含Mo和Ta作为β稳定剂元素的Ti合金在 牙科应用中的电化学行为
来源期刊:中国有色金属学报(英文版)2013年第12期
论文作者:Daniel MARECI Romeu CHELARIU Georgiana BOLAT Adrian CAILEAN Viorel GRANCEA Daniel SUTIMAN
文章页码:3829 - 3836
关键词:Ti12Mo;Ti60Ta;Cp-Ti;开路电位;动电位极化;电化学阻抗
Key words:Ti12Mo; Ti60Ta; Cp-Ti; open-circuit potential; potentiodynamic polarization; electrochemical impedance spectroscopy
摘 要:研究具有相同Mo质量分数(12%)的Ti12Mo和Ti60Ta合金以及当前常用的Cp-Ti金属生物材料在牙科应用中的电化学行为。采用电化学方法,在37 °C下,研究样品在人工唾液以及加氟人工唾液(含0.1% F-)两种电化学媒质中的电化学性能,如开路电位、动电位极化曲线和电化学阻抗。在牙膏、牙科凝胶和牙科冲洗等方面,通常含有氟化物以防止龋齿和缓解牙齿敏感。观察在两种媒质中所有钛合金样品的电化学行为,结果表明:在两种电化学媒质中,Ti60Ta合金具有比Ti12Mo和Cp-Ti更优异的抗腐蚀性能。
Abstract: Corrosion behaviour of the studied Ti12Mo and Ti60Ta alloys with the same Mo equivalent values (12%, mass fraction) together with the currently used metallic biomaterials Cp-Ti were investigated for dental applications. The electrochemical properties of the samples were examined using electrochemical techniques: such as open-circuit potential, potentiodynamic polarization curves and electrochemical impedance spectroscopy (EIS), in two electrochemical media of artificial saliva and fluoridated artificial saliva (0.1% fluoride ions, F-) at 37 °C. Fluoride is commonly included in toothpastes, odontological gels and dental rinses to prevent dental caries and relieve dental sensitivity. The passive behaviour for all the titanium samples is observed for both solutions. The Ti60Ta alloy appears to possess superior corrosion resistance than the Ti12Mo and Cp-Ti in both electrochemical media.
Trans. Nonferrous Met. Soc. China 23(2013) 3829-3836
Daniel MARECI1, Romeu CHELARIU2, Georgiana BOLAT1, Adrian CAILEAN1, Viorel GRANCEA2, Daniel SUTIMAN1
1. Faculty of Chemical Engineering and Environmental Protection, “Gheorghe Asachi” Technical University of Iasi, Iasi 700050, Romania;
2. Faculty of Materials Science and Engineering, “Gheorghe Asachi” Technical University of Iasi, Iasi 700050, Romania
Received 21 March 2013; accepted 4 September 2013
Abstract: Corrosion behaviour of the studied Ti12Mo and Ti60Ta alloys with the same Mo equivalent values (12%, mass fraction) together with the currently used metallic biomaterials Cp-Ti were investigated for dental applications. The electrochemical properties of the samples were examined using electrochemical techniques: such as open-circuit potential, potentiodynamic polarization curves and electrochemical impedance spectroscopy (EIS), in two electrochemical media of artificial saliva and fluoridated artificial saliva (0.1% fluoride ions, F-) at 37 °C. Fluoride is commonly included in toothpastes, odontological gels and dental rinses to prevent dental caries and relieve dental sensitivity. The passive behaviour for all the titanium samples is observed for both solutions. The Ti60Ta alloy appears to possess superior corrosion resistance than the Ti12Mo and Cp-Ti in both electrochemical media.
Key words: Ti12Mo; Ti60Ta; Cp-Ti; open-circuit potential; potentiodynamic polarization; electrochemical impedance spectroscopy
1 Introduction
Most of the used materials for dental applications include NiCr alloys [1-5], CoCr alloys [4-7], or titanium based materials [8-11]. Titanium and its alloys remain the first choice for implants due to their biological advantages and excellent corrosion resistance.
For medical application, commercial pure titanium (Cp-Ti) and Ti6Al4V (ASTM F-1472, ASTM F-136, ISO 5832-3) have been used since the 1960s, with Ti6Al4V gradually replacing Cp-Ti due to the increased mechanical strength of nails, screws, plates and end prosthesis [12,13].
