
Preparation of lead sulfate powder directly from galena concentrates
QIN Wen-qing(覃文庆), LIU Hui(刘 辉), TANG Shuang-hua(唐双华), SUN Wei(孙 伟)
School of Resources Processing and Bioengineering, Central South University, Changsha 410083, China
Received 29 April 2008; accepted 17 December 2008
Abstract: A new approach to prepare PbSO4 powder is studied. Using the methods of the leaching of galena concentrates in the ferric chloride media, selective purification and chemical sedimentation, PbS concentrate can be converted into PbSO4. The conversion recovery is 97.39%, the purity of PbSO4 powder is above 99% and its average crystallite size is about 42 nm. In this process, emission of lead vapour and SO2 cannot occur. The experimental results demonstrate the feasibility of realizing a green route to prepare the lead sulfate powder.
Key words: lead sulfate; galena; preparation; leaching; purification
1 Introduction
Lead sulfate (PbSO4) as an important chemical product can be widely used in white pigment, lead storage battery and so on[1-2]. It is well known that the traditional process to prepare PbSO4 involves fire metallurgy to produce crude lead from lead concentrate and electrolysis of the crude lead to produce electrolytic lead, then chemical synthesis[3-5]. Thus, there is serious pollution due to the emission of SO2 and lead vapour as well as lead filled dust during processes of lead metallurgy and electrolysis. The emissions of these pollutants not only do harm to the health of operators, but also result in local atmosphere and water pollution. Under the current pressures of strict environmental regulations, seeking much efficient ways to produce PbSO4 is very necessary.
Many researchers have done extensive work on hydrometallurgical lead production process. The ferric chloride leaching of galena has received considerable attention over the last 20 years or so[6-10]. This process is based on the rapidity of the reaction between FeCl3 and PbS, on the predominant formation of elemental sulphur, and on the elevated solubility of PbCl2 in hot concentrated chloride media. KOBAYASHI and DUTRIZAC[11] reviewed these works. Then, many studies have been carried out on the kinetic model of dissolution of galena[12-13]. DUTRIZAC and CHEN [14] investigated the ferric sulphate leaching of galena. A new process was proposed by CHEN et al[15-17], in which the conversion of galena to PbCO3 in ammonium carbonate solution was simple and realizable, but very fine particles of ore and longer leaching time are required for the complete conversion of galena into PbCO3. The study on the leaching of galena in fluosilicate medium was reported by ALAN et al[18]. It can be found that the methods of hydrometallurgical lead production process mainly involve lead sulfide concentrates leaching in some medium, followed by fused-salt electrolysis to produce electrolytic lead. However, previous investigations were just developed at the bench or pilot-plant scale. What’s more, little research work has been carried out on the direct preparation for lead salts by the method of hydro-chemistry.
Hence, in this work, a new approach of preparing PbSO4 from galena concentrates in ferric chloride media coupled with selective purification and chemical sedimentation was proposed. Compared with traditional process, this new method will simplify the production process and decrease the energy consumption, as well as realize cleaner production of PbSO4. The aim of this work is to investigate the conversion of lead sulfide concentrates to lead sulfate, and to demonstrate the feasibility of realizing a green route to prepare lead sulphate.
2 Experimental
2.1 Samples
The samples used in the experiments came from Fankou Lead-zinc Mine in Guangdong Province of China. Microscopic examinations in thin slides of the groundmass showed that the lead minerals are mainly galena, and gangue minerals are quartz, gypsum, dickite, and sericite (fine grained mica) in both crystalline and microcrystalline forms. Table 1 describes the chemical composition of the ore. X-ray diffraction(XRD) analysis of the ore showed that the ore contains mainly about 77% galena (PbS), 0.6% sphalerite (ZnS), 12% pyrite (FeS2), and 3% quartz(SiO2). Table 2 shows the mineral composition.
