Effect of surface dissolution on flotation separation of fine ilmenite from titanaugite
来源期刊:中国有色金属学报(英文版)2011年第5期
论文作者:朱阳戈 张国范 冯其明 鄢代翠 王维清
文章页码:1149 - 1154
关键词:钛铁矿;钛辉石;浮选;表面溶解
Key words:ilmenite; titanaugite; flotation; surface dissolution
摘 要:
通过红外光谱(FT-IR)和X射线光电子能谱(XPS)分析,研究钛铁矿与钛辉石的表面溶解行为对其浮选分离的影响。实验结果表明,弱酸性条件下的表面溶解有利于提高钛铁矿与钛辉石的可浮性差异。在弱酸性条件下,由于钛铁矿与油酸钠的作用以Fe为主,而表面溶解有利于其在钛铁矿表面的氧化,使钛铁矿可浮性得到提高;同时,油酸钠与Ca和Mg的作用导致了钛辉石的可浮选,但表面溶解降低了钛辉石表面Ca和Mg的含量,使钛辉石可浮性明显下降。对于原矿TiO2品位为8.41%的攀枝花钛铁矿,经表面溶解处理后浮选可将粗选精矿TiO2的品位由26.7%提高到31.73%。
Abstract:
The influence of surface dissolution on flotation separation of fine ilmenite from titanaugite was investigated through infrared spectroscopic (FT-IR) analysis and X-ray photoelectron spectroscopy (XPS) test. The results show that surface dissolution in weak acid solution is helpful to enlarge the floatability difference between ilmenite and titanaugite. In weak acidic solution, as sodium oleate mainly interacts with Fe which results in ilmenite flotation, and surface dissolution is beneficial to its oxidation, the floatability of ilmenite after surface dissolution is increased; meanwhile, sodium oleate interacts with Ca and Mg which results in titanaugite flotation, and the quantities of Ca and Mg on the surface of titanautite are decreased due to the surface dissolution, so the floatability of titanaugite after surface dissolution is depressed. For an ilmenite ore obtained from Panzhihua with TiO2 grade of 8.41%, flotation after surface dissolution treatment could increase TiO2 grade of rough concentrate from 26.7% to 31.73%.

ZHU Yang-ge, ZHANG Guo-fan, FENG Qi-ming, YAN Dai-cui, WANG Wei-qing
School of Resources Processing and Bioengieering, Central South University, Changsha 410083, China
Received 8 June 2010; accepted 21 September 2010
Abstract: The influence of surface dissolution on flotation separation of fine ilmenite from titanaugite was investigated through infrared spectroscopic (FT-IR) analysis and X-ray photoelectron spectroscopy (XPS) test. The results show that surface dissolution in weak acid solution is helpful to enlarge the floatability difference between ilmenite and titanaugite. In weak acidic solution, as sodium oleate mainly interacts with Fe which results in ilmenite flotation, and surface dissolution is beneficial to its oxidation, the floatability of ilmenite after surface dissolution is increased; meanwhile, sodium oleate interacts with Ca and Mg which results in titanaugite flotation, and the quantities of Ca and Mg on the surface of titanautite are decreased due to the surface dissolution, so the floatability of titanaugite after surface dissolution is depressed. For an ilmenite ore obtained from Panzhihua with TiO2 grade of 8.41%, flotation after surface dissolution treatment could increase TiO2 grade of rough concentrate from 26.7% to 31.73%.
Key words: ilmenite; titanaugite; flotation; surface dissolution
1 Introduction
Due to a certain solubility for all minerals, lattice ions in minerals can transfer from the surface of the mineral to the solution during flotation. The surface dissolution will influence the flotation separation of the minerals in two aspects. One is the changes of the distribution and states of the elements on mineral’s surface, which influence surface properties of mineral; the other is that as ions enter into the liquid, the solution chemistry environment of flotation will be changed[1]. The solubility of oxidized or salt-type minerals is relatively high, which will affect more remarkablely. The effect of surface dissolution on flotation has been extensively studied in various systems. For instance, in the flotation of scheelite-calcite, scheelite-fluorite, fluorite-calcite and apatite-calcite systems, the surface dissolution and conversion of the two minerals will affect the separation[2]. The pre-treatment with acid could increase the floatability of kyanite[3]. During the flotation of spodumene and beryl, the adsorption of the collector will be improved by stirring and scrubbing in strong alkaline solution before flotation[4].
