Advances in microwave assisted synthesis of ordered mesoporous materials
来源期刊:中国有色金属学报(英文版)2009年增刊第3期
论文作者:曹渊 魏红娟 夏之宁
文章页码:656 - 664
Key words:microwave assisted synthesis; ordered mesoporous materials (OMMs); ordered mesoporous silicas (OMSs); periodic mesoporous organosilicas (PMOs)
Abstract: Mesoporous materials with uniform pores and high specific areas are used in many fields including catalysts, separation and adsorbents, etc. In order to find faster and more economical synthesis routes, the use of microwave heating was deeply studied. Compared to the hydrothermal method, microwave energy can heat the samples to crystallization temperature rapidly and uniformly result in homogeneous nucleation and shorten crystallization time. The basic principles of microwave assisted synthesis and advantages of microwave heating, and the obtained progress concerning ordered mesoporous materials through microwave synthesis were summarized.
基金信息:the National Natural Science Foundation of China
China Postdoctoral Science Foundation

CAO Yuan(曹 渊)1, 2, WEI Hong-juan(魏红娟)1, XIA Zhi-ning(夏之宁)1, 2
1. College of Chemistry and Chemical Engineering, Chongqing University, Chongqing 400044, China;
2. College of Bioengineering, Chongqing University, Chongqing 400044, China
Received 10 August 2009; accepted 15 September 2009
Abstract: Mesoporous materials with uniform pores and high specific areas are used in many fields including catalysts, separation and adsorbents, etc. In order to find faster and more economical synthesis routes, the use of microwave heating was deeply studied. Compared to the hydrothermal method, microwave energy can heat the samples to crystallization temperature rapidly and uniformly result in homogeneous nucleation and shorten crystallization time. The basic principles of microwave assisted synthesis and advantages of microwave heating, and the obtained progress concerning ordered mesoporous materials through microwave synthesis were summarized.
Key words: microwave assisted synthesis; ordered mesoporous materials (OMMs); ordered mesoporous silicas (OMSs); periodic mesoporous organosilicas (PMOs)
Mesoporous materials with uniform pores and high specific areas are given great attention from material researchers because of their potential applications including catalysts[1-2], separation and adsorption for large organic molecules[3], and guest–host chemical supporters[4], etc.
The pioneer work on the synthesis of mesoporous materials can be traced to 1992[5]. During the past 20 years, mesoporous molecular sieves were developed rapidly. Mesoporous materials are divided into two categories which are silicon-based and non-siliceous mesoporous materials. And silicon-based mesoporous materials (or called mesoporous silicates) are divided into pure silicate and modified ones. Pure silicate materials include MCM, SBA, HMS, MSU, and so on, while modified silicates include ones doped with metal ions (such as doped with Al, Ti, V, Mn, Fe, B, Cu, Co, etc) and organic molecules modified ones (such as chloride-trimethoxysilane, mercaptopropyl trime- thoxysilane, sulfonic acid, etc). Non-siliceous mesoporous materials include transition metal oxides (such as zirconia, titanium dioxide, tin oxide, manganese oxide, niobium oxide, tantalum oxide, etc) and non-metallic oxides (such as phosphate, sulfate and mesoporous carbon). The traditional synthesis method of mesoporous molecular sieves is hydrothermal synthesis, the general process of which is a certain amount of surfactants, and acid or alkali are added to compose mixed aqueous. Next, inorganic sources are added to generate a water gel, and then heated in the autoclave at a certain temperature to crystallize, and after filtering, washing, drying, calcination or extraction to remove the template agent. Finally, ordered mesoporous materials are obtained. However, there are some disadvantages of time consuming and energy dissipation.
Microwaves have been used to synthesize mesoporous materials for several years. So, ordered mesoporous silicas (OMSs) with some good qualities have been prepared by using microwave-assisted synthesis nowadays (in Table 1). Compared with the traditional hydrothermal method, microwave-assisted synthesis method has some advantages of fast response rate (in Table 2), low energy consumption [9, 21] and the product of uniform size[6, 8, 22]. Thus it attracts extra attention.