Table 1 Chemical composition of galena concentrate (mass fraction, %)

Table 2 Mineral composition of galena concentrate (mass fraction, %)

The size distribution of the concentrate was 80% passing 0.074 mm and 42% passing 0.039 mm.
2.2 Reagents and apparatus
The experimental agents were all in reagent grade including 1 mol/L H2SO4, FeCl3·6H2O, NaCl and 0.1 mol/L HCl used for pH control.
A 500 mL reactor was used. Agitation of solution was conducted with electronic agitator of D60 type. The reactor was maintained in a thermostat water bath with DK-8 type to keep the temperature constant (±1 ℃). The pHS-3C type of potential-pH meter was used to adjust pH. Filtration appliance was evacuated from filtration extractor.
The products prepared were identified by XRD (X-ray diffraction) with D/Max-ra type (Cu Kα radiation; λ=0.154 056 nm), and its surface morphology was investigated by SEM(scanning electronic microscope) of KYKY-2800 type.
2.3 Experimental principle and methods
2.3.1 Leaching principles
On account of the lowest valence state of sulphur in PbS, the insoluble PbS can be transformed into soluble lead salts by strong oxidation of ferric chloride with rapid reaction and the moderate solubility of lead chloride in concentrated chloride media[5-7]. During this leaching process, chloride ion plays an important role, especially in saturated NaCl solution system. Chloride ion influences the solubility of lead chloride by the formation of chloride-complex such as PbCl42-, which can increase the PbCl2 diffusion gradient. So, galena concentrates are leached in saturated NaCl solution system. Amounts of hydrochloric acid are added into the solution to guarantee the value of pH less than 2.0 so as to prevent the hydrolysis of ferric and lead chloride. The leaching reactions is considered competition between non-oxidative and oxidative dissolution as follows.
Oxidative mechanism (ferric attack of mineral)
PbS(s)+2Fe3+(aq)+2Cl-(aq)→PbCl2(s)+2Fe2++S0(s)
(1)
PbCl2(s)+2Cl-(aq)→PbCl42-(aq) (2)
Non-oxidative mechanism (acid attack of mineral)
PbS(s)+2H+(aq)+2Cl-(aq)→PbCl2(s)+H2S(aq) (3)
H2S(aq)+2FeCl3(aq)→
2FeCl2(aq)+2H+(aq)+2Cl-(aq)+S0(s) (4)
For example, in the initial stage, the Fe3+/H+ concentration ratio is relatively high, the kinetics is almost governed by the oxidative process, and therefore, the oxidative reaction, ferric ion attack, is predominant in the dissolution of galena. This conclusion is consistent with the observation that no H2S smell is detected in the course of the experiments.
2.3.2 Procedures
At first, FeCl3·6H2O, NaCl and 200 mL distilled water were placed in the 500 mL glass reactor,and pH was adjusted below 2 with HCl. When the lixiviant solution temperature reached the targeted value, a certain amount of lead concentrate was quickly added into the reactor and stirred. At the predetermined reaction time, take the speed motor off, and the leaching solution was immediately filtered. After cooling filtration solution for 2 h in the mixture of ice and water, the lead chloride crystals would be crystallized. In this experiment, by adding Cl2 to filter solution, FeCl2 can be reconverted into FeCl3, which resulted in facile regeneration of leaching reagents in cycling processes. Besides, by using NaOH, the H2S produced can be transformed into Na2S, a by-product. The flow diagram of this leaching process is presented in Fig.1.
2.3.3 Principles and procedures of preparation for lead sulfate
The reaction mechanism to prepare PbSO4 with PbCl2 is as follows.
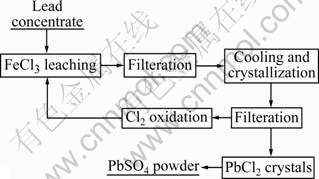
Fig.1 Flow diagram of leaching process and preparation of PbSO4 products
PbCl2+H2SO4→PbSO4↓+2HCl,
?G298 K=-3 844.77 J (5)
The solubility constants of PbCl2 and PbSO4 are 1.6×10-5 and 1.7×10-8, respectively. This reaction can react easily. Hence, the method of chemical sedimentation was adapted.
