Enhanced HMF yield from glucose with H-ZSM-5 catalyst in water–tetrahydrofuran/2-butanol/2-methyltetrahydrofuran biphasic systems
来源期刊:中南大学学报(英文版)2019年第11期
论文作者:肖国民 徐思泉 潘东辉
文章页码:2974 - 2986
Key words:5-hydroxymethylfurfural; H-ZSM-5 zeolite; glucose; biphasic systems
Abstract: With the aim of achieving a high 5-hydroxymethylfurfural (HMF) yield from glucose with H-ZSM-5 catalyst at low cost, three inexpensive biphasic reaction systems, H2O-tetrahydrofuran (THF), H2O-2-methyltetrahydrofuran (MeTHF) and H2O-2-butanol, were discovered and proved to be particularly effective in promoting the formation of HMF from glucose over H-ZSM-5 zeolite. In order to determine the optimal process conditions, the effects of various experimental variables, such as reaction temperature, reaction time, catalyst dosage, volume of organic solvent, as well as inorganic salt type on glucose conversion to HMF in three systems were investigated in detail. It was found that under optimal reaction conditions, H2O-THF, H2O-2-butanol and H2O-MeTHF allowed the glucose dehydration process to achieve HMF yields of up to 61%, 59%, and 50%, respectively. Moreover, in the three biphasic systems, the H-ZSM-5 catalyst was also demonstrated to maintain excellent stability. Thus, the catalytic approach proposed in this paper can be believed to have potential prospects for industrially efficient and low-cost production of HMF.
Cite this article as: XU Si-quan, PAN Dong-hui, XIAO Guo-min. Enhanced HMF yield from glucose with H-ZSM-5 catalyst in water–tetrahydrofuran/2-butanol/2-methyltetrahydrofuran biphasic systems [J]. Journal of Central South University, 2019, 26(11): 2974-2986. DOI: https://doi.org/10.1007/s11771-019-4229-x.

J. Cent. South Univ. (2019) 26: 2974-2986
DOI: https://doi.org/10.1007/s11771-019-4229-x
XU Si-quan(徐思泉), PAN Dong-hui(潘东辉), XIAO Guo-min(肖国民)
School of Chemistry and Chemical Engineering, Southeast University, Nanjing 211189, China
 Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2019
Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2019
Abstract: With the aim of achieving a high 5-hydroxymethylfurfural (HMF) yield from glucose with H-ZSM-5 catalyst at low cost, three inexpensive biphasic reaction systems, H2O-tetrahydrofuran (THF), H2O-2-methyltetrahydrofuran (MeTHF) and H2O-2-butanol, were discovered and proved to be particularly effective in promoting the formation of HMF from glucose over H-ZSM-5 zeolite. In order to determine the optimal process conditions, the effects of various experimental variables, such as reaction temperature, reaction time, catalyst dosage, volume of organic solvent, as well as inorganic salt type on glucose conversion to HMF in three systems were investigated in detail. It was found that under optimal reaction conditions, H2O-THF, H2O-2-butanol and H2O-MeTHF allowed the glucose dehydration process to achieve HMF yields of up to 61%, 59%, and 50%, respectively. Moreover, in the three biphasic systems, the H-ZSM-5 catalyst was also demonstrated to maintain excellent stability. Thus, the catalytic approach proposed in this paper can be believed to have potential prospects for industrially efficient and low-cost production of HMF.
Key words: 5-hydroxymethylfurfural; H-ZSM-5 zeolite; glucose; biphasic systems
Cite this article as: XU Si-quan, PAN Dong-hui, XIAO Guo-min. Enhanced HMF yield from glucose with H-ZSM-5 catalyst in water–tetrahydrofuran/2-butanol/2-methyltetrahydrofuran biphasic systems [J]. Journal of Central South University, 2019, 26(11): 2974-2986. DOI: https://doi.org/10.1007/s11771-019-4229-x.
1 Introduction
5-hydroxymethylfurfural (HMF), a valuable platform compound known as “sleeping giant”, through catalytic conversion, can be further synthesized into high value chemicals [1], pharmaceutical intermediates [2] and furan-based polymers [3], and therefore has attracted wide attention in recently years. In general, the desired HMF yield was readily given by using fructose as starting reactant under acidic conditions [4, 5]. Unfortunately, actual processing of fructose requires expensive chromatography steps to achieve the target concentration [6]. So, taking into account the cost price, glucose with higher abundance and cheaper advantages was more preferred as feedstock to synthesize HMF [7].
It was widely believed that the HMF generation from glucose is a complex tandem process, involving the Lewis acid catalyzed isomerization to form fructose and subsequently dehydration in the presence of Br nsted acid to produce HMF [8, 9]. In view of this conversion route, a great deal of effort has been devoted to the development of bifunctional catalysts having Lewis and Br
nsted acid to produce HMF [8, 9]. In view of this conversion route, a great deal of effort has been devoted to the development of bifunctional catalysts having Lewis and Br nsted acidity, such as resins [10], sulfonated carbon/metal oxides [11], mesoporous silica [12], zeolites [13] and heteropolyacids [14]. Compared to most heterogeneous catalysts with high cost and complex preparation processes, H-ZSM-5 zeolite, which is the most common and inexpensive solid acid catalyst used in petrochemicals, not only has good hydrothermal stability, appropriate specific area and pore structure, but also possesses Lewis and Br
nsted acidity, such as resins [10], sulfonated carbon/metal oxides [11], mesoporous silica [12], zeolites [13] and heteropolyacids [14]. Compared to most heterogeneous catalysts with high cost and complex preparation processes, H-ZSM-5 zeolite, which is the most common and inexpensive solid acid catalyst used in petrochemicals, not only has good hydrothermal stability, appropriate specific area and pore structure, but also possesses Lewis and Br nsted acidity [15, 16]. Therefore, if the commercialized inexpensive H-ZSM-5 can directly catalyze glucose to achieve the desired HMF yield, this will promote the actual production of HMF in the future.
nsted acidity [15, 16]. Therefore, if the commercialized inexpensive H-ZSM-5 can directly catalyze glucose to achieve the desired HMF yield, this will promote the actual production of HMF in the future.
