Co(CH3COO)2·4H2O and Mn(CH3COO)2·4H2O at a molar ratio of 8:1:1 was examined with TG-DSC-TGA on a NETZSCH STA 449F3 at 35-850 °C. The heating rate was 10 °C/min and the high-purity inert gas flow rate was 100 mL/min.
2.2 LiNi0.8Co0.1Mn0.1O2 preparation
Four different procedures, A, B, C and D, were applied for LiNi0.8Co0.1Mn0.1O2 synthesis. For each procedure, Li(CH3COO)·2H2O, Li2CO3, LiOH·H2O and LiNO3 were used, respectively, the transition metal sources were the tetrahydrates, and the use of raw materials was stoichiometric. In procedure A, the lithium source and tetrahydrates were ground and sintered at 800 °C in an O2 atmosphere for 24 h to produce LiNi0.8Co0.1Mn0.1O2 samples A1, A2, A3 and A4 (subscripts 1, 2, 3 and 4 refer to Li(HC3COO)·2H2O, Li2CO3, LiOH·H2O and LiNO3), respectively. In procedure B, the lithium source and tetrahydrates were ground and calcined at 550 °C in air for 8 h, and the calcined product was ground and sintered at 800 °C in an O2 atmosphere for 24 h to derive LiNi0.8Co0.1Mn0.1O2 samples B1, B2, B3 and B4, respectively. In procedure C, the tetrahydrates were ground and calcined at 550 °C in air for 8 h, and the calcined product was ground with a lithium source and sintered at 800 °C in O2 atmosphere for 24 h to prepare LiNi0.8Co0.1Mn0.1O2 samples C1, C2, C3 and C4, respectively. Procedure D differed from procedure C only in the calcining temperature of 700 °C instead of 550 °C and the obtained LiNi0.8Co0.1Mn0.1O2 samples were D1, D2, D3 and D4, respectively. In all cases, the heating rate was 3 °C/min and O2 flow rate was 40 mL/min.
2.3 X-ray powder diffraction
As-prepared LiNi0.8Co0.1Mn0.1O2 was characterized with X-ray powder diffraction (XRD) performed on a Philips X-pert powder diffractometer in the range of 5-90° at a step of 0.02° with Cu Kα radiation.
2.4 Charge–discharge test
As-prepared LiNi0.8Co0.1Mn0.1O2 was charged- discharged in CR2025 coin cells. The electrolyte was 1 mol/L LiPF6 in a mixed solvent of ethylene carbonate, dimethylene carbonate and ethylmethyl carbonate at a volume ratio of 1:1:1. The lithium disc was used as the anode. A Celgard 2400 microporous polypropylene membrane was used as the separator. LiNi0.8Co0.1Mn0.1O2, PVDF binder and acetylene black with a mass ratio of 8:1:1 were ground in N-methyl pyrrolidinone solvent and the resultant slurry was evenly spread on an aluminum foil. The wet cathode was dried at 120 °C under vacuum. Cathode discs were punched for coin cell assembly. The loading of LiNi0.8Co0.1Mn0.1O2 was around 4 mg/cm2 in each cathode. The coin cell was assembled in an argon atmosphere glovebox. The charge–discharge test was conducted galvanostatically on a LAND BTI-40 in 3-4.35 V at 0.2C at room temperature.
3 Results and discussion
3.1 TG-DSC-DTG of mixed tetrahydrate acetates
Figure 1 reveals three successive mass loss steps on the TG curve, 95.1-221 °C, 221-361.7 °C and 361.7-387.7 °C, and the corresponding mass loss rates are 31.60%, 33.93% and 7.33%, respectively, with a total loss of 72.86%. All the three steps are endothermic. The first mass loss at 95.1–221 °C can be attributed to the dehydration of crystallized water in the hydrates. In the mixture of Ni(CH3COO)2·4H2O, Co(CH3COO)2·4H2O and Mn(CH3COO)2·4H2O with a molar ratio of 8:1:1, the theoretical content of crystalliferous water is 25.75%. As a result, more mass than the crystalliferous water is lost. The acetate admixture contains 80% nickel acetate whose thermal response must be inherited by the former. The additional loss at 95.1-221 °C can be ascribed to the evolution of acetic acid from nickel acetate, resulting in (1-x)Ni(CH3COO)2·xNi(OH)2 [8]. Both cobalt acetate and manganese acetate may exhibit a similar behavior [9,10]. Meanwhile, manganese acetate melts above 80 °C.