There are abundant titanium resource in China, and more than 90% of them are stored in Panzhihua vanadic titanomagnetite ore deposit[5]. The difficulty of the flotation of this kind of titanium resource lies on the separation of ilmenite and titanaugite, the main gangue minerals. There are elements such as Ti, Fe, Ca and Mg both on the surface of ilmenite and titanaugite, which decrease the selectivity of the reagents[6]. Meanwhile, the relative large surface area, due to the high content of fine particles in raw mineral (0-0.019 mm fine particles amounting to more than 30% of the mine ore), makes the influence of dissolution more obvious[7-8]. Recently, the research on the flotation of ilmenite focused on the improvement of the flotation reagents and process flow[9-11], while it rarely concerned with the surface dissolution and the influence on flotation of minerals in this system. However, there were some researches on indirectly testifying that surface dissolution process of ilmenite could exert certain influence on its floatability. The researches by ZHONG and CUI[12] and GUTIERREZA[13] showed that the oxidization of the ilmenite was helpful to increase its floatability, and the researches made by Soviet Union believed that H2SO4 could play the role in surface cleaning[14]. But it is still not clear about its internal mechanism. The aim of this work is to explore the influence of the surface dissolution on the separation of fine ilmenite and titanaugite, the change of the distribution and states of the elements before and after the surface dissolution, and the adsorption of collector on mineral surface.
2 Experimental
2.1 Minerals and reagents
The ilmenite and titanaugite samples used in the experiments were obtained from Panzhihua, Sichuan Province in China. Samples were repeatedly purified by physical methods several times, such as low or high intensity magnetic separation, gravity separation, and electric separation. Each mineral was ground to a diameter smaller than 0.019 mm by a CP-20 jet mill. No chemical treatment was carried out during purification and grinding. The purity of the ilmenite was above 95% while that of the titanaugite was above 90% based on mineralogical analysis, chemical analysis and X-ray diffractometry. The chemical compositions of the samples are listed in Table 1. The specific surface area was 1.39 m2/g for ilmenite and 2.55 m2/g for titanaugite. To prepare samples after surface dissolution, the purified mineral particles (2 g) were placed in a beaker, which was then filled with distilled water (30 mL). The suspension was agitated for 10 min by a magnetic stirrer at pH=5.8. After centrifugation, the liquid was poured out. The remaining solid was dried below 60 °C under vacuum.
Table 1 Chemical composition of pure minerals (mass fraction, %)

Ilmenite ore used in the bench-scale flotation tests was tailings from the iron beneficiation of Panzhihua vanadic titanomagnetite ore. The sample with TiO2 head assay of 9.81% was mainly composed of 15.0% ilmenite, 31.4% titanaugite, 30.5% plagioclase, 16.9% chlorite and 2.8% titanomagnetite by the X-ray diffraction analysis and mineralogical analysis. The mass fraction of particles smaller than 0.019 mm in the ore was 24.73%.
The chemically pure sodium oleate was adopted as flotation collector in the purified minerals flotation. H2SO4 and NaOH solutions were used to adjust pH values of the system. The synthesized OL-T1 and CS were separately used as collector and depressant in the bench-scale flotation tests. Butyl xanthate and pine camphor were adopted to remove sulphide minerals in the ore flotation.
2.2 Micro-flotation
The purified mineral particles (2 g) were placed in a plexiglass cell (40 mL), which was then filled with distilled water. Then the pH value was adjusted. After keeping for 3 min, the collector was added. The suspension was agitated for 3 min and pH was measured before the flotation, while the flotation was conducted for 3 min. The concentrates were weighed after filtration and drying, and the recovery rate was calculated.
2.3 Bench scale flotation
500 g ilmenite ores were ground to 83% passing mesh with pure size of 0.074 mm in a XMB-type steel mill of d200 mm×400 mm at a pulp density of 60% (mass fraction). Then pH regulator, depressant and collector were added in a 1.5 L-XFD-type flotation machine at a pulp density of 30% (mass fraction).
2.4 FT-IR spectroscopy
The FT-IR spectra were obtained with NEXU670 FT-IR (Nicolet Corporation, USA) to characterize the nature of the interaction between the collector and the minerals. The mineral sample was ground to smaller than 0.002 mm before contacting with the collector.