At present, the works on the glucose conversion catalyzed by H-ZSM-5 zeolite to HMF have been reported. For instance, JADHAV et al [17] performed the process of H-ZSM-5 zeolite- catalyzed glucose conversion into HMF in ionic liquids butylmethylimidazolium chloride ([Bmim]Cl) and tetrabutylammonium chloride (TBAC), and obtained a moderate 45% and an ideal 56% yield of HMF, respectively. Although the ionic liquid as the reaction system exhibited high efficiency for the HMF formation from glucose with H-ZSM-5. However, the disadvantages of high price, difficulty in separation and recycling make the desire to further applying it in the practical production of HMF defeat. Later, MORENO- RECIO et al [9] discovered that H2O-methyl isobutyl ketone (MIBK) biphasic system with the merits of low price and easy separation facilitated the HMF formation from glucose with H-ZSM-5 catalyst, and obtained a 42% yield of HMF. Such progressive work not only achieved a good HMF yield, but also provided a path for the realization of alternatives to the ionic liquid reaction system, which is to effectively reduce the cost of HMF formation from glucose by using an efficient and inexpensive reaction system. Given the above background, a reaction system that is cheap and efficient seems to be urgent to break through the bottleneck of the process of H-ZSM-5 zeolite-catalyzed glucose conversion to HMF. Therefore, we presented here three inexpensive and efficient biphasic systems (H2O-THF, H2O-2-butanol and H2O-MeTHF) that help to enhance the HMF production from glucose over H-ZSM-5 zeolite. To our knowledge, the work related to the application of these three systems to H-ZSM-5-catalyzed glucose conversion to HMF has not been reported in detail. Under the optimal reaction conditions, HMF yields of up to 61%, 59%, and 50% were obtained in the H2O-THF, H2O-2-butanol and H2O-MeTHF biphasic systems, respectively. Moreover, the H-ZSM-5 zeolite catalyst maintained excellent stability in the three reaction systems, and after four consecutive cycles, the structure and catalytic performance of H-ZSM-5 zeolite were still well preserved.
2 Experimental
Commercial H-ZSM-5 zeolite was purchased from the Catalyst Plant of Nankai University Catalyst Co., Ltd (Tianjin, China) with the mole ratio of SiO2 to Al2O3 of 27. Prior to use, the H-ZSM-5 zeolite catalyst was calcined in a muffle furnace at 550 °C for 2 h to ensure hydrogen-type. 5-hydroxymethylfurfural (HMF) and glucose were supplied by Aladdin (Shanghai, China). All the other chemicals were obtained from Sinopharm Chemical Reagent Co., Ltd (Shanghai, China). The H-ZSM-5 zeolite catalyst was characterized by XRD, N2 adsorption-desorption, TG, NH3-TPD, Py-FTIR and SEM. Details of characterization methods and instruments are given in supporting information.
3 Results and discussion
3.1 Characterization
The textural properties of H-ZSM-5 catalyst were determined using N2 adsorption isotherm, while the relevant results are presented in Table 1. The BET surface area and pore volume were 389 m2/g and 0.231 cm3/g, respectively. Moreover, it should be noted that the microporous surface area was slightly lower than the corresponding BET data, which implied the existence of mesopores. The isotherm for H-ZSM-5 zeolite is displayed in Figure S1, from which it can be seen that the N2 adsorption isotherm exhibited high nitrogen uptakes at low relative pressure and a plateau at high relative pressure, which was a typical type I isotherm of microporous material. In addition, the appearance of hysteresis loops further confirmed the existence of mesopores.
Table 1 Textural and acidity properties of H-ZSM-5 catalyst

NH3-TPD was employed to evaluate the acid strength of the H-ZSM-5 catalyst. It was generally accepted that the desorption peaks in the temperature range of 100-300, 300-500 and 500-700 °C correspond to weak, moderate and strong acid sites, respectively [18, 19]. As depicted in Figure S2, an intense desorption peak around 150 °C and a weak desorption peak around 440 °C were observed in the NH3-TPD profile of the H-ZSM-5 catalyst, which were attributed to the weak acid and moderate acid sites, respectively. The result confirmed that more weak acid sites and a few moderate acid sites exist in the H-ZSM-5 zeolite. IR-Py was utilized to further quantify the Br nsted and Lewis acid sites of the H-ZSM-5 catalyst and the results are displayed in Table 1. It can be clearly seen from Figure 1 that two intense bands appear at 1450 and 1540 cm-1, which are attributed to Lewis acid and Br
nsted and Lewis acid sites of the H-ZSM-5 catalyst and the results are displayed in Table 1. It can be clearly seen from Figure 1 that two intense bands appear at 1450 and 1540 cm-1, which are attributed to Lewis acid and Br nsted acid sites, respectively [9, 18], indicating that the H-ZSM-5 zeolite could be applied as solid bifunctional catalyst with Br
nsted acid sites, respectively [9, 18], indicating that the H-ZSM-5 zeolite could be applied as solid bifunctional catalyst with Br nsted and Lewis acidity.
nsted and Lewis acidity.
3.2 Screening of reaction system
In order to seek an inexpensive and efficient reaction system suitable for H-ZSM-5 zeolite- catalyzed glucose conversion to HMF, numerous systems including monophasic and biphasic systems were first investigated (Table 2). It can be seen that compared to H2O or organic solvents as monophasic system, the yields and selectivity of HMF were significantly improved when the H-ZSM-5-catalyzed glucose dehydration was carried out in biphasic system composed of H2O and organic solvent. In this regard, the explanation was given that the organic solvent as an extraction phase has the ability to continuously transfer the HMF produced in the aqueous phase, suppressing the preferential condensation and polymerization of HMF into other undesired byproducts such as soluble polymers and humins [20, 21], thereby contributing to the production of HMF. To verify this inference, subsequently, the carbonaceous deposition of H-ZSM-5 zeolite catalyst after use in different reaction systems was studied by thermogravimetric analysis. Figure 2 shows the mass loss of H-ZSM-5 zeolites after use in monophasic and biphasic reaction systems during the thermal treatment in air from room temperature up to 900 °C. It can be observed that when the catalytic conversion of glucose was performed in the monophasic system of H2O, the mass loss of the used H-ZSM-5 sample was 28.9%. The relative mass loss was reduced to 23.1%, 21.1%, and 16.8% as H2O-2-butanol, H2O-THF and H2O-MeTHF were applied as biphasic systems, respectively. The results meant that in the biphasic reaction system, the deposition of insoluble byproducts in the H-ZSM-5 zeolite catalyst was significantly reduced, further reflecting that the biphasic reaction system can effectively inhibit the occurrence of side reactions in the HMF formation from the H-ZSM-5-catalyzed glucose conversion.