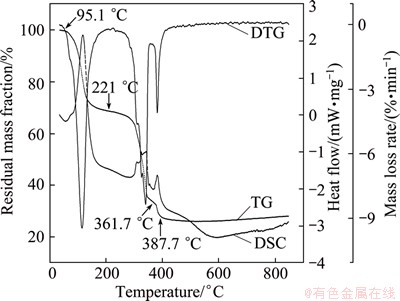
Fig. 1 TG-DSC-DTG of mixture of Ni(CH3COO)2·4H2O, Co(CH3COO)2·4H2O and Mn(CH3COO)2·4H2O at molar ratio of 8:1:1
The mass loss at 221-361.7 °C is caused by the decomposition of the dehydrated intermediate. The theoretical mass loss in this step is 44.34% if there is only dehydration in the first step and the acetates are converted into NiO, CoO and MnO, but the tested value is only 33.93%, which can be imputted to the additional mass loss in the first step at 95.1-221 °C and final mass loss at 361.7-387.7 °C. The last endothermic response at 361.7-387.7 °C gives rise to a mass loss of 7.33%. When nickel acetate was calcined in an inert atmosphere, a reduction to Ni was found, but this was not observed when the calcination was carried out in a H2 or air atmosphere [11]. Thus, the reduction to Ni is responsible for the observed mass loss of 72.86% in TG-DSC-DTG, which is greater than the theoretical 70.09% on the formation of NiO, CoO and MnO.
The sample retains its mass after 387.7 °C, and no more thermal effects are observed to 850 °C, which indicates that the residue is stable in state and transition metal oxides maintain their phases. From above, it is clear that the admixture of the tetrahydrates undergoes dehydration, decomposition and Ni formation during heating in an inert atmosphere. As the air atmosphere is used in pretreatment, the reduction does not take place and MnO is oxidized to MnO2. The tetrahydrates lose 70.38%, 69.43% and 69.29% by mass when heated to 387.7, 550 and 700 °C, respectively, in air, which agree with the theoretical values of 70.09%/69.45% on NiO, CoO and MnO/MnO2 formation. Thus, a mixture of transition metal oxides is obtained by calcining the tetrahydrates in air to above 387.7 °C through the successive steps of dehydration and decomposition.
3.2 Physical properties of as-prepared LiNi0.8Co0.1-Mn0.1O2
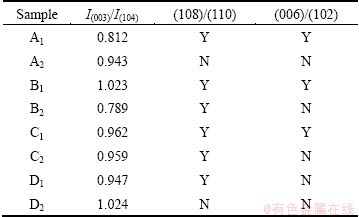
Eight samples were examined by XRD and the results are given in Fig. 2. As shown in Fig. 2(a), the main peaks of (003) and (104) of each pattern are located at the same degrees as the reported layered LiNi0.8Co0.1Mn0.1O2 [2,5-7]. The layered structure is confirmed to be 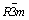 space group as in α-NaFeO2. No impurity phases consisting of lithium, nickel, cobalt and/or manganese are revealed, indicating a high purity for the as-prepared LiNi0.8Co0.1Mn0.1O2. The characteristic peaks in the diffraction pattern are sharp, suggesting a high degree of crystallinity for LiNi0.8Co0.1- Mn0.1O2 crystals. It is, therefore, illustrated that the starting materials and procedures are suitable for preparation of pure and well-crystallized LiNi0.8Co0.1- Mn0.1O2. Figures 2(b) and (c) show that most samples exhibit a well-split (108)/(110), but very few have a well-split (006)/(102).
space group as in α-NaFeO2. No impurity phases consisting of lithium, nickel, cobalt and/or manganese are revealed, indicating a high purity for the as-prepared LiNi0.8Co0.1Mn0.1O2. The characteristic peaks in the diffraction pattern are sharp, suggesting a high degree of crystallinity for LiNi0.8Co0.1- Mn0.1O2 crystals. It is, therefore, illustrated that the starting materials and procedures are suitable for preparation of pure and well-crystallized LiNi0.8Co0.1- Mn0.1O2. Figures 2(b) and (c) show that most samples exhibit a well-split (108)/(110), but very few have a well-split (006)/(102).