This present review attempts to summarize the obtained progress in ordered mesoporous materials through microwave synthesis. At beginning, there is a brief introduction of microwave assisted synthesis. The basic physical principles and advantages of microwave heating are then discussed. Furthermore, so far some literatures on the microwave synthesis of ordered mesoporous materials are summarized. Finally, an outlook on the future development of ordered mesoporous materials is given.
Table 1 Conditions of microwave synthesis of ordered mesoporous materials

Table 2 Synthesis time under microwave irradiation and conventional method

After several years of microwave research, microwave-assisted synthesis has become a highly useful technique, and some review articles were published [23, 31]. Mingos have given a thorough explanation of the microwave heating theory[30]. NUCHTER et al[26] have given a technology overview mostly on reaction engineering in microwave field. We will describe briefly microwave heating in the below.
Microwaves with wavelengths between 0.01 and 1 m are operated in a frequency range between 0.3 and 30 GHz. All the reported microwave experiments are conducted at 2 450 MHz. One of the reasons is that the microwave energy absorption by liquid water is maximal near to this frequency. Another one is that 2450 MHz magnetrons are most available in the commercial microwave equipments. Interaction of dielectric materials with microwaves is due to a net polarization of the substance. There are several mechanisms that are responsible for this, including electronic, ionic, molecular (dipole) and interfacial (space-charge) polarization. In the presence of an oscillating field, dipolar molecules try to orient themselves or be in phase with the field. However, their motion is restricted by resisting forces (inter-particle interaction and electric resistance), which restrict their motion and generate heat.
Compared with conventional heating, microwave heating has these advantages for chemical synthesis. The introduction of microwave energy into a chemical reaction can lead to much higher heating rates. The microwave energy is introduced without direct contact between the energy source and the reacting chemicals. It is volumetric heating with no wall or heat diffusion effects. It can realize selective heating.
2 Microwave synthesis of ordered meso- porous materials
Since the synthesis of MCM-41 by Mobil, ordered mesoporous materials have evoked extensive research interests. They exhibit tunable pore size, well-defined pore arrangement, relatively large specific surface area, large pore volume, and so on. So, we introduce some cases on microwave synthesis.
2.1 Microwave synthesis of mesoporous silicates
MCM-41 is a new type of uni-dimensional regular array with hexagonal channels, highly ordering, uniform pore size ranging among 2-20 nm and large specific surface area. The synthesis of MCM-41 is characterized by the largest template, which is different from the synthesis of the traditional molecule sieve using a single organic molecule or metal ions as template agents.
In 1996, WU et al[32] investigated thermal stable molecular sieve MCM-41 with hexagonal channels in a temperature-controlled microwave oven from aged precursor gels within about 1 h. Silica ester was used as silica source, and cetyl trimethy ammoniumbromide (CTABr) as the template. It is indicated that microwave irradiation may make the synthesis more quickly and convenient.
After two years, mesoporous material MCM-41 was obtained by microwave treatment of precursor gel at 100-120 ℃ for 1 h or less. In addition to shorten synthesis time, microwave irradiation provided a way to control its crystallinity and morphology, especially the addition of a small amount of ethylene glycol (EG) in synthetic mixture, as shown in Fig.1[6].
MCM-48 with Ia3d cubic structure contains two sets of three-dimensional pore systems which are separated but mutually entangling. Compared with the one- dimensional pore MCM-41 system, its structure is more beneficial to the proliferation of reactants, the movement of which is not obstructed. MCM-48 was first synthesized in polypropylene bottles under ambient pressure using the microwave hydrothermal method in 2005. The final molar fraction of the synthesis mixture was TEOS 1%, CTABr 0.15%, NaOH 0.5%, and H2O 80%. The final gel was divided in two parts and poured in Teflon autoclaves for microwave heating. The gel was allowed to heat at 100 ℃ for 1 and 2 h. However, from Fig.1, it is clear that there is structural disorder. Besides MCM-48, a mesoporous wormhole like structure coexists. This is reflected in the lowering of surface area and pore volume compared with MCM-48 materials prepared by conventional method [16].