Reservations have been expressed concerning the presence in long-term implants of elements such as vanadium which are toxic both in the elemental state and oxides [14-17]. According to PIAZZA et al [18], Al is poorly absorbed within the gastrointestinal tract, and very little gets into the blood stream. The association between Al and Alzheimer disease has been concerned, but not confirmed [19,20]. For this reason, novel titanium alloys with greater biocompatibility and lower elastic modulus are desirable. In recent years, attempts were made to develop β type titanium alloys with biomechanical compatibility, low modulus and biochemical compatibility [9]. The β-stabilizing elements, such as Ta, Zr, Mo and Sn, are selected as safe alloying elements to titanium, which are judged to be non-toxic and non-allergic [21]. Additionally, single phase β-structure alloys have better corrosion resistance than two-phase α+β alloys [22].
The stability of the β-phase in the case of titanium alloys is expressed as the sum of the weighted averages of the alloying elements in mass fraction known as the Mo equivalent [23,24]. A value of Mo equivalent being approximately in the range of 8%-24% (mass fraction) indicates β-metastable titanium alloys because the β-stabilizers content is high enough to prevent any martensitic transformation in the β phase upon quenching to room temperature [23,24].
Currently, dental gels and rinses containing fluoride are popular for prevention of plaque and caries formation.
In this work, the electrochemical properties of the two β-titanium alloys (Ti12Mo and Ti60Ta alloys) for dental application are compared in order to establish which alloying element increases the corrosion resistance. For comparative purposes, the same electrochemical measurements were also performed on Cp-Ti material.
2 Experimental
2.1 Materials
The origin and nominal chemical compositions of the studied titanium alloys are listed in Table 1.
Table 1 Origin and chemical composition of investigated titanium alloys

Both Ti12Mo and Ti60Ta alloys were synthesized using the procedure described elsewhere [25,26]. Using the equation of Mo equivalent indicated in Ref. [23], the Mo equivalent values of the both investigated alloys are 12% for Ti12Mo and Ti60Ta. This indicates that both titanium alloys are β-metastable.
The samples were cut into 0.95 cm2, ground with SiC abrasive paper up to 2000 grit, and polished with 1 μm alumina suspension. Then, the samples were degreased with ethyl alcohol followed by ultrasonic cleaning with deionised water and dried under a hot air stream.
2.2 Electrochemical media
Fresh Fusayama’s artificial saliva [27] was selected as it has been shown to produce results that were consistent with the clinical experience of dental alloys [28]. It was composed of 0.400 g NaCl, 0.400 g KCl, 0.795 g CaCl2×2H2O, 0.780 g NaH2PO4×2H2O, 0.005 g Na2S×9H2O, 1.000g NH2CONH2 and distilled water of 1000 mL. The pH was measured with a CONSORT 831C multiparameter analyser. The pH of the reference saliva corresponding to the first medium was 5.6.
The second medium used, named fluoridated artificial saliva, had the same contents as the first but was doped with F- with a concentration of 0.1%. The concentration of F- selected for this work may be considered as similar to the one used in fluoridated odontological gels [29].
2.3 Material characterization
The microstructures of Ti12Mo and Ti60Ta alloys were examined using a LEICA DMI5000 M metallographic microscope equipped with a dedicated digital camera connected to a personal computer and analyzed with the Leica Application Suite software program. The phase constitutions of the Ti12Mo and Ti60Ta alloys were analyzed by X-ray diffraction (XRD) analysis with an X’Pert PRO MRD, PANalytical Holland diffractometer and Cu Kα radiation.
2.4 Electrochemical setup
Electrochemical measurements were performed under open air solution at 37 °C using a PARSTAT 4000 potentiostat (Princeton Applied Research, USA) controlled by a personal computer and specific software program (VersaStudio, PAR).
A glass corrosion flow cell kit (C145/170, Radiometer, France) with a platinum counter-electrode and a saturated calomel reference electrode (SCE) were used to perform the electrochemical measurements. The C145/170 was fitted with a PCTFE sample holder and a freely adjustable Luggin capillary. All potentials referred to in this work are with respect to SCE.
The following sequence of electrochemical experiments was adopted:
1) Cronoamperometric polarization at -1 V (vs SCE) for 60 s in artificial saliva;
2) Open circuit potential (OCP) measurement for 2 h in artificial saliva and for 1 h after doping the artificial saliva with NaF resulting in final concentration of 0.1% F-, named fluoridated artificial saliva (φ1);
3) EIS measurement at open circuit potential, φ1, in fluoridated artificial saliva;
4) Potentiodynamic cathodic polarization from φ1 to -1 V (vs SCE) with 1 mV/s potential sweep rate;
5) OCP measurement for 1 h in fluoridated artificial saliva (φ2);
6) EIS measurement at open-circuit potential, φ2, in fluoridated artificial saliva;
7) Potentiodynamic anodic polarization from φ2 to 1 V (vs SCE) with 1 mV/s potential sweep rate.