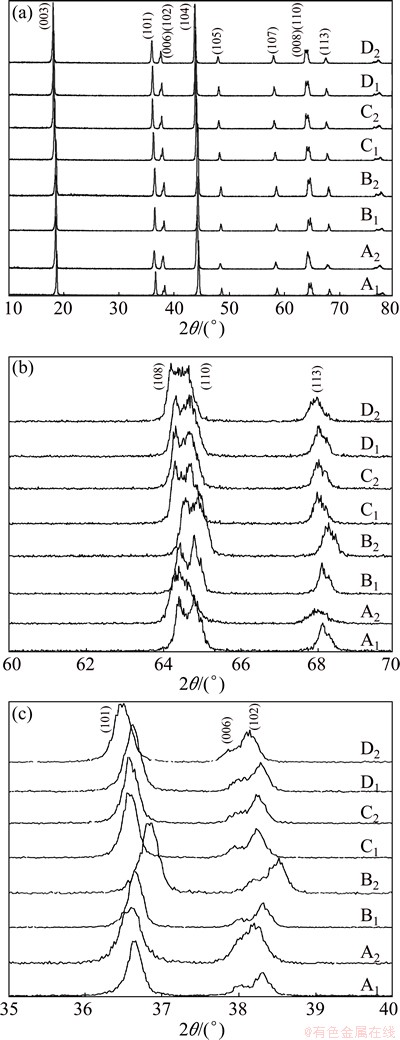
Fig. 2 XRD patterns for as-prepared LiNi0.8Co0.1Mn0.1O2 samples (a), magnified (108)/(110) (b) and (006)/(102) (c)
The ratio between I(003) and I(104) reflects the cation mixing between Ni2+ and Li+ which reside in 3a and 3b sites, respectively, in an ideal layered structure. A higher value for I(003)/I(104) endows a lower lattice disorder, and vice versa. Actually, in Ni-rich LiNi0.8Co0.1Mn0.1O2, there is definitely a high cation disorder. The elimination of this defect needs a n(Co)/n(Ni) ratio greater than 1:1 and a careful heating and cooling in an O2 atmosphere for cation ordering in LiNiyMnzCo1–y–zO2 [12,13].
Several parameters of the samples derived from their XRD data are listed in Table 1. Different lithium sources and procedures lead to LiNi0.8Co0.1Mn0.1O2 with differences in structure parameters. B1 and D2 exhibit the highest I(003)/I(104) and in other words best layeredness and lowest cation disorder, but D2 has unsplit (108)/(110) and (006)/(102). A1, B1 and C1 display well-split (006)/(102) and (108)/(110), B2 has the lowest I(003)/I(104) but well-split (108)/(110). All the samples prepared from transition metal acetates exhibit very low I(003)/I(104) values in comparison with those reported [2,5-7], which merits further understanding. A possible reason is that the sintering atmosphere is not oxidizing enough to oxidize Ni2+ [6].
Table 1 Structure parameters of LiNi0.8Co0.1Mn0.1O2 samples (Y: split, N: unsplit)
3.3 Charge–discharge behavior of as-prepared LiNi0.8Co0.1Mn0.1O2
The charge-discharge performance and capacity retention of the LiNi0.8Co0.1Mn0.1O2 samples are compared in accordance with the lithium source used, as shown in Fig. 3. All the charge-discharge profiles are indicative of one phase lithium extraction–insertion in 3-4.35 V for the as-prepared cathode. The initial capacities are 166.8, 168.1, 191.3 and 142.2 mA·h/g, the highest capacities are 172.8, 168.1, 191.3 and 149.2 mA·h/g, the average values are 157.8, 154.6, 173.4 and 139.5 mA·h/g, and the capacity retentions are 88.3%, 82.6%, 84.7% and 92.1%, in the first 20 cycles for A1, B1, C1 and D1, respectively. A1 and B1 exhibit similar performance, which can be attributed to the Li(CH3COO)·2H2O melting at 56 °C and seeping evenly in the mixture of transition metal acetates. Thus, a second grinding process fails to further improve the electrochemical behavior of the final product. In this group, C1 exhibits the best electrochemical performance, the smallest polarization in discharge and the highest capacities. As a result, a low temperature treatment of the tetrahydrates and a subsequent lithiation using Li(CH3COO)·2H2O result in LiNi0.8Co0.1Mn0.1O2 with a relatively high I(003)/I(104) value and well-split (006)/(102) and (108)/(110), giving rise to a good electrochemical behavior. D1 displays the worst performance due to the high temperature pretreatment of the tetrahydrates at 700 °C. This high temperature treatment may lead to agglomeration, which is unfavorable for mass transfer in lithiation.