Another kind of ordered mesoporous material SBA-15 is a very promising material with highly ordered hexagonal pore structure. The diameter is 5-50 nm which is tunable. And pore wall thickness (typically 3-9 nm) makes the material good hydrothermal stability. In 2006, microwave technology made a temperature programmed microwave-assisted synthesis (TPMS) of ordered mesoporous silicas available. This work took advantage of the existing capabilities of modern microwave systems to program the temperature and time. The major advantage is the feasibility of temperature and time programming, which had been demonstrated by the synthesis of one of the most popular SBA-15 over an unprecedented temperature range from 40 to 200 ℃. These SBA-15 samples exhibited better thermal stability. This study show that the simplicity and capability of temperature and time programming in TPMS allowed one not only to tune the adsorption and structural properties of OMSs, but also to easily screen a wide range of conditions in order to optimize and scale up their preparation, as well as to significantly reduce the synthesis time from days to hours[7].

Fig.1 SEM images of MCM-41 molecular sieves prepared by hydrothermal heating (a) and microwave induced heating with ethylene glycol (EG) content of 0 (b), 2% (c) and 4% (d)[21]
Then, SBA-16 materials with a rhombdodecahedral or decaoctahedral shape were synthesized under microwave irradiation within 2 h using sodium silicate as silica source and a tri-block copolymer F127 as structure directing agent in 2004. The stirring time and reaction temperature governed the structure of the product and the reaction time influencing the particle size and morphology. The optimal conditions for highly crystallinity SBA-16 at stirring time of 30 min and microwave irradiation at 100 ℃ for 120 min were established. The results show that microwave irradiation time is important for the well-defined morphology of SBA-16 without changing mesostructures[8].
In particular, in 2004, ordered mesoporous silicas FDU-1 with large cage-like pores, synthesized by using tri-block copolymer EO39BO47EO39 as a template were hydrothermally treated in a microwave oven at 100 ℃ for different times. The best sample was obtained after the microwave treatment of 60 min, which was reflected by narrow pore size distribution, uniform pore size entrances and thick mesopore walls. Longer time microwave treatment increased the nonuniformity of the pore entrance sizes by changing the hysteresis loops of nitrogen adsorption isotherms[33].
2.2 Microwave synthesis of metal-doped mesoporous silicates
These metal-doped mesoporous materials have often been doped with heteroatoms and the intention of developing materials that exhibit the required activity and selectivity for specific catalytic reactions. The chromium-doped mesoporous materials Cr-MCM-41 and Cr-MCM-48 were prepared by microwave-assisted synthesis. They were completed within 1-2 h, whereas the conventional hydrothermal method took at least 12-24 h for completion. These XRD patterns show evidently that the reflections are more developed for the samples obtained by microwave-assisted synthesis with higher long-range ordering than for those from hydrothermal synthesis,as shown in Fig.2. The parameters values, such as SBET(Bsunauer-Emmett-Teller surface area), VP,BJH(Barrett-Joyner-Halendal pore volume) and dP,BJH(Barrett-Joyner-Halendal pore diameter), were in good agreement with those of typical ordered mesoporous materials (shown in Table 3)[1].
Mesoporous Co-MCM-41 with different amounts of cobalt was synthesized by the microwave-assisted synthesis method. The results show that these synthesized materials have typical mesoporous structure of MCM-41. Also, specific surface area and pore volume decreased with the amount of cobalt added increasing, and mesoporous ordering also decreased. When the molar ratio of SiO2 and CoO in the starting material is 1.0?0.05, mesoporous ordering of Co-MCM-41 was the best among all the contents. On the other hand, thermal and hydrothermal tests show that Co-MCM-41 after calcination at 750 ℃ for 3 h or hydrothermal treatment at 100 ℃ for 5 d still existed in mesostructure. However, mesoporous framework was entirely damaged after calcination at 850 ℃ for 3 h[9].