The EIS spectra were recorded in the frequency range of 10-2 Hz to 104 Hz. The applied alternating potential signal had amplitude of 10 mV. The EIS experimental data were analyzed in terms of equivalent circuits (EC) using ZSimpWin 3.22 software. Since the measured capacitive response was not generally ideal due to certain heterogeneity of the electrode surface, a constant phase element (CPE) was introduced for fitting the spectra, instead of an ideal capacitance element [30].
2.5 SEM of corroded surfaces
In order to observe the occurrence of the surface effects of the corrosion after anodic polarization treatment, the corroded surfaces were observed by Quanta 3D scanning electron microscope (AL99/D8229).
3 Results and discussion
3.1 Microstructural characteristics
Figure 1 shows the XRD patterns of Ti12Mo and Ti60Ta alloy samples measured at room temperature. Besides the β phase peaks, the obvious diffraction peaks of α″(110) for Ti12Mo alloy sample were observed, as shown in Fig. 1. In contrast, it can be observed that the Ti60Ta alloy sample only comprises single β phase.

Fig. 1 XRD patterns of Ti12Mo and Ti60Ta alloys
The microstructures of the Ti12Mo and Ti60Ta alloys are shown in Fig. 2. The two titanium alloys have similar microstructures characterized by an average grain size of 350 μm and equiaxed β grains morphology. However, the Ti12Mo sample exhibits an acicular α″ phase formed within primary β grains, as identified by the XRD patterns. The Cp-Ti microstructure is characterized by equiaxed α grains morphology, and an average size of 35 μm. The structural characterization of the Cp-Ti was detailed elsewhere [31].
3.2 Electrochemical tests
The open-circuit potential (OCP) is the potential at which the material is in equilibrium with the specific environment. The OCP of a metal varies as function of the time. In Fig. 3, the variation of OCP with time for all the Ti alloys in artificial saliva and in fluoridated artificial saliva is shown. These results are based on the Ti samples studied after 2 h of immersion in artificial saliva and 1 h of immersion in fluoridated artificial saliva. Prior to each measurement, the electrode was cathodically polarized at -1 V (vs SCE) in the artificial saliva for 60 s in order to remove any spontaneously formed surface film. OCP variation is similar for all the titanium samples in both aerated solution.
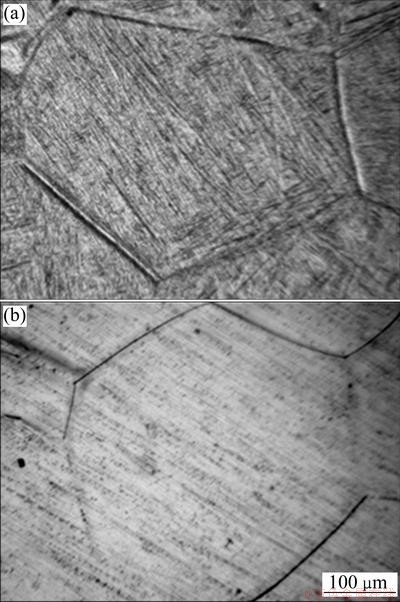
Fig. 2 Optical microstructures of Ti12Mo (a) and Ti60Ta (b) alloys

Fig. 3 Variation of OCP with time for Ti alloys maintained for 3 h in artificial saliva without or with F-
Figure 3 shows that all the samples have a tendency to form a passive film by the shift of OCP to more positive (noble) direction with respect to time. An abrupt OCP displacement towards positive potentials is noticed in Fig. 3 during a period of 10 min. This initial increase seems to be related to the formation and thickening of the oxide film on the metallic surface. Afterwards, the OCP increases slowly, suggesting the growth of the oxide film. The protecting of the oxide film increases the corrosion resistance. Stable potentials in open-circuit measurements are obtained after exposuring in artificial saliva for 60 min, which means that the oxide film becomes stable. The OCP for Ti12Mo or Ti60Ta alloy in artificial saliva is more positive than that for Cp-Ti, which is due to the positive contribution of the Mo and Ta alloying elements in the formation of oxide film. After 1 h of immersion in fluoridated artificial saliva, there is not a significant change of the OCP. 0.1% F- has no significant influence on the OCP. This behaviour can be essentially attributed to the presence of the oxide film. Finally, after 3 h immersion in artificial saliva without or with F-, the highest OCP (φ1) was found for Ti60Ta alloy followed by Ti12Mo and Cp-Ti alloy.