A2, B2, C2 and D2 exhibit initial capacities of 90.3, 183.6, 160.8 and 157.4 mA·h/g, highest capacities of 91.4, 200.8, 163.8 and 160.3 mA·h/g, average values of 80.6, 188.1, 161.7 and 152.7 mA·h/g, and capacity retentions of 84.8%, 95.5%, 99.6% and 93.3%, respectively, in the first 20 cycles. C2 and D2 have similar initial and highest capacities, but the former has a higher average capacity and better capacity retention. Therefore, it is shown again that a higher temperature treatment of the tetrahydrates prior to lithiation impairs the electrochemical property of LiNi0.8Co0.1Mn0.1O2. B2 shows a much better performance than A2, indicative of the second grinding process being capable of greatly improving the electrochemical behavior of LiNi0.8Co0.1Mn0.1O2. Li2CO3 has a melting point of 723 °C and is more stable than the tetrahydrates. While the acetates dehydrate and decompose, Li2CO3 may segregate to a certain extent. A second grinding process is just appropriate for addressing the issue.
A3, B3, C3 and D3 deliver initial capacities of 144.7, 151.9, 176.5 and 173.8 mA·h/g, highest capacities of 179.1, 197.0, 180.9 and 182.1 mA·h/g, average capacities of 155.2, 150.4, 148.6 and 151.0 mA·h/g, and capacity retentions of 92.1%, 85.6%, 68.3% and 73.7%, respectively, in the first 20 cycles. The average capacities achieved by the 4 samples synthesized by using LiOH·H2O are very similar and lower compared with most of those exhibited by the samples prepared with Li(CH3COO)·2H2O and Li2CO3. LiOH·H2O has a melting point of 471 °C and is more basic than the other three lithium sources and its reaction with transition metal oxides in sintering is different. B3 has a highest capacity of 197.0 mA·h/g but its capacity fade is marked on cycling.
Neither A4 nor B4 demonstrates an electrochemical capacity in 3-4.35 V, and the research for finding the reasons is underway. In the first 20 cycles, C4 and D4 are observed to have initial capacities of 145.1 and 155.9 mA·h/g, highest capacities of 153.6 and 157.3 mA·h/g, average capacities of 147.6 and 141.0 mA·h/g, and capacity retentions of 94.1% and 79%, respectively.