Table 3 Characterization of Cr-MCM-41 and Cr-MCM-48 by chemical analysis and N2 adsorption [1]
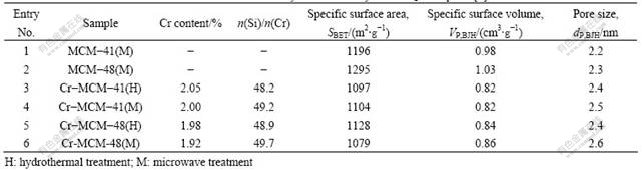

Fig.2 XRD patterns of Cr-MCM-41 and Cr-MCM-48 obtained by microwave (M) and conventional hydrothermal (H) synthesis[1]
The mesoporous Zr-SBA-15 has received considerable attention, because Zr incorporated mesoporous materials are not only good catalysts[2, 34], but also suitable catalyst supports[35], even acidic solid catalysts by sulfation[11, 36-39]. Recently, several novel routes have been developed for direct synthesis of Zr-SBA-15 or sulfated Zr-SBA-15[22, 11, 40]. For example, NEWALKAR et al[23] reported direct synthesis of Zr-SBA-15 under microwave irradiation. Zr-SBA-15 with different mole ratios of Si and Zr were successfully synthesized at 100 ℃ under microwave hydrothermal conditions within about 2 h. The analysis confirmed that, the structure, high surface area and uniform pore size distribution are maintained in the crystallized samples with n(Si)/n(Zr) of 20.
2.3 Microwave synthesis of non-siliceous mesoporous materials
Since self-synthesized mesoporous TiO2 was reported by ZHANG et al[41], the research progress of non-siliceous mesoporous materials have been made, and a variety of new synthetic materials constantly appeared. From the synthetic point of view, because of non-siliceous material with different chemical properties, there is an inevitable requirement for researchers to develop many appropriate synthesis methods. Especially, some groups have researched the synthesis of non-siliceous system specifically.
In recent years, mesoporous titanium dioxide have attracted great interest of many researcher for their peculiar properties, such as large specific surface areas, superior photocatalytic activities and chemical stabilities. Titanium dioxide (TiO2) is regarded as the best photocatalyst for environmental remediation. The synthesis of pure mesoporous TiO2 and its modification have been reviewed in this paper. TiCl4 and SnCl4 were used as raw materials, urea as precipitator, active carbon as template and soluble starch as anti-agglomerating reagent. Thus, Sn doped mesoporous TiO2 was synthesized under the microwave. The TEM image of the sample is shown in Fig.3. From Fig.3, the particle was in spherical shape and the average diameter was 20 nm. Then, the highest photocatalytic efficiency can be obtained when the content of Sn was 10 % (molar fraction), and the decolorization rate of navy blue dye could be as high as 100% after being illuminated by sunlight for 70 min [41].

Fig.3 TEM images of Sn doped mesoporous TiO2[41]
Another non-siliceous mesoporous material mesoporous carbon exhibits a higher electrocatalytic activity for methanol electrooxidation and controlled particle sizes as catalyst supports. In 2007, mesoporous carbon with ordered hexagonal structure derived from the co-assembly of tri-block copolymer F127 and resol was employed as the carbon support of Pt catalysts. Structural characterizations revealed that the mesoporous carbon exhibited large surface area and uniform mesopores. The Pt nanoparticles supported on the novel mesoporous carbon were synthesized by microwave- assisted synthesis process. Besides, CTAB was expected to improve the wet ability of carbon support as well as the dispersion of Pt nanoparticles. The Pt nanoparticles were uniform in size and highly dispersed on the mesoporous carbon supports [4].
2.4 Microwave synthesis of organically modified mesoporous silicates
Organically modified mesoporous silicates are widely used at separation, catalysis, and so on. Because of non-existence of higher chemical reactivity inherent, it greatly limited its practical applications. For the realization of potential applications of organosilicas, scientists have employed organically modification to improve its hydrothermal stability and chemical reactivity. So, many researchers attempt to synthesize them under the microwave irradiation.
SU et al[10]. synthesized directly amino functionalized SBA-15 from the hydrolysis and co-condensation of aminopropyltriethoxysilane (APTES) and sodium silicate under a strong acidic condition by using microwave. The amino functionalized SBA-15 catalysts had very short channels in the range of 200-300 nm perpendicular to the hexagonal platelet morphologies. And they greatly improved catalytic activities over the typical SBA-15 having long channels. Obviously, the easy diffusion and rapid mass transfer of substrate into the short channel mesopores have a dramatic effect on the significant improvement in the catalytic activities.