After recording the φ1, the samples were subjected to the cathodic polarization tests. Plots in semi- logarithmic scale of current densities corresponding to all samples after 2 h in artificial saliva and 1 h in fluoridated artificial saliva traced between φ2 to -1 V (vs SCE) are displayed in Fig. 4. All curves present the same feature.

Fig. 4 Potentiodynamic cathodic polarisation curves of Ti alloys maintained in artificial saliva for 2 h and maintained in fluoridated artificial saliva for 1 h on semi-logarithmic axes with 1 mV/s potential sweep rate at 37 oC
After cathodic polarization, the oxide film is reduced, the second OCP (φ2) returns to the same potential range (around to φ1). This behavior suggests that all the titanium alloys exhibit a spontaneous passive state and cannot be activated by cathodic treatment (Fig. 5).
Potentiodynamic anodic polarisation curves of Ti alloys tested in fluoridated artificial saliva from φ2 to 1 V (vs SCE) are displayed in Fig. 6. This test was performed in order to analyze the continuity and stability of the passive oxide film formation.
Figure 6 clearly proves that Ti60Ta alloy possesses a superior corrosion resistance than Cp-Ti or Ti12Mo alloy in fluoridated artificial saliva.
Passive current densities (Jpass) were determined from the potentiodynamic anodic diagram at different potentials (0, 0.5, 1.0 V (vs SCE)).
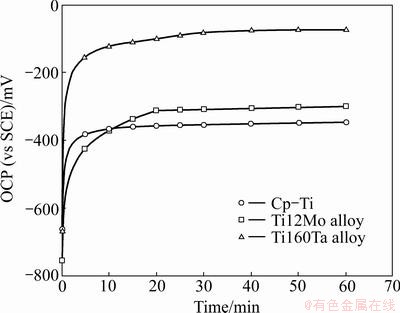
Fig. 5 Variation of OCP with time for Ti alloys maintained in fluoridated artificial saliva (φ2) for 1 h after cathodic polarization test
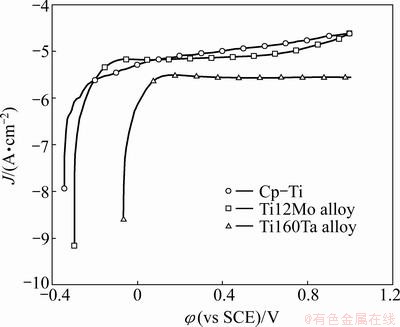
Fig. 6 Potentiodynamic anodic polarisation curves of Ti samples tested in fluoridated artificial saliva on semi- logarithmic axes with 1 mV/s potential sweep rate at 37 oC
Moreover, a coulometric analysis was performed. The method proposed here consists of the partition of the anodic polarization curve into two separate zones: the first zone (zone I) from φ2 up to 0.4 V (vs SCE) and second zone (zone II) from 0.4 to 0.7 V (vs SCE). The separation is somewhat arbitrary, but the potential range of zone I is close to clinical conditions. It is known that pure titanium in human body may be exposed to potentials up to 0.4 V (vs SCE) [32]. For analysis, the surfaces under polarization curves are integrated for each zone. The Jpass values and the quantities of electrical charge consumed by the corrosion phenomenon are listed in Table 2. It is worthy of noting that the Cp-Ti and Ti12Mo alloys have a lower corrosion resistance in this electrolyte comparative with the Ti60Ta alloy.
Observation by SEM of the surface after anodic polarization indicates a uniform oxidation for all Ti alloys (Fig. 7). No pitting cracks or other defect appeared on the Ti alloys surfaces except the presence of polishing scratches. Fluoride ions can cause localized corrosion and partial dissolution of the passive protective film formed on the Ti alloys [33]. In this case, the results suggest a non-predominant fluoride effect on the passive behaviour of Ti alloys.
Table 2 Main parameters of corrosion process measured for Ti12Mo and Ti60Ta alloys (with the same Mo equivalent values of 12%) and Cp-Ti in fluoridated artificial saliva


Fig. 7 Surface morphologies of Cp-Ti alloy (a), Ti12Mo alloy (b) and Ti60Ta alloy (c)
Impedance spectroscopy results for Ti alloys in fluoridated artificial saliva at φ1 and φ2 potential values are presented as Bode plots (Fig. 8).

