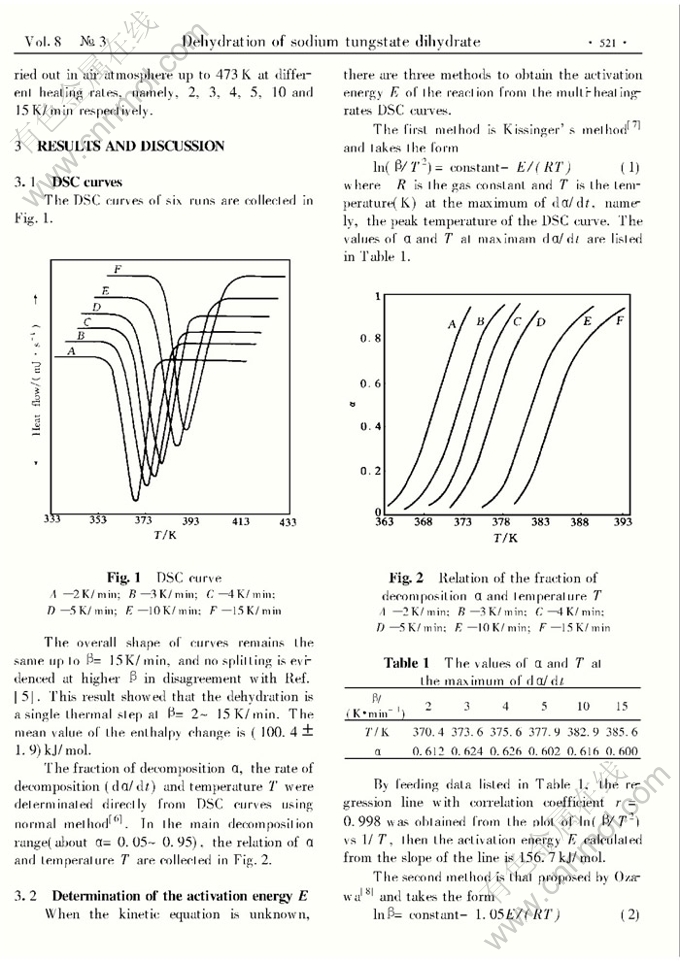
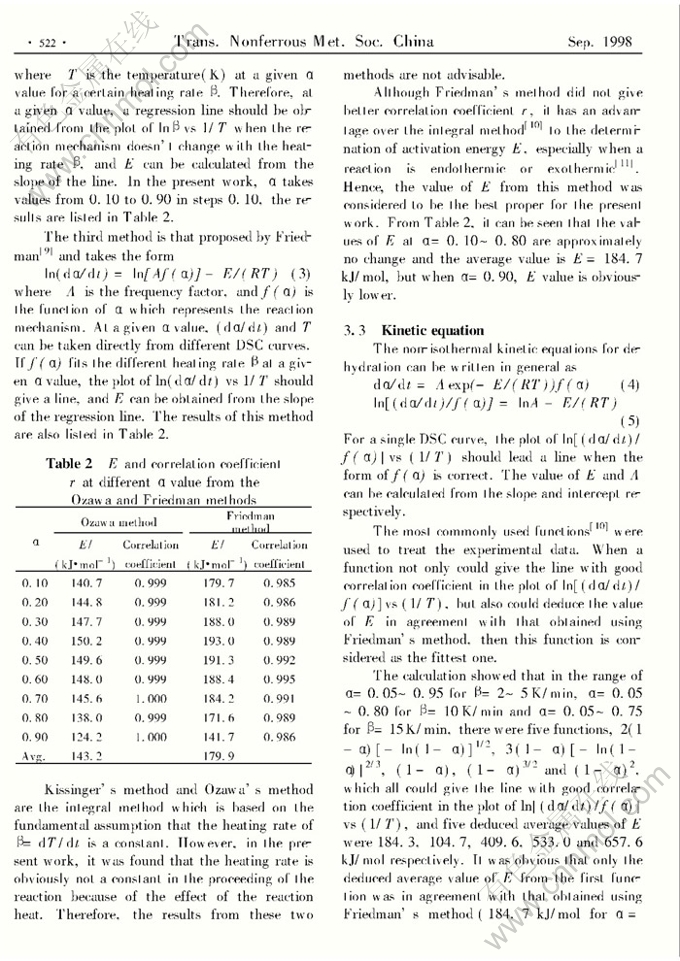
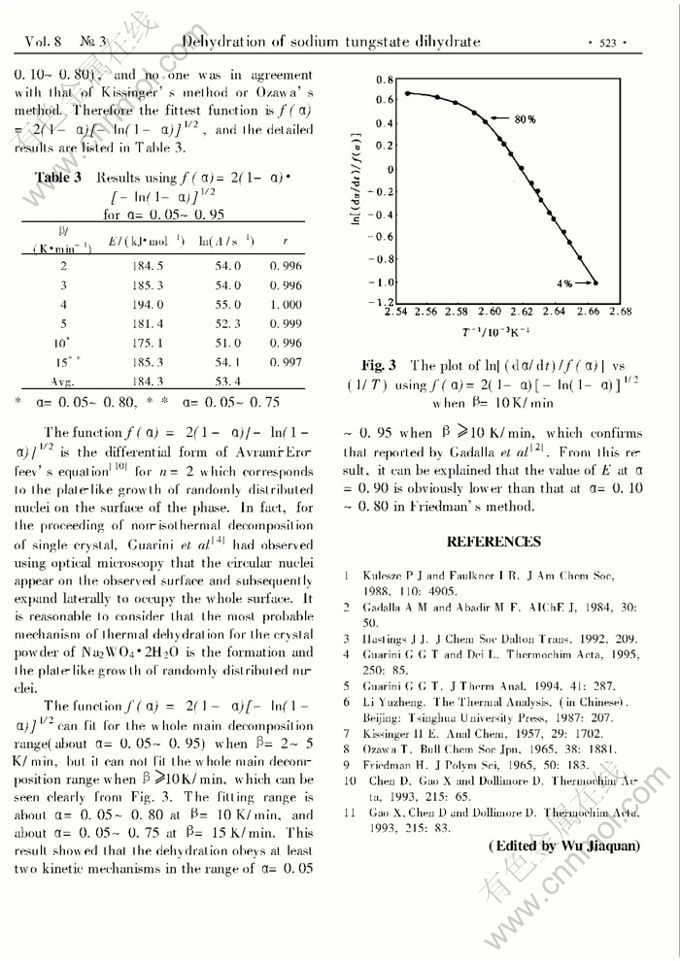
NON-ISOTHERMAL KINETIC STUDY ON THE THERMAL DEHYDRATION OF SODIUM TUNGSTATE DIHYDRATE
来源期刊:中国有色金属学报(英文版)1998年第3期
论文作者:Liu Shijun Chen Qiyuan Zhang Pingmin
文章页码:520 - 523
Key words:non-isothermal kinetics; dehydration; sodium tungstate dihydrate
Abstract: The thermal dehydration of sodium tungstate dihydrate has been studied by using differential scanning calorimetry (DSC). It was found that the dehydration takes place in a single thermal step at heating ratesβ=2~15K/min, and the enthalpy change is (100.4±1.9)kJ/mol. In the main decomposition range of α=0.05~0.95 whenβ=2~5K/min,α=0.05~0.80 whenβ=10K/min andα=0.05~0.75 whenβ=15K/min, the fittest kinetic equation was deduced, and from this equation the calculated activation enegyE was 184.3 kJ/mol. According to this equation, the mechanism of dehydration should be the formation and the plate-like growth of randomly distributed nuclei. Whenβ≥10 K/min, the dehydration obeys at least two kinetic equations.