Later, the chloropropyl functionalized mesoporous silicas with various mesostructural phases of hexagonal p6mm, cubic Ia-3d, and cubic Im3m in the presence of P123 (EO20PO70EO20) and F127 (EO106PO70EO106) tri-block copolymers as structure directing agents under acidic conditions were synthesized directly. The mesostructural phases of these mesoporous silicas were closely related to the amount of CPTS when P123 was used as structure directing agent. A hexagonal mesostructure of SBA-15 was obtained at low concentration of CPTS (n(Cl)/n(Si)=0.05). On the other hand, the amount of CPTS did not alter the mesostructure of the materials templated by F127 structure directing agent. In this way, mesoporous materials with an Im3m cubic phase of SBA-16 could be synthesized with molar ratios of Cl and Si as high as 0.2. The successful assembly of highly ordered chloropropyl functionalized mesoporous silicas was contingent upon the successful formation of chloropropyl-silicate-block copolymer micelle. And the formation of block copolymer was dependent on the addition sequence of the silica source, acid catalyst and CPTS. The hydrophobic chloropropyl moiety of CPTS controlled the overall growth of ordered silica mesostructures [12].
Besides, mesoporous silicas with organic groups and niobium species were prepared at the same year. The new materials were synthesized by self-assembly of bridged silsesquioxane precursor containing ethane- bridging group using nonionic template Pluronic123 as a structure-directing agent under microwave irradiation. The mixture of P123, BTEE, ammonium trisoxalate complex of niobium (V) in HCl and H2O was transferred into microwave reaction vessel (10 cm3) and treated in the CEM microwave system under stirring at 100 ℃ for 1 h. It was indicated that microwave allowed the hydrothermal treatment time to reduce from 20 to 1 h while maintaining the mesoporous ordering. Above all, the microwave-assisted synthesis method could be attributed to the long-range regularity of Nb-PMO rods because of using microwave heating [13].

Fig.4 TEM images of ethane-containing PMO(Sample ES-24) [36]
In 2009, ethane and disulfide-bridged periodic mesoporous organosilicas were synthesized under microwave conditions. These materials were obtained by co-condensation of 1, 2-bis(triethoxysilyl) ethane and bis(triethoxysilylpropyl) disulfide organosilica precursors and P123 tri-block copolymer template under weakly acidic conditions. The samples have the specific surface area, single-point pore volume and pore size of 960-1 220 m2/g, 1.14-1.56 cm3/g, and 7.1-8.4 nm, respectively. TEM images(in Fig.4) show the domains of hexagonally ordered mesopores in these samples, although this ordering was much less pronounced as in the case of purely siliceous SBA-15[14].
3 Formation mechanism of mesoporous materials under microwave irradiation
Microwave heating may reduce synthesis time. It is generally explained by the rapid heat-up of the sample and a better heat transfer which results in rapid and sufficient heating of the synthesis mixture [42]. The effects of the electromagnetic wave causing ion oscillation and water dipole rotation on crystallization mechanism may be different from those of conventional heating. However, the formation mechanism of MCM-41 under microwave heating is apparently identical to that of conventional heating, since physical properties of MCM-41 prepared by microwave method are different from that under reaction conditions. It seems that microwave heating not only increases the rate of crystallization, but also directs the crystallization mechanism. An essential difference between conventional and microwave heating is the enhancement of the Brownian motion and the rotation dynamics of the water molecules[43]. In the case of the rotational motion, more hydrogen bonds of water molecules are destroyed, producing active water molecules. The active water molecules have a higher potential to dissolve gel because the lone pairs and OH groups of the active water molecules are available to block gel bond[43].
Furthermore, PARK et al[6] found that even though water has higher dielectric constant than that of glycol, the utilization of microwave energy on the crystallinity and morphology of MCM-41 is more effective in the presence of glycol. And absorbing microwave energy generally depends on the dissipation factor of the sample. In the microwave synthesis of SAPO (silicoalumino-phosphate molecular sieve)-11, it was found that the rate of heating primarily influenced the nucleation time of microwave and conventional heating. A more uniform morphology and narrower size distribution were formed using microwave heating compared to conventional heating. This suggests that rapid and even nucleation is the enhancement mechanism [21].