Figure 1 FTIR spectra of pyridine adsorption on H-ZSM-5 zeolite catalyst at different temperatures
Table 2 Effect of different reaction systems on H-ZSM-5 zeolite catalyzed glucose conversion to HMF a


Figure 2 TG (a) and DTG (b) curves of H-ZSM-5 catalyst after use in different reaction systems (Reaction condition: glucose (1.0 g), H-ZSM-5 zeolite (0.2 g), NaCl (3.5 g), H2O (10 mL), organic solvent (30 mL), reaction temperature (160 °C), reaction time (90 min))
It was worth noting that, in a variety of biphasic systems, the three reaction systems H2O-2-butanol, H2O-THF and H2O-MeTHF allow the H-ZSM-5 zeolite-catalyzed glucose dehydration process to obtain a considerable yield of HMF exceeding 45% (Table 2, entries 7-9). Especially, the H2O-THF system, which provided a superior 61% HMF yield (Table 2, entry 8). With the aim of exposing the reason for the effectiveness of these three systems, their partition coefficient (R) for HMF was subsequently calculated. One fact can be observed from Table 2 that organic solvents 2-butanol, THF and MeTHF possessed a higher partition coefficient for HMF than other organic solvents (Table 2, entries 4-9). The results suggested that 2-butanol, THF and MeTHF were capable of maximally efficiently extracting the HMF continuously produced in the aqueous phase into the organic phase, alleviating the further degradation of HMF into unwanted by-products, thus achieving an ideal HMF yield.
3.3 Effect of organic phase volume and inorganic salt type on HMF yield
For the process of glucose dehydration in the biphasic reaction system, the volume of the organic phase (extraction phase) plays a key role in the HMF yield. Less extractant is not conducive to the transfer of HMF, resulting in increased rehydration rate [22]. On the other hand, excessive extractants have a negative effect on the HMF yield due to the potential for enhanced humus formation [22, 23]. Therefore, after the biphasic systems H2O-2-butanol, H2O-THF and H2O-MeTHF were believed to be applicable as reaction systems for the H-ZSM-5 zeolite-catalyzed glucose conversion to produce HMF and the effect of the organic phase volume on HMF yield was also investigated. From Figure 3, it was observed that for the three biphasic reactions of H2O-2-butanol, H2O-THF and H2O- MeTHF, when the volume of the organic phase was fixed at 30 mL, the optimal HMF yield can be provided up to 45%, 61%, and 50%, respectively. As a comparison, the lower (20 mL) and higher (40 mL) organic phase volumes did not contribute to the H-ZSM-5 zeolite-catalyzed glucose conversion to HMF reaction. Therefore, considering the above results, 30 mL was determined as the optimal organic phase volume for these three efficient biphasic systems and selected as the test standard throughout the next parameter evaluation.

Figure 3 Effect of organic phase volume on HMF yield (Reaction condition: glucose (1.0 g), H-ZSM-5 zeolite (0.2 g), NaCl (3.5 g), H2O (10 mL), reaction temperature (160 °C), reaction time (90 min))
In the previous study in Ref. [24], the beneficial effects of inorganic salts in the biphasic system were demonstrated. That was to change the intermolecular bonding interaction between liquid components through the salting-out effect. In the presence of inorganic salts, the mutual compatibility between the aqueous phase and the organic phase was not only reduced, but the upper critical solution temperature was also increased, thus ensuring a biphasic state even at high temperatures. In view of this, we subsequently added several inorganic salts (NaCl, KBr, MgCl2) that have been proven to be effective into the three biphasic systems to further study the changes in HMF yield [9, 24]. In a blank control without any inorganic salt addition, only less than 28% of the HMF yield was given in the three biphasic systems H2O-2-butanol, H2O-THF and H2O-MeTHF (Figure 4). After three inorganic salts including NaCl, KBr and MgCl2 were added into biphasic systems, the process of HMF formation catalyzed by H-ZSM-5 zeolite was obviously promoted, and the HMF yield was greatly enhanced. From Figure 4, it can be found that adding NaCl in the three systems was a particularly effective way to promote the HMF formation from glucose with the H-ZSM-5 zeolite catalyst, compared with KBr and MgCl2. Similar results were also confirmed by the investigate previously reported by in Ref. [24].
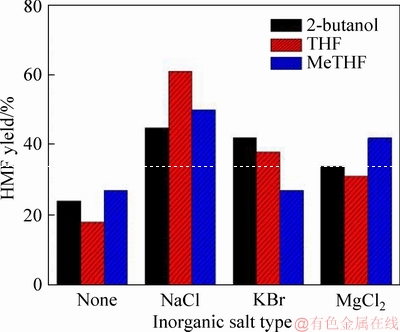
Figure 4 Effect of inorganic salt type on HMF yield (Reaction condition: glucose (1.0 g), H-ZSM-5 zeolite (0.2 g), inorganic salt (3.5 g), H2O (10 mL), organic solvent (30 mL), reaction temperature (160 °C), reaction time (90 min))
3.4 Effect of reaction temperature and time on HMF yield
In order to further increase the HMF yield, we also investigated the change of the corresponding HMF yield in the temperature range of 150 to 190 °C in the three systems H2O-2-butanol, H2O-THF and H2O-MeTHF. As depicted in Figure 5, the process of H-ZSM-5-catalyzed glucose conversion to HMF in the three systems was significantly affected by the reaction temperature. When the reaction was conducted at 150 °C, the HMF yield was low, and only 28%, 28% and 37% of HMF were supplied in the H2O-2-butanol, H2O-MeTHF and H2O-THF biphasic systems, respectively. Later, as the temperature gradually increased, the HMF yield was also enhanced. Also for the glucose conversion process performed in H2O-MeTHF and H2O-THF systems, the optimal 50% and 61% of HMF were achieved at a reaction temperature of 160 °C, indicating that mild reaction conditions favor the generation of HMF in both systems. Further increasing the reaction temperature to over 160 °C, the HMF yields obtained in the H2O-MeTHF and H2O-THF systems started to show a decreasing trend. It can be attributed to the fact that high temperature led to the formation of soluble and insoluble polymers and humins [6, 25], which can be inferred from the color change of the used H-ZSM-5 zeolite as well as the browning solvent(Figures S3 and S4). However, for the H2O-2-butanol system, the highest HMF yield of 59% was achieved after the temperature was raised to 180 °C. It seemed to reveal that the high temperatures are more beneficial for the process of catalytic glucose conversion to form HMF in the H2O-2-butanol reaction system by the H-ZSM-5 zeolite catalyst. Taken together above results, 160 °C, 160 °C and 180 °C were recognized to be the optimum temperatures for the H-ZSM-5 zeolite-catalyzed glucose dehydration reaction in H2O-MeTHF, H2O-THF and H2O-2-butanol reaction systems, respectively.