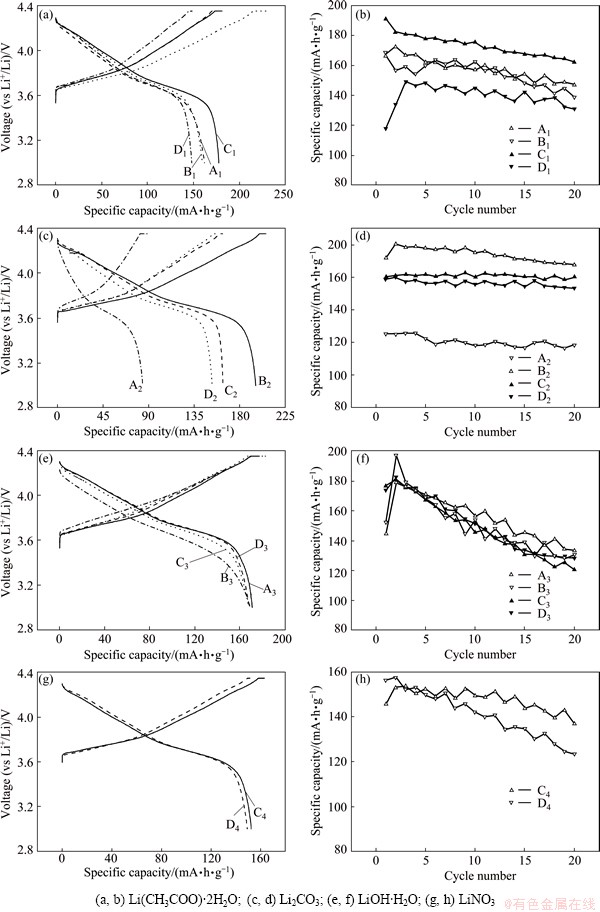
Fig. 3 Typical charge–discharge profiles and rate performance for as-prepared LiNi0.8Co0.1Mn0.1O2 at 0.2C
Under the current experimental conditions, the adoption of LiNO3 as a lithium source for LiNi0.8Co0.1Mn0.1O2 synthesis is not appealing, though it is successfully used in other cases [1]. C4 has a better cyclability than D4, reaching the same conclusion that a very high temperature pretreatment of the tetrahydrates is unfavorable for synthesizing LiNi0.8Co0.1Mn0.1O2 with superior electrochemical performance.
The adoption of LiOH·H2O or LiNO3 cannot achieve LiNi0.8Co0.1Mn0.1O2 with high capacities in the work, which by no means excludes the possibility of using the two lithium sources to produce high-quality LiNi0.8Co0.1Mn0.1O2. Among all the samples, A2 exhibits a very inferior electrochemical performance due to the possible lithium segregation in sintering, leading to unsplit (006)/(102) and (108)/(110), which is an indication of inferior layeredness. Though it has the lowest cation order, B2 shows the highest capacities, which is contrary to Ref. [7]. In fact, even cation-disordered cathode materials have very good electrochemical performance [14]. B1 shows a comparatively high I(003)/I(104) value and well-split (108)/(110) and (006)/(102) but its electrochemical performance is not superior. The reason is that the layeredness is not the sole factor determining the electrochemical behavior of LiNi0.8Co0.1Mn0.1O2. Other parameters, such as conductivity and morphology, may greatly affect the performance of the cathode [4]. More detailed reasons need to be revealed by other modern analysis tools.
The two procedures, a low temperature pretreatment of transition metal acetates followed by a lithiation using lithium acetate at an elevated temperature and a pretreatment of lithium carbonate and transition metal acetates at a low temperature and a second sintering at a high temperature, are capable of synthesizing high-capacity LiNi0.8Co0.1Mn0.1O2. When LiOH·H2O is not used, the two steps of a pretreatment at a low temperature and a sintering at a high temperature are preferred for LiNi0.8Co0.1Mn0.1O2 synthesis. More work should be done to know the reasons why the use of LiOH·H2O leads to exceptions.
One phenomenon worth noting is the evident capacity fade for all the samples synthesized in this work. Actually, the worst drawback of Ni-rich LiNi0.8Co0.1Mn0.1O2 is its cycling instability. Mechanisms for LiNi0.8Co0.1Mn0.1O2 failure include phase transition, side reactions between Ni4+ and electrolyte, formation of inactive and low conductivity NiO phase on the surface of LiNi0.8Co0.1Mn0.1O2 particles and O2 evolution at a high charge cutoff voltage [15-17]. Strategies, such as doping, coating, morphology engineering, and so on, are effective to tackle these problems. Based on the high capacities achieved by C1 and B2, the subsequent work is worth carrying out to optimize the involved process parameters and adopt strategies to stabilize the electrochemical performance of the Ni-rich cathode for practical use.
4 Conclusions
1) Well-crystallized and layered LiNi0.8Co0.1Mn0.1O2 was synthesized by using transition metal acetates and different lithium sources via different synthesis procedures. The mixture of the transition metal acetates is dehydrated and decomposed successively during heating. All the as-prepared LiNi0.8Co0.1Mn0.1O2 exhibit an unusual low value for I(003)/I(104). The lithium source and synthesis procedure greatly affect the charge– discharge performance of the final product.