2.5 XPS test
XPS spectra were measured with TU-1810 X-ray photoelectron spectroscope to study the distribution density and binding energy of the elements on the mineral surface. The reagents were added according to the condition of micro-flotation.
3 Results and discussion
3.1 Effects of surface dissolution on flotation of ilmenite and titanaugite
Figure 1 presents the relationship between the floatability of pure minerals and pH values before and after surface dissolution. The result indicates that the floatability of ilmenite is better than that of titanaugite through direct floatation. The floatability of ilmenite is good at the pH value above 5.0, while titanaugite floats relatively well in the neutral or strong alkaline solution. After surface dissolution, the floatability of ilmenite is increased in the acid solution of pH below 5.5, while it becomes bad in the neutral or basic solution. And the floatability of titanaugite is obviously decreased in the whole pH range and it is even not able to be floated under the condition of pH above 9.5.
Figure 2 shows the effect of sodium oleate concentration on the floatability of ilmenite and titanaugite at pH 5.8. Figure 2 indicates that the floatability of ilmenite increases after surface dissolution while that of titanaugite remarkably decreases. It could be concluded that the floatability difference of two minerals is remarkably enlarged by surface dissolution in weak acid solution.
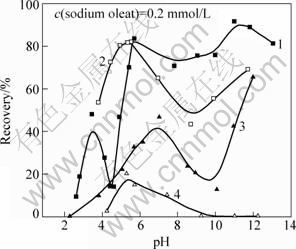
Fig.1 Effect of pH and surface dissolution on flotation behaviour of minerals: 1—Ilmenite; 2—Ilmenite after surface dissolution; 3—Titanaugite without surface dissolution; 4—Titanaugite after surface dissolution

Fig.2 Effect of surface dissolution and dosage of sodium oleate on flotation behaviour of minerals: 1—Ilmenite without surface dissolution; 2—Ilmenite after surface dissolution; 3—Titanaugite without surface dissolution; 4—Titanaugite after surface dissolution
3.2 Effects of surface dissolution on distribution of surface elements on ilmenite and titanaugite
In the crystal structures of ilmenite and titanaugite, metal ions of Ti, Fe, Ca and Mg are in different chemical situations[6]. Therefore, the dissolution behaviors of these metal ions in solution are different, leading to the distribution change of surface elements on the surface of minerals after surface dissolution. Table 2 lists the distribution of surface elements of Ti, Fe, Ca and Mg before and after surface dissolution at pH 5.8. It is indicated that the contents of Ti and Fe on the surface of ilmenite are more than the contents of Ca and Mg, while the contents of Ti and Fe on the surface of titanaugite are less than the contents of Ca and Mg. After surface dissolution, the content of Fe in ilmenite increases from 9.36% to 10.45% while the contents of Ca decreases from 4.26% to 3.39% and Mg from 4.13% to 2.79% separately. For titanaugite, the tendency after surface dissolution is similar to ilmenite. Therefore, surface dissolution results in a remarkable change on distribution of surface elements of the two minerals, and the relative contents of Ca and Mg are markedly reduced, while the contents of Ti and Fe show a mild increase on the surface of ilmenite.
Table 2 Relative content on mineral surface of Ti, Fe, Ca and Mg (mole fraction, %)

3.3 FT-IR analysis of sodium oleate adsorbed on ilmenite and titanaugite
The FT-IR spectra of sodium oleate, ilmenite and titanaugite are presented in Fig.3. The FT-IR spectra for minerals conditioned with sodium oleate in weak acid or weak alkaline solution are indicated in Fig.4.
In 3 000-2 500 cm-1, new bands around 2 925 and 2 855 cm-1 for minerals with sodium oleate are previously attributed to —CH2 stretching of acyclic compounds. It is evidenced that sodium oleate is adsorbed on the mineral surface. New bands at 1 585 cm-1 and 1 467 cm-1 at pH 5.5 for ilmenite may be attributed to iron oleate[15-17], while new bands at 1 570 cm-1 and 1 437 cm-1 at pH 9.5 for ilmenite may be attributed to calcium oleate or magnesium oleate[18-21]. And new bands around 1 570 cm-1 and 1 541 cm-1 for titanaugite at pH 5.5 and 9.5 are observed, which may be attributed to calcium oleate or magnesium oleate.