At room temperature, 1 mol/L H2SO4 was dropped into PbCl2 solution with a dropping bottle, then was stirred with constant speed. When the reaction was end, the solution should be stood for 2 h, filtrated, washed with distilled water and dried in evacuated drier. At last, PbSO4 powder could be obtained.
3 Results and discussion
3.1 Factors experiments
Several factors, affecting the leaching processes, which involve stirring speed, reaction time, reaction temperature and lixiviant concentration, were studied. Unless otherwise noted, the ferric chloride leaching solution contained 0.1 mol/L HCl and L/S=20.
Stirring speeds were varied from 1 200 to 1 800 r/min in the reactor. The experimental results were expressed in terms of the lead leaching rate vs time, as shown in Fig.2. Over the stirring speed range, there was no significant effect of string speed on the reaction rate. Based on these results, a stirring speed of 1 600 r/m was chosen for all subsequent experiments.
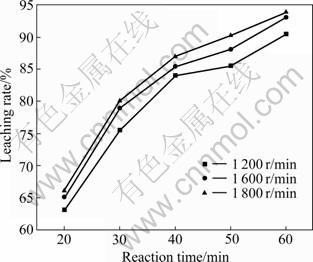
Fig.2 Effect of stirring speed on leaching rate of galena concentrate at 80 ℃ in 200 mL lixiviant containing 65 g/L FeCl3·6H2O, 250 g/L NaCl
Two experiments were performed to determine the dependence of the rate of lead leaching on the reaction time and the ferric ion concentration. The results are plotted in Fig.3 and Fig.4.
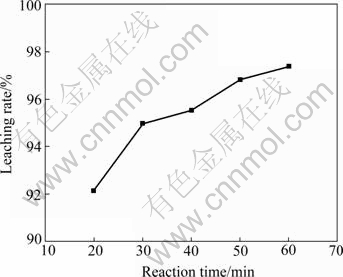
Fig.3 Effect of reaction time on leaching rate (Stirring speed 1 600 r/min, 80 ℃, lixiviant 200 mL containing 75 g/L FeCl3·6H2O and 250 g/L NaCl)
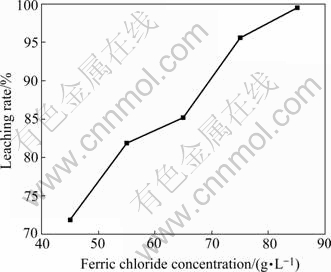
Fig.4 Effect of ferric chloride concentration on leaching rate (Stirring speed 1 600 r/min, 80 ℃, reaction time 40 min, lixiviant 200 mL containing 250 g/L NaCl)
Over the temperature range of 20-80 ℃, the effects of temperature on the leaching rate are shown in Fig.5. The rate of lead leaching increased dramatically with temperature increasing. From the energy consumption point of view, reaction temperature of 80 ℃ was suitable for practical leaching process.
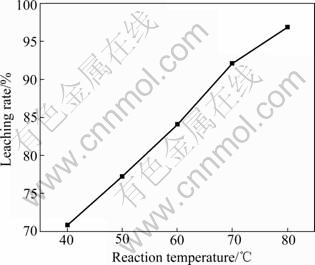
Fig.5 Effect of temperature on leaching rate (Stirring speed 1 600 r/min, lixiviant 200 mL containing 75 g/L FeCl3·6H2O and 250 g/L NaCl)
The effects of NaCl concentration on the leaching rate are demonstrated in Fig.6. The maximum leaching rate appeared to be reached after 250 g/L NaCl concentration, followed by a small but perceptible decrease in leaching.