Fig. 8 Bode plots recorded at selected potentials φ1 (a) and φ2 (b) in fluoridated artificial saliva at 37 °C
The maximum phase angle observed for Ti12Mo and Ti60Ta alloys at φ1 potentials was found to lie in the range of approximately -70° to -80°. High impedance values (about 106 W·cm2) were obtained from medium to low frequencies for Ti12Mo and Ti60Ta alloys at φ1, suggesting high corrosion resistance in fluoridated artificial saliva. It can be seen in Fig. 8(a) that the modulus of impedance (|Z|) for Ti12Mo or Ti60Ta alloys is higher than that of the Cp-Ti.
The impedance parameters supplied quantitative support for discussions of EIS results and were obtained with the ZSimpWin software by adopting the well-known equivalent circuit (EC). The EC from Fig. 9 was used to model the experimental spectra, and good agreement between experimental data and fitted data were obtained. PAN et al [34] used a physical model based on the formation and growth of a duplex titanium oxide layer on the bare titanium surface exposed to phosphate buffered saline solution. This duplex layer consisted of an inner barrier layer and an outer porous layer. The presence of duplex layer on surfaces of the titanium and titanium alloys was intensively reported in Refs. [10, 35-39].
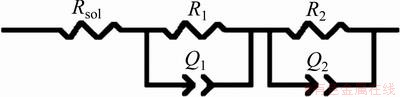
Fig. 9 Equivalent circuit (EC) used to fit impedance data
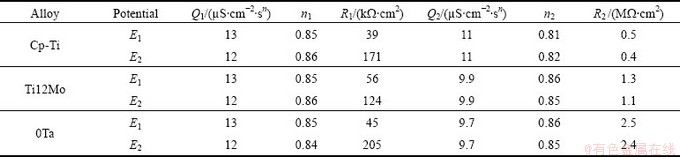
The high-frequency parameters R1 and Q1 represent the properties of the reactions at the outer porous passive film/solution interface. The parameters R2 and Q2 describe the processes at the electrolyte/compact passive film interface (inner barrier layer). Rsol is the ohmic resistance of the electrolyte. The chi-square value (χ2) around of 5×10-4 points to excellent agreement between the experimental data and simulated values. The fitted parameters values of the EC are listed in Table 3.
High values of R2 (about 1 MW·cm2) are observed at φ1 potentials for both Ti12Mo and Ti60Ta alloys, confirming the formation of a compact layer with high corrosion protection ability. R2 is greater than R1 (Table 2), showing that the resistance of the oxide film on the all the sample at φ1 potentials is due to this layer. R1 is around 104 W·cm2 for all the Ti alloys at φ1 potentials.
The EIS spectra recorded for φ2 were fitted and the model Rsol(R1Q1)(R2Q2) from Fig. 9 was proposed. The values of R2 remain around 1 MW·cm2, for both Ti12Mo and Ti60Ta alloys, at φ2 potentials, indicating high corrosion resistance.
The higher R2 values of Ti samples at φ1 and φ2 potentials indicate that Ti60Ta alloy (with Mo equivalent values of 12%) possesses a superior corrosion resistance than Ti12Mo alloy or Cp-Ti in fluoridated artificial saliva.
In terms of EIS analysis, the corrosion resistance of Ti samples immersed in fluoridated artificial saliva is improved with addition of β-stabilizing elements. Probable, the additions of the β-stabilizing elements have a positive contribution to the formation of the passive oxide film. However, the corrosion resistance of Ti alloys is influenced by the type of β alloying elements.
Titanium possesses a better corrosion resistance due to a thin, stable oxide film on the surface, which mainly consisted of TiO2 oxide film [40,41]. OKAZAKI and GOTOH [15] explained that when Zr, Nb and Ta were added to Ti alloy, the resultant ZrO2, Nb2O5 and Ta2O5 strengthened the TiO2 on the Ti alloy and promoted a better resistance. ZHOU et al [42] reported that Ta2O5 oxide film formed on TiTa alloys was stronger and more stable than the TiO2 oxide film. Molybdenum was used as alloying element for titanium because it had a good thermodynamic stability [43]. ZHOU and LUO [44] confirmed that the passive oxide layer formed on the surface of Ti10Mo alloy consisted of a mixture of both TiO2 and MoO3. A modification of the TiO2 passive layer by Ta2O5 or MoO3 improved the integrity of the oxide layer. Probable, the difference in the corrosion electrochemical behavior of Ti12Mo and Ti60Ta was composition of the Mo and Ta alloying elements.
The corrosion resistance of the three titanium alloys increased in the following order: Ti60Ta alloy (with 12% Mo equivalent values)>Ti12Mo alloy>Cp-Ti alloy.