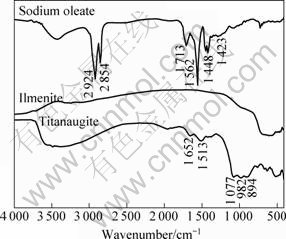
Fig.3 FT-IR spectra of sodium oleate, ilmenite and titanaugite
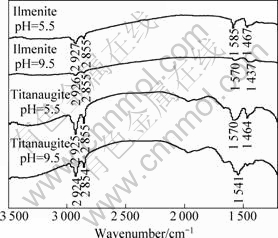
Fig.4 FT-IR spectra of minerals with sodium oleate at different pH values
Therefore, it can be concluded that sodium oleate mainly interacts with Fe on the surface of ilmenite in weak acid solution, which results in the flotation of ilmenite; sodium oleate mainly interacts with Ca and Mg on the surface of ilmenite in weak alkline solution. After surface dissolution in weak acid solution, the contents of Ca and Mg on ilmenite surface decrease (Table 2), and then the floatability of ilmenite in weak alkaline solution decreases (Curve 2 in Fig.1). Ca and Mg interact with sodium oleate in both weak acid and weak alkaline solutions. As Ca and Mg are dissolved a lot during surface dissolution, the floatability is decreased seriously during the whole pH range (Curve 4 in Fig.1).
3.4 Effects of surface dissolution on oxidation on surface of ilmenite
It was proved that sodium oleate mainly interacts with Fe in ilmenite in weak acid solution by FT-IR analysis, and that the ability of Fe3+ reacting with sodium oleate is more intensive than that of Fe2+[22]. To provide direct evidence of oxidation on ilmenite surface and its influence on reaction with sodium oleate, XPS test of Fe 2p3/2 was carried out. In order to determine the relative contents and chemical shifts of Fe2+ and Fe3+ separately, scanning spectra were analyzed by peak fitting and separation[23], and the results are shown in Fig.5 and Table 3. In Fig.5, peak areas represent the relative contents of Fe2+ and Fe3+, while peak positions indicate the binding energy.
Figure 5 and Table 4 show that Fe on the original surface of ilmenite is mainly in form of Fe2+. Besides, the content of Fe3+ only accounts for 7.14 % of total iron, and increases to 45.14% after surface dissolution, which suggests that surface dissolution significantly promotes the oxidation of Fe2+. After the reaction of the mineral with sodium oleate, the chemical shift of Fe2+ is not obvious, while that of Fe3+ reaches -0.79 eV, which indicates the intense transform of the chemical environment. Therefore, it can be concluded that surface dissolution promotes the oxidation of Fe2+ to Fe3+, and Fe3+ reacts with sodium oleate intensively, which results in the adsorption of sodium oleate on the surface of ilmenite and the increase in the floatability.
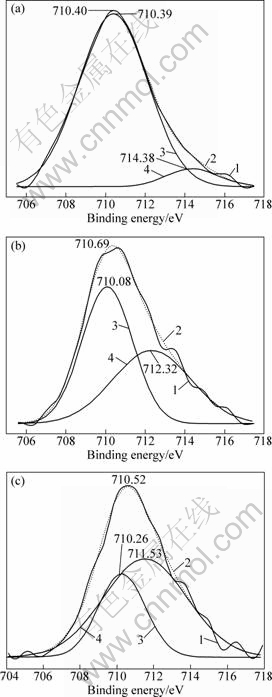
Fig.5 XPS spectra of Fe 2p3/2 on surface of ilmenite: (a) Original surface; (b) Surface after dissolution; (c) Sodium oleate adsorbed surface (1—Scanning spectrum of Fe 2p3/2; 2—Fitting spectrum of Fe 2p3/2; 3—Spectrum of Fe2+ after peak fitting and separation; 4—Spectrum of Fe3+ after peak fitting and separation)
3.5 Flotation of ilmenite ore
Based on the results of aforementioned basic studies, a flotation process has been designed for Panzhihua ilmenite ore. The detailed flow chart of flotation is shown in Fig.6 and the results are summarized in Table 4. In the process of flotation after surface dissolution, 2 000 g/t H2SO4 was added to pulp, and conditioned for 10 min, then the pulp was settled for 10 min and the supernatant fluid was poured out, and the pH value was then adjusted to 5.5-6.0. After this period, regulator and collector were added to the solution. The flotation without surface dissolution was directly carried out at pH value of 5.5-6.0 by 1 000 g/t H2SO4. The reagent system for the processes with surface dissolution and without surface dissolution are same. The results (Table 4) indicate that TiO2 grade for the rough ilmenite concentrate increases from 26.7% to 31.73% after surface dissolution treatment. Meanwhile, the recovery is not obviously decreased.