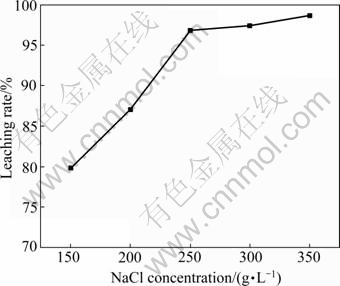
Fig.6 Effect of NaCl concentration on leaching rate (Stirring speed 1 600 r/min, 80 ℃, reaction time 40 min, lixiviant 200 mL containing 75 g/L FeCl3·6H2O)
3.2 Confirmation of optimum leaching conditions
According to above experimental results, the optimum leaching conditions are as follows:
1) Solution: 0.1 mol/L HCl, pH<2, L/S=20;
2) NaCl concentration: 250 g/L;
3) FeCl3·6H2O concentration: 75 g/L;
4) Reaction temperature and time: 80 ℃ and 40 min;
5) Stirring speed: 1 600 r/min.
The conversion rate of galena concentrate into PbCl2 reached 97.39%.
3.3 Characterization and purity analysis of products
Fig.7 shows X-ray diffraction pattern of the final products. It is clear that the position of different peaks and peak intensities are almost in agreement with standard XRD data of PbSO4 sample by comparing Table 3 with Fig.7, which expresses these products are PbSO4 powders. This also shows that the diffraction peaks are very sharp, which demonstrates that both the state of crystallization and purity of PbSO4 are high. Using Scherrer formula[19-20], the average crystallite size of PbSO4 crystal is about 42 nm.
Table 3 XRD data of PbSO4 sample
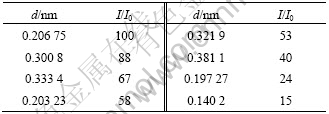
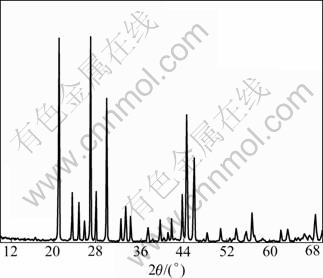
Fig. 7 XRD pattern of PbSO4 powder
Fig.8 shows the SEM patterns of PbSO4 powder. Most parts of powders adhere together, which shows there is intensive cluster phenomenon in the course of reaction.
According to analysis, main impurities contents in galena concentrate, PbCl2 crystal and PbSO4 powder, are listed in Table 4. The results showed that impurities content in PbSO4 powder was almost below 0.01% and the purity of PbSO4 was not less than 99%.
Table 4 Content of individual lead compound

4 Conclusions
1) The maximum leaching rate of PbCl2 from galena concentrate is 97.39%. The optimum leaching conditions are 250 g/L NaCl, 75 g/L FeCl3?6H2O, 0.1 mol/L HCl, 40 min, L/S=20, pH<2, 1 600 r/min.
2) The experimental results express that the processes of hot-filtration, crystallization and chemical
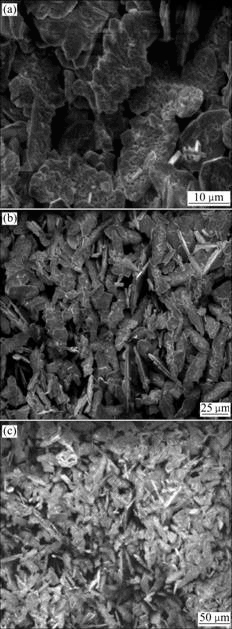
Fig.8 SEM morphologies of PbSO4 powder
sedimentation has the function of purification. The purity of PbSO4 products is higher than 99%. Its average crystallite size is about 42 nm, but cluster phenomenon is intensive.