Figure 5 Effect of reaction temperature on HMF yield (Reaction condition: glucose (1.0 g), H-ZSM-5 zeolite (0.2 g), NaCl (3.5 g), H2O (10 mL), organic solvent(30 mL), reaction time (90 min))
Then, the effect of reaction time on the HMF yield was evaluated between 60 and 180 min. As shown in Figure 6, at the optimal reaction temperature for each of the three systems, a shorter reaction time ensured that the H-ZSM-5 zeolite catalyzed the glucose conversion process to obtain a considerable HMF yield. Also when the catalytic reaction was proceeded for 90 min, the optimal HMF yield (more than 50%) was achieved in all the three systems. With the extension of reaction time, the HMF yields gradually decreased after reaching the peak. It was due to the fact that the dehydration process was maintained at a longer reaction time leading to the occurrence of side reactions and the formation of undesirable by-products [25, 26], thus resulting in a decrease in the HMF yield. It was worth mentioning, however, that only negligible levulinic acid was detected in H2O-THF system after the reaction was completed, but not in the other two biphasic systems. In other words, the H2O-2-butanol and H2O-MeTHF systems can effectively avoid rehydration reactions that occur during the H-ZSM-5 zeolite catalyzed glucose conversion to HMF, and the main by-products formed were humins, soluble polymers or aldol condensation products [27, 28]. So, 90 min were identified as the optimum reaction time for all three systems.

Figure 6 Effect of reaction time on HMF yield (Reaction condition: glucose (1.0 g), H-ZSM-5 zeolite (0.2 g), NaCl (3.5 g), H2O (10 mL), organic solvent (30 mL), reaction temperature (H2O/THF and H2O-MeTHF systems: 160 °C; H2O-2-butanol system: 180 °C))
3.5 Effect of H-ZSM-5 dosage on HMF yield
Once the most suitable reaction conditions were established, the effect of catalyst dosage on the glucose conversion to HMF was also studied. Figure 7 indicates that high yield of HMF strongly depended on the use of H-ZSM-5 zeolite in the three systems. In a comparative experiment without any catalyst addition, the HMF yields given by the three systems were relatively low, ranging from 17% to 26%. After the H-ZSM-5 zeolite was loaded as a catalyst, the HMF yields were significantly increased, and the presence of 0.2 g of H-ZSM-5 zeolite in systems H2O-2-butanol, H2O-THF and H2O-MeTHF could achieve optimal 59%, 61% and 50% HMF, respectively. Later, the use of H-ZSM-5 zeolite in the three systems was further increased to 0.3 and 0.4 g, resulting in a corresponding decrease in the HMF yields. It was mainly due to the excess H-ZSM-5 zeolite facilitated side reactions, particularly excess Lewis acid sites, which inevitably promoted the degradation reaction and led to the formation of undersired compounds[29, 30]. Therefore, for systems H2O-2-butanol, H2O-THF and H2O-MeTHF, 0.2 g of H-ZSM-5 zeolite was considered to be the most reasonable catalyst dosage.
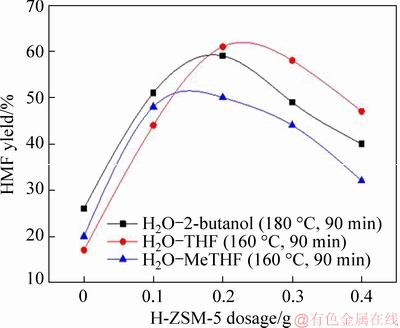
Figure 7 Effect of H-ZSM-5 dosage on HMF yield (Reaction condition: glucose (1.0 g), NaCl (3.5 g), H2O (10 mL), organic solvent (30 mL), reaction temperature (H2O-THF and H2O-MeTHF systems: 160 °C; H2O-2- butanol system: 180 °C), reaction time (90 min))
3.6 Stability of H-ZSM-5 zeolite catalyst in different reaction systems
Whether the catalyst can maintain good stability and reusability in the reaction system is crucial for evaluating the efficiency of the heterogeneous catalytic systems. Thus, we investigated the cycling experiments of H-ZSM-5- catalyzed glucose conversion in three systems under optimal reaction conditions. After each run, the used H-ZSM-5 catalyst was collected by filtration, washed with water and dried overnight at 100 °C. Subsequently, the recovered H-ZSM-5 catalyst was calcined at 450 °C for 3 h to remove the adsorbed by-products. The relevant results are shown in Figure 8, from which it can be observed that after four consecutive cycles in the H2O-2-butanol, H2O-THF, and H2O-MeTHF reaction systems, the used H-ZSM-5 zeolite catalyst can still provide HMF yields of 51%, 53%, and 44%, respectively. It confirmed that the catalytic activity of H-ZSM-5 zeolite catalyst in these three biphasic systems was still maintained after many cycles of utilization. The slight decrease in the HMF yield was probably due to the partial collapse of the framework of the regenerated catalyst and the decrease in its acidity (Table S1). In addition, the XRD patterns of H-ZSM-5 zeolite regenerated after the fourth cycle were almost the same as that of the fresh one (Figure 9), and match well with that of a typical MFI structure, with main diffraction peaks at 2θ=10.16°, 11.33°, 23.08°, 23.48° and 23.78° (PDF 00-037-0359) [9, 31]. Moreover, it can also be seen from the SEM images that the typical morphology of the H-ZSM-5 zeolite did not change significantly after four cycles in the three systems (Figure 10). All the above results further confirmed the fact that in the three systems H2O-2-butanol, H2O-THF and H2O-MeTHF, the H-ZSM-5 catalyst can maintain good catalytic activity and structural stability.