4 Conclusions
1) Open circuit potentials of the Ti12Mo and Ti60Ta alloys in both electrochemical media are more electropositive than the Cp-Ti due to beneficial effect of the β alloying elements of Mo and Ta.
2) The passive behaviors for all the Ti alloys are observed in both electrochemical media.
3) The EIS results of the samples in fluoridated artificial saliva can be fitted using the model of Rsol(R1Q1)(R2Q2). These results confirm the presence of a two-layer film consisting of an inner barrier responsible for the corrosion protection, and other porous layer on the surface of the samples.
4) Values of barrier layer indicate that the β alloying elements improve the electrochemical corrosion behaviour of Ti alloys in both electrochemical media, compared to the Cp-Ti, and are influenced by the type of β alloying elements; the corrosion resistance is in the following order: Ti60Ta alloy>Ti12Mo alloy>Cp-Ti alloy.
Table 3 Values of fitted parameters of equivalent circuits as function of selected potential Ti samples in fluoridated artificial saliva
References
[1] WYLIE C M, SHELTON R M, FLEMING G J P, DAVENPORT A J. Corrosion of nickel-based dental casting alloys [J]. Dental Materials, 2007, 23: 714-723.
[2] LIN H Y, BOWERS B, WOLAN J T, CAI Z, BUMGARDNER J D. Metallurgical, surface, and corrosion analysis of Ni-Cr dental casting alloys before and after porcelain firing [J]. Dental Materials, 2008, 24: 378-385.
[3] RECLARU L, UNGER R E, KIRKPATRICK C J, SUSZ C, ESCHLER P Y, ZUERCHER M H, ANTONIAC I, LUTHY H. Ni-Cr based dental alloys: Ni release, corrosion and biological evaluation [J]. Materials Science and Engineering C, 2012, 32: 1452-1460.
[4] AMEER M A, KHAMIS E, AL-MOTLAQ M. Electrochemical behaviour of recasting Ni-Cr and Co-Cr non-precious dental alloys [J]. Corrosion Science, 2004, 46: 2825-2836.
[5] SAJI Viswanathan S, CHOE Han-Cheol. Electrochemical behavior of Co-Cr and Ni-Cr dental cast alloys [J]. Transactions of Nonferrous Metals Society of China, 2009, 19(4): 785-790.
[6] MARECI D, SUTIMAN D, CAILEAN A, BOLAT G. Comparative corrosion study of Ag–Pd and Co–Cr alloys used in dental applications [J]. Bulletin of Materials Science, 2010, 33: 491-500.
[7] QIU Jing, YU Wei-qiang, ZHANG Fu-qiang. Effects of the porcelain-fused-to-metal firing process on the surface and corrosion of two Co–Cr dental alloys [J]. Journal of Materials Science, 2011, 46: 1359-1368.
[8] CHEN Yu-yong, XU Li-juan, LIU Zhi-guang, KONG Fan-tao, CHEN Zi-yong. Microstructures and properties of titanium alloys Ti-Mo for dental use [J]. Transactions of Nonferrous Metals Society of China, 2006, 16(2): s824-s828.
[9] GUO Yong-yuan, CHENG Meng-qi, CHEN De-sheng, XUE Xiao-bing, ZHANG Xian-long. In vitro corrosion resistance and cytotoxicity of novel TiNbTaZr alloy [J]. Transactions of Nonferrous Metals Society of China, 2012, 22(1): s175-s180.
[10] MARECI D, CHELARIU R, DAN I, GORDIN D M, GLORIANT T. Corrosion behaviour of β-Ti20Mo alloy in artificial saliva [J]. Journal of Materials Science: Materials in Medicine, 2010, 21: 2907-2913.
[11] XU Li-juan, XIAO Shu-long, TIAN Jing, CHEN Yu-yong, HUANG Yu-dong. Microstructure and dry wear properties of Ti-Nb alloys for dental prostheses [J]. Transactions of Nonferrous Metals Society of China, 2012, 22: s639-s644.
[12] ZAVANELLI R A, HERRIQUES G P E, FERREIRA I, ROLLO M D A. Corrosion-fatigue life of commercially pure titanium and Ti-6Al-4V alloys in different storage environments [J]. The Journal of Prosthetic Dentistry, 2000, 84: 274-279.
[13] SITTING C, TEXTOR M, SPENCER N D, WIELAND M, VALLOTTON P H. Surface characterization [J]. Journal of Materials Science: Materials in Medicine, 1999, 10: 35-46.