2) A pretreatment of transition metal acetates at 550 °C followed by a lithiation using lithium acetate at 800 °C resulted in LiNi0.8Co0.1Mn0.1O2 showing a highest capacity of 191.3 mA·h/g and an average of 173.4 mA·h/g in the first 20 cycles at 0.2C. Through preheating at 550 °C, grinding and sintering at 800 °C, the mixture of lithium carbonate and transition metal acetates is converted into LiNi0.8Co0.1Mn0.1O2 exhibiting a highest capacity of 200.8 mA·h/g and an average of 188.1 mA·h/g in the first 20 cycles at 0.2C.
3) Under our experimental conditions, the use of LiOH·H2O or LiNO3 fails to achieve high-capacity LiNi0.8Co0.1Mn0.1O2. Except when LiOH·H2O is used, the two steps of a low temperature pretreatment and a high temperature sintering are preferable for LiNi0.8Co0.1- Mn0.1O2 synthesis. Transition metal acetates are suitable for the synthesis of high-capacity LiNi0.8Co0.1Mn0.1O2. The cyclability of the as-prepared LiNi0.8Co0.1Mn0.1O2 must be greatly improved by optimization of synthesis conditions and use of improving strategies for application.
References
[1] SUN Y K, MYUNG S T, PARK B C, PRAKASH J, BELHAROUAK I, AMINE K. High-energy cathode material for long-life and safe lithium batteries [J]. Nature Materials, 2009, 8(4): 320-324.
[2] LU Lei, ZHONG Wei-pan, YANG Hui. Synthesis and characterization of spherical LiNi0.8Co0.1Mn0.1O2 particles with a high tap-density [J]. Journal of Inorganic Materials, 2012, 27(3): 258-264. (in Chinese)
[3] DU Ke, HUANG Jin-long, HU Guo-rong, PENG Zhong-dong, CAO Yan-bin, TANG Cao-pu, WANG Wei-gang. Research on synthesis and performance of composite cathode material Li[Ni0.92Co0.04- Mn0.04]O2 with gradient-coating layer as lithium ion battery [J]. Chinese Journal of Inorganic Chemistry, 2013, 29(5): 2031-1036. (in Chinese)
[4] LI Ling-jun, LI Xin-hai, WANG Zhi-xing, WU Ling, ZHENG Jun-chao, LI Jin-hui. Synthesis of LiNi0.8Co0.1Mn0.1O2 cathode material by chloride co-precipitation method [J]. Transactions of Nonferrous Metals Society of China, 2010, 20(S1): s279-s282.
[5] LU H, ZHOU H, SVENSSON A M, FOSSDAL A, SHERIDAN E, LU S, VULLUM-BRUER F. High capacity Li[Ni0.8Co0.1Mn0.1]O2 synthesized by sol-gel and co-precipitation methods as cathode materials for lithium-ion batteries [J]. Solid State Ionics, 2013, 249-250: 105-111.
[6] ANG Xi-min, WANG Xian-you, YI Si-yong, CAO Jun-qi. Synthesis and characteristic of layered LiNi0.8Co0.1Mn0.1O2 cathode material for lithium rechargeable batteries [J]. The Chinese Journal of Process Engineering, 2007, 7(4): 817-821. (in Chinese)
[7] WANG Jie-xi, LI Xin-hai, WANG Zhi-xing, LI Ling-jun, GUO Hua-jun, YUE Peng, WU Ling. Effect of pH value on performance of Ni0.8Co0.1Mn0.1(OH)2 and LiNi0.8Co0.1Mn0.1O2 synthesized via fast co-precipitation process [J]. The Chinese Journal of Nonferrous Metals, 2011, 21(9): 2175-2181. (in Chinese)
[8] de JESUS J C, GONZALEZ I, QUEVEDO A, PUERTA T. Thermal decomposition of nickel acetate tetrahydrate: An intergrated study by TGA, QMS and XPS techniques [J]. Journal of Molecular Catalysis A: Chemical, 2005, 228(1): 283-291.
[9] UAN H, SHAO C, WEN S, CHEN B, GONG J, YANG X. A novel method for preparing Co3O4 nanofibers by using electrospun PVA/cobalt acetate composite fibers as precursor [J]. Materials Chemistry and Physics, 2003, 82(3): 1002-1006.