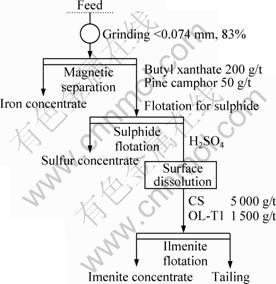
Fig.6 Flotation flowsheet for ilmenite ore
4 Conclusions
1) Surface dissolution in weak acidic solution remarkably decreases the relative contents of Ca and Mg on the surface of ilmenite and titanaugite.
2) Sodium oleate interacts with Fe in weak acid solution, and it interacts with Ca and Mg in weak alkaline solution, which results in ilmenite flotation. And sodium oleate interacts with Ca and Mg, resulting in titanaugite flotation in both weak acidic and weak alkaline solution.
3) In weak acidic solution, the oxidation of Fe2+ to Fe3+ occurs easily after surface dissolution, the reaction between Fe3+ and sodium oleate is more intensive, and then the floatability of ilmenite is increased.
4) It is beneficial to enlarging the floatability difference between ilmenite and titanaugite through surface dissolution. In the flotation of Panzhihua ilmenite ore, TiO2 grade for rough concentrate could be obviously increased by surface dissolution treatment.
Table 3 Binding energy and relative contents of Fe2+ and Fe3+ by peak fitting and separation in XPS of Fe 2p3/2 on surface of ilmenite
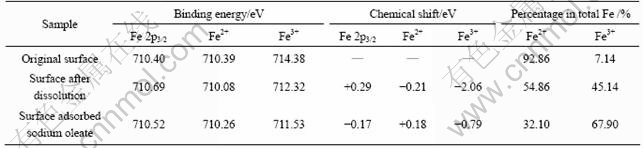
Table 4 Flotation results of ilmenite ore

References
[1] WANG Dian-zuo, HU Yue-hua. Solution chemistry of flotation [M]. Changsha: Hunan Science and Technology Press, 1988: 180-198. (in Chinese)
[2] HU Yue-hua, WANG Dian-zuo. Disslution/surface property of salt-type mineral and design of schemes of flotation separation [J]. Journal of Central South Institute of Mining and Metallurgy, 1992, 23(3): 273-279. (in Chinese)
[3] DONG Hong-jun. A study of crystal structure and flotation behavior of kyanite-species polymorphous minerals and the development of marketable products [D]. Changsha: Central South University of Technology, 1993: 42-49. (in Chinese)
[4] MOON K S, FUERSTENAU D W. Surface crystal chemistry in selective flotation of spodumene (LiAl[SiO3]2) from other aluminosilicates [J]. International Journal of Mineral Processing, 2003, 72(1-4): 11-24.
[5] DENG Chuan-hong, MA Jun-er, ZHANG Guo-fan, FENG Qi-ming, ZHU Yang-ge. Effect of water glass on floatation of ilmenite [J]. The Chinese Journal of Nonferrous Metals, 2010, 20(3): 551-556. (in Chinese)
[6] WEI Min. Research on Panzhihua ilmenite flotation by using TAO collector series [J]. Journal of Guangdong Non-ferrous Metals, 2006, 16(2): 80-83. (in Chinese)
[7] RUBIO J, CAPPONI F, RODRIGUES R T, MATIOLO E. Enhanced flotation of sulfide fines using the emulsified oil extender technique [J]. International Journal of Mineral Processing, 2007, 84(1-4): 41-50.
[8] TATU M, JOHN R, DANIEL F. The limits of fine particle flotation [J]. Mineral Engineering, 2010, 23: 420-437.
[9] ZHU Jian-guang. Study and application of collector for ilmenite in China [J]. Mining and Metallurgical Engineering, 2006, 26(8): 78-85. (in Chinese)
[10] ZOU Jian-xin, ZHOU Jian-guo, ZHOU You-bin. Progress and development trend of beneficiation technology for Panzhihua titanium ores [J]. Mining and Metallurgical Engineering, 2006, 26(3): 38-41. (in Chinese)
[11] ZHU Yang-ge, ZHANG Guo-fan, FENG Qi-ming, OU Le-ming, LU Yi-ping. Autogenous-carrier flotation of fine ilmenite [J]. The Chinese Journal of Nonferrous Metals, 2009, 19(3): 554-560. (in Chinese)
[12] ZHONG Kang-nian, CUI Lin. Influence of Fe2+ ions of ilmenite on its flotability [J]. International Journal Mineral Processing, 1987, 20(3-4): 253-265.