[14] HO W F, CHEN W K, WU S C, HSU H C. Structure, mechanical properties, and grindability of dental Ti–Zr alloys [J]. Journal of Materials Science: Materials in Medicine, 2008, 19: 3179-3186.
[15] OKAZAKI Y, GOTOH E. Comparison of metal release from various metallic biomaterials in vitro [J]. Biomaterials, 2005, 26: 11-21.
[16] BRUNETTE D M, TEXTOR P T M, THOMSEN P. Titanium in medicine [M]. Berlin, Heidelberg, New York: Springer, 2001.
[17] KIM T I, HAN J H, LEE I S, LEE K H, SHIN M C, CHOI B B. New titanium alloys for biomaterials: A study of mechanical and corrosion properties and cytotoxicity [J]. Bio-Medical Materials and Engineering, 1997, 7: 253-263.
[18] PIAZZA S, LO BIUNDO G, ROMANO M C, SUNSERI C, DI QUATRO F. In situ characterization of passive film on Al-Ti alloy by photocurrentt and impedance spectroscopy [J]. Corrosion Science, 1998, 40: 1087-1108.
[19] OKAZAKI Y, ITO Y, KYO K, TATEISHI T. Corrosion resistance and corrosion fatigue strength of new titanium alloys for medical implants without V and Al [J]. Materials Science and Engineering A, 1996, 213: 138-147.
[20] RAO S, OKAZAKI Y, TATEISHI T, USHIDA T, ITO Y. Cytocompatibility of new Ti alloy without Al and V by evaluating the relative growth ratios of fibroblasts L929 and osteoblasts MC3T3-E1 cells [J]. Materials Science and Engineering C, 1994, 4: 311-314.
[21] NIINOMI M. Recent research and development in titanium alloys for biomedical applications and healthcare goods [J]. Science and Technology of Advanced Materials, 2003, 4: 445-454.
[22] MARECI D, CHELARIU R, GORDIN D M, UNGUREANU G, GLORIANT TH. Comparative corrosion study of Ti-Ta alloys for dental applications [J]. Acta Biomaterialia 2009, 5: 3625-3639.
[23] ZHOU T, AINDOW M, ALPAY S P, BLACKBURN M J, WU M H. Pseudo-elastic deformation behavior in a Ti/Mo-based alloy [J]. Scripta Materialia, 2004, 50: 343-348.
[24] ZHANG L C, ZHOU T, AINDOW M, ALPAY S P, BLACKBURN M J. Nucleation of stress induced martensites in a Ti/Mo based alloy [J]. Journal of Materials Science, 2005, 40: 2833-2836.
[25] GORDIN D M, GLORIANT T, TEXIER G, THIBON I, ANSEL D, DUVAL J L, NAGEL M D. Development of a beta-type Ti-12Mo-5Ta alloy for biomedical applications: Cytocompatibility and metallurgical aspects [J]. Journal of Materials Science: Materials in Medicine, 2004, 15: 885-891.
[26] GORDIN D M, DELVAT E, CHELARIU R, UNGUREANU G, BESSE M, LAILLE D, GLORIANT T. Characterization of Ti-Ta alloys synthesized by could crucible levitation melting [J]. Advanced Engineering Materials, 2008, 10: 714-719.
[27] FUSAYAMA T, KATAYORI T, NOMOTO S. Corrosion of gold and amalgam placed in contact with each other [J]. Journal of Dental Research, 1963, 42: 1183-1197.
[28] MCCABE J F. Applied dental materials [M]. 7th ed. Oxford: Blackwell Science Publication, 1994.
[29] SCHIFF N, GROSGOGEAT B, LISSAC M, DALARD F. Influence of fluoride content and pH on the corrosion resistance of titanium and its alloys [J]. Biomaterials, 2002, 23: 1995-2002.
[30] RAISTRICK I D, MACDONALD J R, FRANCSCHETTI D R. Impedance spectroscopy emphasizing solid materials and systems [M]. New York: John Wiley & Sons, 1987.
[31] MARECI D, BOLAT G, CHELARIU R, SUTIMAN D, MUNTEANU C. The estimation of corrosion behaviour of ZrTi binary alloys for dental applications using electrochemical techniques [J]. Materials Chemistry and Physics, 2013, 141: 362-369.
[32] RONDELLI G, VICENTINI B. Effect of copper on the localized corrosion resistance of Ni-Ti shape memory alloy [J]. Biomaterials, 2002, 23: 639-644.
[33] RECLARU L, MEYER J M. Effects of fluorides on titanium and other dental alloys in dentistry [J]. Biomaterials, 1998, 19: 85-92.