[10] YANDRAPALLI N, RAJAN K S. One-pot synthesis of oil dispersible ultra fine manganese (II) oxide nanoparticles [J]. Asian Journal of Scientific Research, 2012, 5(4): 228-237.
[11] HONG J, GUO G, ZHANG K. Kinetic and mechanism of non-isothermal dehydration of nickel acetate tetrahydrate in air [J]. Journal of Analytical and Applied Pyrolysis, 2006, 77(2): 111-115.
[12] WHITTINGHAM M S. Lithium batteries and cathode materials [J]. Chemical Review, 2004, 104(10): 4271-4301.
[13] XU Z, XIAO L, WANG F, WU K, ZHAO L, LI M R, ZHANG H L, WU Q, WANG J. Effects of precursor, synthesis time and synthesis temperature on the physical and electrochemical properties of Li(Ni1-x-yCoxMny)O2 cathode materials [J]. Journal of Power Sources, 2014, 248: 180-189.
[14] LEE J, URBAN A, LI X, SU D, HAUTIER G, CEDER G. Unlocking the potential of cation-disordered oxides for rechargeable lithium batteries [J]. Science, 2014, 343: 519-522.
[15] GOODENOUGH J B, PARK K S. The Li-ion rechargeable battery: A perspective [J]. Journal of the American Chemical Society, 2014, 135(4): 1167-1176.
[16] CHO Y, OH P, CHO J. A new type of protective surface layer for high-capacity Ni-based cathode materials: Nanoscaled surface pillaring layer [J]. Nano Letters, 2013, 13(3): 1145-1152.
[17] PAN Cheng-chi, BANKS C E, SONG Wei-xin, WANG Chi-wei, CHEN Qi-yuan, JI Xiao-bo. Recent development of LiNixCoyMnzO2: Impact of micro/nano structures for imparting improvements in lithium batteries [J]. Transactions of Nonferrous Metals Society of China, 2013, 23(1): 108-119.
采用过渡金属醋酸盐制备高容量LiNi0.8Co0.1Mn0.1O2正极材料
肖政伟,张英杰,王一帆
昆明理工大学 冶金与能源工程学院,昆明 650093
摘 要:采用过渡金属醋酸盐在不同合成条件下制备LiNi0.8Co0.1Mn0.1O2正极材料。使用同步热重–差热–微分热重分析法研究过渡金属醋酸盐混合物。利用X射线粉末衍射和充放电测试对所制备的LiNi0.8Co0.1Mn0.1O2材料进行表征。过渡金属醋酸盐混合物在加热过程中经历脱水和分解。所有测试的LiNi0.8Co0.1Mn0.1O2样品均为层状结构,且具有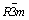 空间群。采用不同锂源和不同合成工艺制备的LiNi0.8Co0.1Mn0.1O2样品表现出的充放电性能差别很大。采用550 °C预处理碳酸锂和过渡金属醋酸盐后在800 °C烧结获得的样品在0.2C倍率下前20次充放电循环过程中的最高容量为200.8 mA·h/g,平均容量为188.1 mA·h/g。
空间群。采用不同锂源和不同合成工艺制备的LiNi0.8Co0.1Mn0.1O2样品表现出的充放电性能差别很大。采用550 °C预处理碳酸锂和过渡金属醋酸盐后在800 °C烧结获得的样品在0.2C倍率下前20次充放电循环过程中的最高容量为200.8 mA·h/g,平均容量为188.1 mA·h/g。
关键词:富镍正极材料;过渡金属醋酸盐;锂源;合成工艺
(Edited by Yun-bin HE)
Foundation item: Project (2010ZC051) supported by the Natural Science Foundation of Yunnan Province, China; Project (20140439) supported by the Analysis and Testing Foundation from Kunming University of Science and Technology, China; Project (14118245) supported by the Starting Research Fund from Kunming University of Science and Technology, China
Corresponding author: Ying-jie ZHANG; Tel: +86-18808804528; E-mail: zhangyingjie09@126.com
DOI: 10.1016/S1003-6326(15)63759-1