[13] GUTIERREZA C. Influence of previous aeration in water or heating in air of ilmenite on its flotation with oleic acid [J]. International Journal of Mineral Processing, 1976, 3(3): 247-256.
[14] LUO Rong-chang, GUO Qing-hua. Flotation of silicate and oxidized ore [M]. Beijing: China Industry Press, 1965: 111-138. (in Chinese)
[15] PECK A S, RABY L H, WADSWORTH M E. An infrared study of the flotation of hematite with oleic acid and sodium oleate [J]. Transactions of the American Institute of Mining, 1966, 235(3): 301-307.
[16] WANG Yu-hua, YU Fu-shun. Effects of metallic ions on the flotation of spodumene and beryl [J]. Journal of China University of Mining & Technology, 2007, 17(1): 35-39.
[17] PRAKASH S, DAS B, MOHANTY J K, VENUGOPAL R. The recovery of fine mineral from quartz and corundum mixtures using selective magnetic coating [J]. International Journal of Mineral Processing, 1999, 57(2): 87-103.
[18] RAO K H, FORSSBERG E. Adsorption of oleate from aqueous solution onto fluorite [C]// Proceeding XVIIth International Mineral Processing Congress. Freiberg: Bergakademie Freiberg, 1991:191-202.
[19] UCAR A, OZDAG H. Mechanism of collector adsorption in fluorite flotation [J]. Mineral Processing and Extractive Metallurgy, 2002, 111(2): 100-105.
[20] ZENG Neng, CHEN Yu-kun. Structure characterizations of the loose nano magnesium hydroxide[J]. Journal of South China University of Technology: Natural Science Edition, 2006, 34(10): 55-61. (in Chinese)
[21] WENSEL R, PENALOZA M, CROSS W, WINTER R, KELLAR J. Adsorption behavior of oleate on Mg(OH)2 as revealed by FT-IR spectroscopy [J]. Langmuir, 1995, 11(11): 4593-4595.
[22] ZHANG Guo-fan, ZHU Yang-ge, FENG Qi-ming, LU Yi-ping, OU Le-ming. Flotation of fine ilmentie by sodium oleate [J]. The Chinese Journal of Nonferrous Metals, 2009, 19(2): 372-377. (in Chinese)
[23] WEN Mei-lan. Study on photoelectron spectroscopy of Magnetic ferrites ZnxFe3-xO4 [D]. Nanjing: Southeast University, 2005. (in Chinese)
朱阳戈,张国范,冯其明,鄢代翠,王维清
中南大学 资源加工与生物工程学院,长沙 410083
摘 要:通过红外光谱(FT-IR)和X射线光电子能谱(XPS)分析,研究钛铁矿与钛辉石的表面溶解行为对其浮选分离的影响。实验结果表明,弱酸性条件下的表面溶解有利于提高钛铁矿与钛辉石的可浮性差异。在弱酸性条件下,由于钛铁矿与油酸钠的作用以Fe为主,而表面溶解有利于其在钛铁矿表面的氧化,使钛铁矿可浮性得到提高;同时,油酸钠与Ca和Mg的作用导致了钛辉石的可浮选,但表面溶解降低了钛辉石表面Ca和Mg的含量,使钛辉石可浮性明显下降。对于原矿TiO2品位为8.41%的攀枝花钛铁矿,经表面溶解处理后浮选可将粗选精矿TiO2的品位由26.7%提高到31.73%。
关键词:钛铁矿;钛辉石;浮选;表面溶解
(Edited by YANG Hua)
Foundation item: Project (2007CB613602) supported by the National Basic Research Program of China; Project (20090162110053) supported by Doctoral Fund of Ministry of Education of China; Project (CX2009B049) supported by Hunan Provincial Innovation Foundation For Postgraduate, China
Corresponding author: ZHANG Guo-fan; Tel: +86-731-88830913; E-mail: zhangguofan2002@163.com
DOI: 10.1016/S1003-6326(11)60835-2