[34] PAN J, THIERRY D, LEYGRAF C. Electrochemical impedance spectroscopy study of the passive oxide film on titanium for implant application [J]. Electrochimica Acta, 1996, 141: 1143-1153.
[35] de LUIZ ASSIS S, WOLYNEC S, COSTA I. Corrosion characterization of titanium alloys by electrochemical tecniques [J]. Electrochimica Acta, 2006, 51: 1815-1819.
[36] CREMASCO A, OSORIO W R, FREIRE C M A, GARCIA A, CARAM R. Electrochemical corrosion behavior of a Ti-35Nb alloy for medical prostheses [J]. Electrochimica Acta, 2008, 53: 4867-4874.
[37] LAVOS-VALERETO I C, WOLYNEC S, RAMIRES I, GUASTALDI A C, COSTA I. Electrochemical impedance spectroscopy characterization of passive film formed on implant Ti6Al7Nb alloy in Hank’s solution [J]. Journal of Materials Science: Materials in Medicine, 2004, 15: 55-59.
[38] MILOSEV I, KOSEC T, STREHBLOW H H. XPS and EIS study of the passive film formed on orthopaedic Ti–6Al–7Nb in Hank’s physiological solution [J]. Electrochimica Acta, 2008, 53: 3547-3558.
[39] WANG B I, ZHENG Y F, ZHAO I C. Electrochemical corrosion behavior of biomedical Ti–22Nb and Ti–22Nb–6Zr alloys in saline medium [J]. Materials and Corrosion, 2009, 60: 788-794.
[40] YU W Q, QIU J, XU L, ZHANG F Q. Corrosion behaviors of TiO2 nanotube layers on titanium in Hank's solution [J]. Biomedical Materials, 2009, 4: 065012, doi:10.1088/1748-6041/4/6/065012.
[41] CHENG Y, HU J, ZHANG C, WANG Z, HAO Y, GAO B. Corrosion behavior of novel Ti-24Nb-4Zr-7.9Sn alloy for dental implant applications in vitro [J]. Journal of Biomedical Materials Research Part B: Applied Biomaterials, 2013, 101B: 287-294.
[42] ZHOU Y L, NIINOMI M, AKAHORI T, FUKUI H, TODA H. Corrosion resistance and biocompatibility of Ti–Ta alloys for biomedical applications [J]. Materials Science and Engineering A, 2005, 398: 28-36.
[43] BOJINOV M, BETOVA I, RAICHEFF R. A model for the transpassivity of molybdenum in acidic sulphate solutions based on ac impedance measurements [J]. Electrochimica Acta, 1996, 41: 1173-1179.
[44] ZHOU Y L, LUO D M. Corrosion behavior of Ti–Mo alloys cold rolled and heat treated [J]. Journal of Alloys and Compounds, 2011, 509: 6267-6272.
Daniel MARECI1, Romeu CHELARIU2, Georgiana BOLAT1, Adrian CAILEAN1, Viorel GRANCEA2, Daniel SUTIMAN1
1. Faculty of Chemical Engineering and Environmental Protection, “Gheorghe Asachi” Technical University of Iasi, Iasi 700050, Romania;
2. Faculty of Materials Science and Engineering, “Gheorghe Asachi” Technical University of Iasi, Iasi 700050, Romania
摘 要:研究具有相同Mo质量分数(12%)的Ti12Mo和Ti60Ta合金以及当前常用的Cp-Ti金属生物材料在牙科应用中的电化学行为。采用电化学方法,在37 °C下,研究样品在人工唾液以及加氟人工唾液(含0.1% F-)两种电化学媒质中的电化学性能,如开路电位、动电位极化曲线和电化学阻抗。在牙膏、牙科凝胶和牙科冲洗等方面,通常含有氟化物以防止龋齿和缓解牙齿敏感。观察在两种媒质中所有钛合金样品的电化学行为,结果表明:在两种电化学媒质中,Ti60Ta合金具有比Ti12Mo和Cp-Ti更优异的抗腐蚀性能。
关键词:Ti12Mo;Ti60Ta;Cp-Ti;开路电位;动电位极化;电化学阻抗
(Edited by Chao WANG)
Foundation item: Project (PN-II-ID-PCE-2011-3-0218) supported by the Romanian National Authority for Scientific Research, CNCS-UEFISCDI
Corresponding author: Georgiana BOLAT; E-mail: georgiana20022@yahoo.com
DOI: 10.1016/S1003-6326(13)62936-2