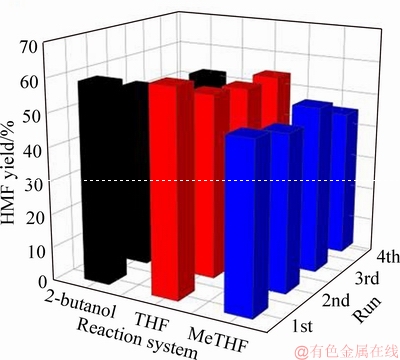
Figure 8 H-ZSM-5 catalyst stability testing in biphasic systems (Reaction condition: glucose (1.0 g), NaCl(3.5 g), H2O (10 mL), organic solvent (30 mL), reaction temperature (H2O-THF and H2O-MeTHF systems: 160 °C, H2O-2-butanol system: 180 °C), reaction time (90 min))

Figure 9 XRD patterns of fresh and regenerated H-ZSM-5 catalysts
3.7 Path of HMF formation from glucose in three systems catalyzed by H-ZSM-5 zeolite.
In the work presented, H-ZSM-5 zeolite with Br nsted and Lewis acidity has been demonstrated to catalyze glucose to achieve the desired HMF yield in the three systems H2O-2-butanol, H2O-THF, and H2O-MeTHF. It was well known that the formation of HMF from glucose was a process of synergistic catalysis by the acid sites of Br
nsted and Lewis acidity has been demonstrated to catalyze glucose to achieve the desired HMF yield in the three systems H2O-2-butanol, H2O-THF, and H2O-MeTHF. It was well known that the formation of HMF from glucose was a process of synergistic catalysis by the acid sites of Br nsted and Lewis, including isomerization and dehydration [9, 32]. And, in the process of synergistic catalysis, levulinic acid was often accompanied as a by-product due to the tendency of HMF to undergo further rehydration reaction[33, 34]. However, as mentioned above, negligible levulinic acid was only detected in system H2O-THF after the reaction was over, but not in systems H2O-2-butanol and H2O-MeTHF. Therefore, taking these results together, the pathway for the conversion of glucose to HMF catalyzed by H-ZSM-5 zeolite in the three systems was summarized. As shown in Figure 11, for the three reaction systems, the following two steps were certain: 1) isomerization of glucose to fructose by Lewis acid sites on the H-ZSM-5 zeolite; 2) further dehydration of the generated fructose to HMF Br
nsted and Lewis, including isomerization and dehydration [9, 32]. And, in the process of synergistic catalysis, levulinic acid was often accompanied as a by-product due to the tendency of HMF to undergo further rehydration reaction[33, 34]. However, as mentioned above, negligible levulinic acid was only detected in system H2O-THF after the reaction was over, but not in systems H2O-2-butanol and H2O-MeTHF. Therefore, taking these results together, the pathway for the conversion of glucose to HMF catalyzed by H-ZSM-5 zeolite in the three systems was summarized. As shown in Figure 11, for the three reaction systems, the following two steps were certain: 1) isomerization of glucose to fructose by Lewis acid sites on the H-ZSM-5 zeolite; 2) further dehydration of the generated fructose to HMF Br nsted acid sites. Since H2O-2-butanol and H2O-MeTHF have the ability to inhibit the rehydration of HMF, the further conversion of HMF to levulinic acid and formic acid occurred only in system H2O-THF. Besides, apparent humins and polymers formed during the catalytic process were observed in the three systems based on the catalyst and the solution that turned dark brown after the reaction (Figures S3 and S4). Therefore, we also speculate that humins and soluble polymers were the major by-products for H-ZSM-5-catalyzed glucose dehydration in the three systems H2O-2-butanol, H2O-THF, and H2O-MeTHF.
nsted acid sites. Since H2O-2-butanol and H2O-MeTHF have the ability to inhibit the rehydration of HMF, the further conversion of HMF to levulinic acid and formic acid occurred only in system H2O-THF. Besides, apparent humins and polymers formed during the catalytic process were observed in the three systems based on the catalyst and the solution that turned dark brown after the reaction (Figures S3 and S4). Therefore, we also speculate that humins and soluble polymers were the major by-products for H-ZSM-5-catalyzed glucose dehydration in the three systems H2O-2-butanol, H2O-THF, and H2O-MeTHF.

Figure 10 SEM images of fresh and regenerated H-ZSM-5 catalysts:
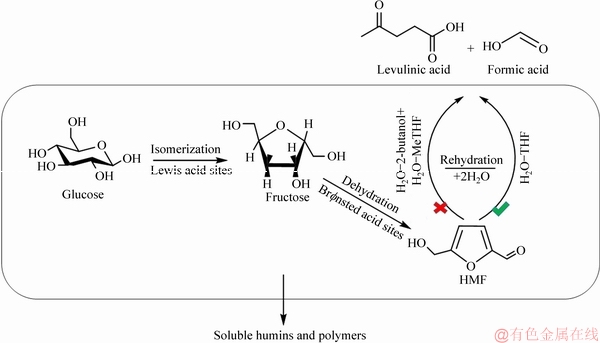
Figure 11 Possible pathways for glucose conversion to HMF in three biphasic systems on H-ZSM-5
4 Conclusions
The utilization of the inexpensive H2O-2-butanol, H2O-THF, and H2O-MeTHF biphasic systems was found to be efficient for H-ZSM-5 zeolite-catalyzed glucose conversion to HMF. Under optimal reaction conditions, more than 50% of the HMF yield was provided in the three reaction systems. Especially, the H2O-THF biphasic system, which enabled the H-ZSM-5 to catalyze glucose dehydration, gave a superior 61% HMF. Furthermore, the H-ZSM-5 zeolite also maintained good stability in the three reaction systems, and after four consecutive cycles, the catalytic activity and structure were maintained. Therefore, the heterogeneous catalytic systems described in this work showed the prospects for further application of HMF production in the future.