References
[1] ZHONG Nai-liang, SHI Rui. Research on the factors affecting the quality of lead sulphate [J]. Journal of Heilongjiang Institute of Science and Technology, 2001, 11(3): 20-22. (in Chinese)
[2] DAI Zhong-xu, WANG Di-hua, ZOU Jin-yun. Studies on the behaviours of PbSO4/Pb and PbO2/PbSO4 electrodes prepared from lead carbonate by powder microelectrode technique [J]. Journal of Wuhan University (Natural Sciences), 2000, 5(4): 474-478. (in Chinese)
[3] ZHANG Bo-yin. Science technology of nonferrous metals [M]. Beijing: Metallurgical Industry Press, 1990: 17-24. (in Chinese)
[4] CHEN Guan-rong, SHI Jun. Cyclopaedia of chemical engineering (Volume 12) [M]. Beijing: Chemistry Industry Press, 1996: 909-913. (in Chinese)
[5] POLING H Y. The development of sulfide mine smelt (Volume 2) [M]. BAO Xiao-bo, Transl. Beijing: Metallurgical Industry Press, 1991: 118-132. (in Chinese)
[6] WANG S, FANG Z, WANG Y, CHEN Y. Electrogenerative leaching of galena with ferric chloride [J]. Minerals Engineering, 2003, 16(9): 869-872.
[7] BALAZ P. Influence of solid state properties on ferric chloride leaching of mechanically activated galena [J]. Hydrometallurgy, 1996, 40(3): 359-368.
[8] BASTL Z, BALAZ P. X-ray photoelectron spectroscopy study of galena dissolution in ferric chloride media [J]. Journal of Materials Science Letters, 1993, 12: 789-790.
[9] LUENGOS M A, AMBROSIO E, BOHE A E, PASQUEVICH D M. Thermal behaviour of galena ore in the chloride atmosphere [J]. Journal of Thermal Analysis and Calorimetry, 2000, 59: 775-789.
[10] DUTRIZAC J E, CHEN T T. The effect of the element sulphur reaction product on the leaching of galena in ferric chloride media [J]. Metallurgical Transactions B, 1990, 21: 935-943.
[11] KOBAYASHI M, DUTRIZAC J E. A critical review of the ferric chloride leaching of galena [J]. Can Metall Q, 1990, 29(3): 201-211.
[12] PRITZKER M. Model for the ferric chloride leaching of galena [J]. Metallurgical and Materials Transactions B, 1998, 29: 953-959.
[13] WRIGHT K, HILLIER I H, VAUGHAN D J, VINCENT M A. Cluster models of the dissociation of water on the surface of galena (PbS) [J]. Chemical Physics Letters, 1999, 299(6): 527-531.
[14] DUTRZAC J E, CHEN T T. The leaching of galena in ferric sulphate media [J]. Metallurgical and Materials Transactions B, 1995, 26: 219-227.
[15] LU Ke-yuan, YU Hong, CHEN Jia-yong. A new approach to lead hydrometallurgy [J]. Progress in Chemistry, 1998, 10(3): 344-346. (in Chinese)
[16] GONG Ya-jun, CHEN Jia-yong. Kinetics of conversion of galena into lead carbonate in ammonium carbonate solution in the presence of cupric ion [J]. Hydrometallurgy, 1993, 33: 177-195.
[17] LU Ke-yuan, CHEN Jia-yong. Conversion of galena to lead carbonate in ammonium carbonate solution—A new approach to lead hydrometallurgy [J]. Hydrometallurgy, 1986, 17: 73-83.
[18] CHEN A A, DREISINGER D B. The ferric fluosilicate leaching of lead concentrates [J]. Metallurgical Transactions B, 1994, 25(5): 473-480.
[19] HUANG Hui-zhong. Analysis of nanometer materials [M]. Beijing: Chemistry Industry Press, 2003: 253. (in Chinese)
[20] LI Shu-tang. Elements of X-ray crystallography [M]. Beijing: Metallurgical Industry Press, 1990. (in Chinese)
Foundation item: Project(50774094) supported by the National Natural Science Foundation of China
Corresponding author: QIN Wen-qing; Tel/Fax: +86-731-8830346; E-mail: qinwenqing1@126.com
DOI: 10.1016/S1003-6326(08)60299-X
(Edited by YANG Bing)