In addition, it is generally accepted that polar solvents and ionic species are the major microwave absorbers and the heat releases from chemical reactions. The formation mechanism of the mesostructured materials with non-ionic templates is generally addressed with the assembly of the mesostructured silica organized by the charge-associated EO units of F127 and cationic silica species in acid media [44-45]. In this way, ionic species accumulated at the interface influence greatly microwave irradiation. In order to support this explanation, HWANG et al[8], attempted to synthesize SBA-16 using tetraorthosilicate (TEOS) instead of sodium metasilicate as silicate source under microwave irradiation.
4 Outlook
Because of excellent performances, mesoporous materials are widely used. Mesoporous materials have potential uses in the separation and purification of biological materials, chemical industry, catalysis, environment, energy, new materials, and so on. As a result, mesoporoas materials are expected to be more practical to meet more need.
The precise control of structure and pore diameter in these materials were studied, so that the systems are virtually tunable for a desired application and an unparalleled ability in the synthesis of materials. From the application point of view, how to improve the performance and function of the mesoporous materials is an important direction of development.
However, non-thermal effects of microwave theory and interpretation of microwave promote agent crystallization in a chemical reaction are not perfect and should be further studied.
References[1] LAHA S C, GL?SER R. Characterization and catalytic performance of [Cr] MCM-41 and [Cr] MCM- 48 prepared by either classical or microwave heating [J]. Microporous and Mesoporous Materials, 2007, 99: 159-166.
[2] WONG M S, HUANG H C, YING J Y. Supramolecular templated synthesis of nanoporous zirconia-silica catalysts [J]. Chem Mater, 2002, 14: 1961-1973.
[3] GRUDZIEN R M, GRABICKA B E,JARONIEC M. Effect of organosilane/polymer ratio on adsorption properties of periodic mesoporous ethane-silica [J]. Colloids Surf A, 2007, 300: 235-244.
[4] ZHOU Jian-hua, HE Jian-ping, JIA Ya-jun, DANG Wang-juan, LIU Xiao-lei, ZHAO Gui-wang, ZHANG Chuan-xiang, ZHAO Ji-shuang, FU Qing-bin, HU Huo-ping. CTAB assisted microwave synthesis of ordered mesoporous carbon supported Pt nanoparticles for hydrogen electro-oxidation [J]. Electrochimica Acta, 2007, 52: 4691-4695.
[5] KRESGE C T, LEONOWICZ M E, ROTH W J, VARTULI J C, BECK J C. Ordered mesoporous molecular sieves synthesized by a liquid-crystal template mechanism [J]. Nature, 1992, 359: 710-712.
[6] PARK S E, KIM D S, CHANG J S KIM W Y. Synthesis of MCM-41 using microwave heating with ethylene glycol [J]. Microporous and Mesoporous Mater, 1998, 44: 301-308.
[7] CELER E B, JARONIEC M. Temperature-programmed microwave-assisted synthesis of SBA-15 ordered mesoporous silica [J]. Am Chem Soc, 2006, 128: 14408-14414.
[8] HWANG Y K, CHANG J S, KWON Y U, PARK S E. Microwave synthesis of cubic mesoporous silica SBA-16 [J]. Microporous and Mesoporous Materials, 2004, 68: 21-27.
[9] JIANG Ting-shun, SHEN Wei, ZHAO Qian, LI Mei, CHU Jin-yu, YIN Heng-bo. Characterization of Co-MCM-41 mesoporous molecular sieves obtained by the microwave irradiation method [J]. Journal of Solid State Chemistry, 2008, 181: 2298-2305.
[10] SU J D, PRASETYANTO E A, PARK S E. Synthesis of short-channeled amino-functionalized SBA-15 and its beneficial applications in base-catalyzed reactions [J]. Applied Catalysis A: General, 2008, 350: 244-251.
[11] CHEN X R, JU Y, MOU C Y. Direct synthesis of mesoporous sulfated silica-zirconia catalysts with high catalytic activity for biodiesel via esterification [J]. Phys Chem C, 2007, 111: 18731-18737.