Supporting information
A Catalyst characterization
The structures of fresh and used H-ZSM-5 zeolite catalysts were characterized by power X-ray diffraction (XRD; Rigaku Ultima IV), using Cu-Kα radiation (0.154 nm, 40 kV, 40 mA) at a scanning rate of 10 (°)/min between 5° and 80°.
The nitrogen adsorption-desorption isotherms of H-ZSM-5 zeolite was recorded at -196 °C using a 3H-2000PS1 gas sorption system (BeiShiDe Instrument Technology Co., Ltd., Beijing, China) to obtain the surface area and pore volume. Before adsorption analysis, the sample was degassed at 200 °C for 12 h. The specific surface area and total pore volume of sample were determined by the BET (Brunauer–Emmett–Teller) method and the BJH (Barrett–Joyner–Halanda) method, respectively.
The thermogravimetric (TG) analysis of the used H-ZSM-5 zeolite catalysts was implemented on a NETZSCH-STA 409PC DSC-SP thermal analyzer with 5–10 mg of sample by increasing the temperature from 40 to 900 °C at 10 °C/min under an air flow rate of 30 mL/min.
The acid strength of H-ZSM-5 zeolite was evaluated by NH3-temperature-programmed desorption (NH3-TPD) using a TP-5076 catalyst analyzer (Xianquan Industrial and Trading Co., Ltd., Tianjing, China) equipped with a thermal conductivity detector (TCD). In a typical acidity measurement, the H-ZSM-5 zeolite sample was degassed at 400 °C for 1 h under a constant He flow (40 cm3/min). Then, the sample was cooled to 50 °C and NH3 was adsorbed for 0.5 h. After saturation, He was purged for 1 h at 20 cm3/min to remove excess NH3 on the zeolite surface. Finally, NH3 desorption was performed from 50 to 700 °C with a heating rate of 10 °C /min under a He flow (20 cm3/min).
FTIR spectra of pyridine adsorption were recorded on a Frontier Fourier Transform Infrared Instrument. H-ZSM-5 samples were previously evacuated at 400 °C and 6×10-2 Pa for 1 h, and then exposed to pyridine vapours at room temperature for 1 h. Finally, they were outgassed at different temperatures (from 100 to 300 °C). The relative concentrations of Br nsted and Lewis acid sites were determined from the area of the absorption bands at 1545 and 1450 cm-1, respectively.
nsted and Lewis acid sites were determined from the area of the absorption bands at 1545 and 1450 cm-1, respectively.
The morphology of fresh and used H-ZSM-5 zeolite catalysts was investigated by using a scanning electron microscope (SEM, SU8010, Hitachi, Japan). Before the SEM observation, the dried powder sample was dispersed on carbon tape supported on a stub and then pretreated with Pt sputtering.
B Catalytic runs
The dehydration process of glucose was performed in a 100 mL autoclave equipped with a temperature-controlled heating-jacket and mechanical stirrer (Xinyuan Chemical Co., Ltd., Weihai, China). In a typical run, a mixture of glucose (1.0 g), H-ZSM-5 zeolite catalyst (0.2 g) and solvent (30 mL THF and 10 mL H2O saturated with NaCl) was loading into the sealed reactor with a stirring rate of 500 r/min. Once the reaction temperature was reached, the monitoring of the reaction started. After the reaction was over, the mixture was cooled to room temperature. The liquid was filtered before further analysis using HPLC.
C Product analysis
The concentration of HMF in the aqueous and organic phases was determined by using HPLC (Shimadzu LC-2010AHT) with an XDB-C18 column and a UV detector at 280 nm. The mobile phase was a solution of water and methanol (3/7, v/v) at a flow rate of 0.5 mL/min and the temperature of the column oven was maintained at 35 °C. The glucose concentration was analyzed by using HPLC (Agilent 1200) equipped with a column (Agilent Hi-Plex H) and a Refractive Index detector. The mobile phase was deionized water at a flow rate of 0.5 mL/min and the temperature of column oven was kept at 60 °C. The conversion of glucose, yield of HMF was defined as follows:
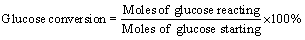



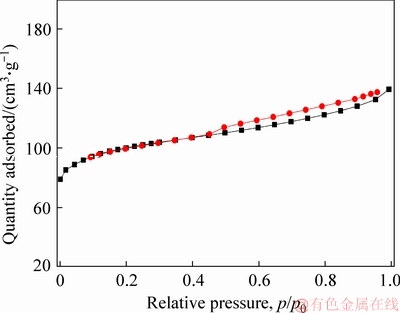
Figure S1 Nitrogen adsorption isotherms of H-ZSM-5 zeolite catalyst

Figure S2 NH3-TPD profile of H-ZSM-5 zeolite catalyst

Figure S3 Biphasic systems obtained after high temperature reaction under reaction condition: glucose 1.0 g, H-ZSM-5 zeolite 0.2 g, NaCl 3.5 g, H2O 10 mL, organic solvent 30 mL, reaction time 90 min

Figure S4 H-ZSM-5 catalyst obtained after high temperature reaction under reaction condition: glucose 1.0 g, H-ZSM-5 zeolite 0.2 g, NaCl 3.5 g, H2O 10 mL, organic solvent 30 mL, reaction time 90 min
Table S1 Physicochemical properties of regenerated catalyst

References
[1] van NGUYEN C, LIAO Y T, KANG T C. A metal-free, high nitrogen-doped nanoporous graphitic carbon catalyst for an effective aerobic HMF-to-FDCA conversion [J]. Green Chemistry, 2016, 18(22): 5957-5961. DOI: 10.1039/ C6GC02118B.
[2] OSATIASHTIANI A, LEE A F, GRANOLLERS M. Hydrothermally stable, conformal, sulfated zirconia monolayer catalysts for glucose conversion to 5-HMF [J]. ACS Catalysis, 2015, 5(7): 4345-4352. DOI: 10.1021/ acscatal.5b00965.
[3] TONG X, MA Y, LI Y. Biomass into chemicals: Conversion of sugars to furan derivatives by catalytic processes [J]. Applied Catalysis A: General, 2010, 385(1, 2): 1-13. DOI: 10.1016/j.apcata.2010.06.049.
[4] XU S, YAN X, BU Q. Highly efficient conversion of carbohydrates into 5-hydroxymethylfurfural using the bi-functional CrPO4 catalyst[J]. RSC Advances, 2016, 6(10): 8048-8052. DOI: 10.1039/C5RA23716E.
[5] BAO Q, QIAO K, TOMIDA D. Preparation of 5-hydroymethylfurfural by dehydration of fructose in the presence of acidic ionic liquid [J]. Catalysis Communications, 2008, 9(6): 1383-1388. DOI: 10.1016/j.catcom.2007.12.002.
[6] ROSATELLA A A, SIMEONOV S P, FRADE R F M. 5-hydroxymethylfurfural (HMF) as a building block platform: Biological properties, synthesis and synthetic applications [J]. Green Chemistry, 2011, 13(4): 754-793. DOI: 10.1039/C0GC00401D.
[7] YONG G, ZHANG Y, YING J Y. Efficient catalytic system for the selective production of 5-hydroxymethylfurfural from glucose and fructose [J]. Angewandte Chemie International Edition, 2008, 47(48): 9345-9348. DOI: 10.1002/anie.200803207.
[8] CHOUDHARY V, MUSHRIF S H, HO C. Insights into the interplay of Lewis and Br nsted acid catalysts in glucose and fructose conversion to 5-(hydroxymethyl) furfural and levulinic acid in aqueous media [J]. Journal of the American Chemical Society, 2013, 135(10): 3997-4006. DOI: 10.1021/ ja3122763.
nsted acid catalysts in glucose and fructose conversion to 5-(hydroxymethyl) furfural and levulinic acid in aqueous media [J]. Journal of the American Chemical Society, 2013, 135(10): 3997-4006. DOI: 10.1021/ ja3122763.
[9] MORENO-RECIO M, SANTAMAR A-GONZ
A-GONZ LEZ J, MAIRELES-TORRES P. Br
LEZ J, MAIRELES-TORRES P. Br nsted and Lewis acid ZSM-5 zeolites for the catalytic dehydration of glucose into 5-hydroxymethylfurfural [J]. Chemical Engineering Journal, 2016, 303: 22-30. DOI:10.1016/j.cej.2016.05.120.
nsted and Lewis acid ZSM-5 zeolites for the catalytic dehydration of glucose into 5-hydroxymethylfurfural [J]. Chemical Engineering Journal, 2016, 303: 22-30. DOI:10.1016/j.cej.2016.05.120.
[10] QI X, WATANABE M, AIDA T M. Catalytic conversion of cellulose into 5-hydroxymethylfurfural in high yields via a two-step process [J]. Cellulose, 2011, 18(5): 1327-1333. DOI: 10.1007/s10570-011-9568-1.
[11] MO H, CHEN X, LIAO X. Sustainable synthesis of 5-hydroxymethylfurfural from waste cotton stalk catalyzed by solid superacid-SO42-/ZrO2 [J]. Journal of Central South University, 2017, 24(8): 1745-1753. DOI: https://doi.org/ 10.1007/s11771-017-3582-x.
[12] XU S, PAN D, WU Y. Catalytic conversion of xylose and xylan into furfural over Cr3+/P-SBA-15 catalyst derived from spent adsorbent [J]. Industrial & Engineering Chemistry Research, 2019, 58(29): 13013-13020. DOI: 10.1021/acs. iecr.9b01821.
[13] XU S, PAN D, WU Y. Direct conversion of wheat straw components into furan compounds using a highly efficient and reusable SnCl2-PTA/β zeolite catalyst [J]. Industrial & Engineering Chemistry Research, 2019, 58(22): 9276-9285. DOI: 10.1021/acs.iecr.9b00984.
[14] FAN C, GUAN H, ZHANG H. Conversion of fructose and glucose into 5-hydroxymethylfurfural catalyzed by a solid heteropolyacid salt [J]. Biomass & Bioenergy, 2011, 35(7): 2659-2665. DOI:10.1016/j.biombioe.2011.03.004.
[15] OLSON D H, KOKOTAILO G T, LAWTON S L. Crystal structure and structure-related properties of ZSM-5 [J]. The Journal of Physical Chemistry, 1981, 85(15): 2238-2243. DOI: 10.1021/j150615a020.
[16] SHIRAZI L, JAMSHIDI E, GHASEMI M R. The effect of Si/Al ratio of ZSM-5 zeolite on its morphology, acidity and crystal size [J]. Crystal Research and Technology: Journal of Experimental and Industrial Crystallography, 2008, 43(12): 1300-1306. DOI:10.1002/crat.200800149.
[17] JADHAV H, TAARNING E, PEDERSEN C M. Conversion of D-glucose into 5-hydroxymethylfurfural (HMF) using zeolite in [Bmim]Cl or tetrabutylammonium chloride (TBAC)/CrCl2 [J]. Tetrahedron Letters, 2012, 53(8): 983-985. DOI:10.1016/j.tetlet.2011.12.059.
[18] RAMLI N A S, AMIN N A S. Fe/HY zeolite as an effective catalyst for levulinic acid production from glucose: Characterization and catalytic performance [J]. Applied Catalysis B: Environmental, 2015, 163: 487-498. DOI: 10.1016/j.apcatb.2014.08.031.
[19] LONG R Q, YANG R T. Reaction mechanism of selective catalytic reduction of NO with NH3 over Fe-ZSM-5 catalyst [J]. Journal of Catalysis, 2002, 207(2): 224-231. DOI: 10.1006/jcat.2002.3528.
[20] PAGAN-TORRES Y J, WANG T, GALLO J M R. Production of 5-hydroxymethylfurfural from glucose using a combination of Lewis and Br nsted acid catalysts in water in a biphasic reactor with an alkylphenol solvent [J]. ACS Catalysis, 2012, 2(6): 930-934. DOI:10.1021/cs300192z.
nsted acid catalysts in water in a biphasic reactor with an alkylphenol solvent [J]. ACS Catalysis, 2012, 2(6): 930-934. DOI:10.1021/cs300192z.
[21] XIA H, HU H, XU S. Catalytic conversion of glucose to 5-hydroxymethyfural over Fe/β zeolites with extra- framework isolated Fe species in a biphasic reaction system [J]. Biomass & Bioenergy, 2018, 108: 426-432. DOI: 10.1016/j.biombioe.2017.12.007.