[12] SU J D, PRASETYANTO E A, LEE S C, PARK S E. Microwave synthesis of large pored chloropropyl functionalized mesoporous silica with p6mm, Ia-3d, and Im3m structures [J]. Microporous and Mesoporous Materials, 2009, 118: 134-142.
[13] KATARZYNA W, IZABELA N. Microwave-assisted synthesis of nioboorganosilicates and microwave-accelerated catalytic activity of thereof [J]. Catalysis Today, 2009, 142: 293-297.
[14] GRABICKA B E, JARONIEC M. Microwave-assisted synthesis of periodic mesoporous organosilicas with ethane and disulfide groups [J]. Microporous and Mesoporous Materials, 2009, 119: 144-149.
[15] PARK S S, PARK J H, PARK J H, HONG S S, PARK H C, LEE G D. The preparation of fibrous MCM-41 using microwave heating [J]. Key Engineering Materials, 2007, 1: 1316-1319.
[16] BANDYOPADHYAY M, GIES H, CHIMIE C R. Synthesis of MCM-48 by microwave-hydrothermal process [J]. Comptes Rendus Chimie, 2005, 8: 621-626.
[17] LIU X B, SUN H, CHEN Y, YANG Y H, BORGNA A. Preparation of spherical large-particle MCM-41 with a broad particle-size distribution by a modified pseudomorphic transformation [J]. Microporous and Mesoporous Materials, 2009, 121: 73-78.
[18] SCHUMACHER K, GR?N M, UNGER K K. Novel synthesis of spherical MCM-48 [J]. Microporous and Mesoporous Materials, 1999, 27: 201-206.
[19] ROMANNIKOV V N, FENELONOV V B, PAUKSHTIS E A, DEREVYANKIN A Y, ZAIKOVSKII V I. Mesoporous basic zirconium sulfate: structure, acidic properties and catalytic behaviour [J]. Microporous and Mesoporous Materials, 1998, 21: 411-419.
[20] WANG Y, NOGUCHI M, TAKAHASHI Y, OHTSUKA Y. Synthesis of SBA-15 with different pore sizes and the utilization as supports of high loading of cobalt catalysts [J]. Catalysis Today, 2001, 68: 3-9.
[21] GHARIBEH M,TOMPSETT G A,CONNER WC. Microwave reaction enhancement: The rapid synthesis of SAPO-11 molecular sieves [J]. Topics in Catalysis, 2008, 49: 157-166.
[22] NEWALKAR B L, OLANREWAJU J, KOMARNENI S. Microwave-hydrothermal synthesis and characterization of zirconium substituted SBA-15 mesoporous silica [J]. Phys Chem B, 2001, 105: 8356-8360.
[23] CUNDY C S. Microwave techniques in the synthesis and modification of zeolite catalysts: A review [J]. Chem Commun, 1998, 63: 1699-1723.
[24] TOMPSETT G A, CONNER W C, YNGVESSON K S. Microwave synthesis of nanoporous materials [J]. Chem Phys Chem, 2006, 7: 296-319.
[25] KAPPE C O. Controlled microwave heating in modern organic synthesis [J]. Angew Chem, 2004, 43: 6250-6284.
[26] NUCHTER M, ONDRUSCHKA B, BONRATH W, GUM A. Microwave assisted synthesis—a critical technology overview [J]. Green Chem, 2004, 6: 128-141.
[27] N?CHTER M, M?LLER U, ONDRUSCHKA B, TIED A, LAUTENSCHL?GER W. Microwave-assisted chemical reactions [J]. Chem Eng Tech, 2003, 26: 1207-1216.
[28] LIDSTR?M P, TIERNEY J, WATHEY B, WESTMAN J. Microwave assisted organic synthesis—a review [J]. Tetrahedron, 2001, 57: 9225-9283.
[29] RAO K J, VAIDHYANATHAN B, GANGULI M, RAMAKRISHNAN P A. Synthesis of inorganic solids using microwaves [J]. Chem Mater, 1999, 11: 882-895.
[30] GABRIEL C, GABRIEL S, GRANT E H, GRANT E H, HALSTEAD B S J, MINGOS D M P. Dielectric parameters relevant to microwave dielectric heating [J]. Chem Soc Rev, 1998, 27: 213-223.
[31] DE LA HOZ A, DIAZ-ORTIZ A, MORENO A. Microwaves in organic synthesis thermal and non-thermal microwave effects [J]. Chem Soc Rev, 2005, 34: 164-178.
[32] WU C G, BEIN T. Microwave synthesis of molecular sieve MCM-41 [J]. Chem Commun, 1996, 8: 925-926.
[33] FANTINI M C A, MATOS J R, L C CIDES DA SILVA, MERCURI L P, CHIERECI G O, CELER E B, JARONIEC M. Ordered mesoporous silica: microwave synthesis [J]. Materials Science and Engineering B, 2004, 112: 106-110.
[34] BARRECA D, COPLEY M P, GRAHAM A E, HOLMES J D, MORRIS M A, SERAGLIA R, SPALDING T R, TONDELLO E. Methanolysis of styrene oxide catalysed by a highly efficient zirconium-doped mesoporous silica [J]. Appl Catal A, 2006, 304: 14-20.
[35] GUTI?RREZ O Y, FUENTES G A, SALCEDO C, KLIMOVA T. SBA-15 supports modified by Ti and Zr grafting for NiMo hydrodesulfurization catalysts [J]. Catal Today, 2006, 116: 485-497.
[36] CHEN C L, CHENG S, LIN H P, WONG S T, MOU C Y. Sulfated zirconia catalyst supported on MCM-41 mesoporous molecular sieve [J]. Appl Catal A, 2001, 215: 21-30.
[37] CHEN C L, LI T, CHENG S, LIN H P, WONG S T, BHONGALE C J, MOU C Y. Direct impregnation method for preparing sulfated zirconia supported on mesoporous silica [J]. Microporous and Mesoporous Materials, 2001, 50: 201-208.
[38] SUN Yin-yong, ZHU Lei, LU Hui-juan, WANG Run-wei, LIN Sen, JIANG Da-zheng, XIAO Feng-shou. Sulfated zirconia supported in mesoporous materials [J]. Appl Catal A, 2002, 237: 21-31.
[39] WANG Y, LEE K Y, CHOI S, LIU J, WANG L Q, CHARLES H, PEDEN F. Grafting sulfated zirconia on mesoporous silica [J]. Green Chem, 2007, 9: 540-544.
[40] CHEN S Y, JANG L Y, CHENG S. Synthesis of Zr-incorporated SBA-15 mesoporous materials in a self-generated acidic environment [J]. Chem Mater, 2004, 16: 4174-4180.
[41] ZHANG Mei-hong, DING Shi-wen, ZHANG Zhen-xing. Synthesis of mesoporous nano-TiO2 doped with Sn by auto-assembly method and photo-catalytic property [J]. Science in China Series B: Chemistry, 2005, 48: 436-441.
[42] SLANGEN P M, JANSEN J C, VAN BEKKUM H. The effect of ageing on the microwave synthesis of zeolite NaA [J]. Microporous and Mesoporous Materials, 1997, 92: 59-65.
[43] JANSEN J C, ARAFAT A, BARAKAT A K, VAN BEKKUM H M L, OCCELLI H E. Synthesis of microporous materials [J]. Molecular Sieves, 1992, 2: 507-521.
[44] ZHAO Dong-yuan, HUO Qi-sheng, FENG Jiang-lin, CHMELKA B F, STUCKY G D, TRIBLOCK G D, SHIN H J, RYONG R Y. Copolymer and Oligomeric Surfactant Syntheses of Highly Ordered, Hydrothermally Stable, Mesoporous Silica Structures [J]. Am Chem Soc, 1998, 120: 6024-6036.
[45] SAKAMOTO Y, KANEDA M, TERASAKI O, ZHAO D Y, KIM J M, STUCKY G, SHIN H J, RYOO R. Direct imaging of the pores and cages of three-dimensional mesoporous materials [J]. Nature, 2000,408:449-453.
(Edited by LI Yan-hong)
Foundation item: Project(20775096/B050104) supported by the National Natural Science Foundation of China; Project(20080440696) supported by China Postdoctoral Science Foundation
Corresponding author: CAO Yuan; Tel: +86-15923344273; E-mail: caoyuan@cqu.edu.cn