[22] LI H, DENG A, REN J. A modified biphasic system for the dehydration of D-xylose into furfural using SO42-/TiO2-ZrO2/La3+ as a solid catalyst [J]. Catalysis Today, 2014, 234: 251-256. DOI:10.1016/j.cattod.2013.12.043.
[23] MORAIS A R C, BOGEL-LUKASIK R. Highly efficient and selective CO2-adjunctive dehydration of xylose to furfural in aqueous media with THF[J]. Green Chemistry, 2016, 18(8): 2331-2334. DOI:10.1039/C5GC02863A.
[24] COMBS E, CINLAR B, PAGAN-TORRES Y, DUMESIC J A, SHANKS B H. Influence of alkali and alkaline earth metal salts on glucose conversion to 5-hydroxymethylfurfural in an aqueous system [J]. Catalysis Communications, 2013, 30: 1-4. DOI: 10.1016/j.catcom. 2012.10.011.
[25] TSILOMELEKIS G, ORELLA M J, LIN Z. Molecular structure, morphology and growth mechanisms and rates of 5-hydroxymethyl furfural (HMF) derived humins [J]. Green Chemistry, 2016, 18(7): 1983-1993. DOI: 10.1039/ C5GC01938A.
[26] PATIL S K R, HELTZEL J, LUND C R F. Comparison of structural features of humins formed catalytically from glucose, fructose, and 5-hydroxymethylfurfuraldehyde [J]. Energy & Fuels, 2012, 26(8): 5281-5293. DOI: 10.1021/ ef3007454.
[27] JIM NEZ-MORALES I, MORENO-RECIO M, SANTAMAR
NEZ-MORALES I, MORENO-RECIO M, SANTAMAR A-GONZ
A-GONZ LEZ J. Production of 5-hydroxymethylfurfural from glucose using aluminium doped MCM-41 silica as acid catalyst [J]. Applied Catalysis B: Environmental, 2015, 164: 70-76. DOI: 10.1016/j.apcatb. 2014.09.002.
LEZ J. Production of 5-hydroxymethylfurfural from glucose using aluminium doped MCM-41 silica as acid catalyst [J]. Applied Catalysis B: Environmental, 2015, 164: 70-76. DOI: 10.1016/j.apcatb. 2014.09.002.
[28] HU S, ZHANG Z, ZHOU Y. Conversion of fructose to 5-hydroxymethylfurfural using ionic liquids prepared from renewable materials [J]. Green Chemistry, 2008, 10(12): 1280-1283. DOI:10.1039/B810392E.
[29] OTOMO R, YOKOI T, KONDO J N. Dealuminated Beta zeolite as effective bifunctional catalyst for direct transformation of glucose to 5-hydroxymethylfurfural [J]. Applied Catalysis A: General, 2014, 470: 318-326. DOI: 10.1016/j.apcata.2013.11.012.
[30] OTOMO R, TATSUMI T, YOKOI T. Beta zeolite: A universally applicable catalyst for the conversion of various types of saccharides into furfurals [J]. Catalysis Science & Technology, 2015, 5(8): 4001-4007. DOI: 10.1039/ C5CY00719D.
[31] FOGER K, SANDERS J V, SEDDON D. Channel arrangements and activity of some ZSM zeolites [J]. Zeolites, 1984, 4(4): 337-345. DOI:10.1016/0144-2449(84)90009-5.
[32] SWIFT T D, NGUYEN H, ERDMAN Z. Tandem Lewis acid/Br nsted acid-catalyzed conversion of carbohydrates to 5-hydroxymethylfurfural using zeolite beta [J]. Journal of Catalysis, 2016, 333: 149-161. DOI: 10.1016/j.jcat.2015. 10.009.
nsted acid-catalyzed conversion of carbohydrates to 5-hydroxymethylfurfural using zeolite beta [J]. Journal of Catalysis, 2016, 333: 149-161. DOI: 10.1016/j.jcat.2015. 10.009.
[33] GIRISUTA B, JANSSEN L, HEERES H J. A kinetic study on the decomposition of 5-hydroxymethylfurfural into levulinic acid [J]. Green Chemistry, 2006, 8(8): 701-709. DOI: 10.1039/b518176c.
[34] PENG L, LIN L, ZHANG J. Catalytic conversion of cellulose to levulinic acid by metal chlorides [J]. Molecules, 2010, 15(8): 5258-5272. DOI:10.3390/molecules15085258.
(Edited by YANG Hua)
中文导读
H-ZSM-5分子筛在H2O-四氢呋喃/2-丁醇/2-甲基四氢呋喃反应体系中催化葡萄糖转化制备5-羟甲基糠醛
摘要:在本文中,H2O-四氢呋喃、H2O-2-丁醇、H2O-2-甲基四氢呋喃三种廉价的双相反应体系被发现并证实可以高效地促进H-ZSM-5分子筛催化葡萄糖转化制备5-羟甲基糠醛(HMF)。为了在三种反应体系中获得最佳的HMF产率,研究了包括反应温度、反应时间、催化剂用量、有机相体积以及无机盐种类在内的不同反应参数对HMF产率的影响。研究发现,最佳反应条件下,H-ZSM-5分子筛催化葡萄糖转化制备HMF在H2O-四氢呋喃、H2O-2-丁醇、H2O-2-甲基四氢呋喃三种体系中分别可获得高达61%、59%以及50%的产率。这些结果证实上述三种高效的反应体系可以实现对昂贵的离子液体反应体系进行替代。而且更重要的是,在上述三种反应体系中,H-ZSM-5分子筛在经历多次循环使用后依然可以保持催化活性和物质结构的稳定性。因此我们相信,本文中所呈现的高效、廉价的催化体系在HMF的实际生产过程中将会具有极大的应用前景。
关键词:5-羟甲基糠醛;H-ZSM-5分子筛;葡萄糖;双相体系
Foundation item: Project( 3207049713) supported by the Scientific Research Foundation of Graduate School of Southeast University, China
Received date: 2018-07-14; Accepted date: 2019-09-10
Corresponding author: XIAO Guo-min, PhD, Professor; Tel: +86-25-52090612; E-mail: xiaogm@seu.edu.cn; ORCID: 0000-0001-8482- 1276

