Oxidation and hot corrosion behaviors of HVAF-sprayed conventional andnanostructured NiCrC coatings
来源期刊:中国有色金属学报(英文版)2009年第5期
论文作者:陶凯 周香林 崔华 张济山
文章页码:1151 - 1160
Key words:corrosion; nanocrystalline materials; coating; thermal spray; nickel-based alloys
Abstract: The oxidation and hot corrosion behaviors of HVAF-sprayed conventional and nanostructured NiCrC coatings were studied. The oxidation experiment was conducted in air, and the hot corrosion was conducted in the Na2SO4-30%K2SO4 environment, in the temperature range of 550-750 ℃ for periods up to 160 h. The corrosion kinetics was tested with the thermogravimetric method. The corrosion products were characterized by scanning electron microscopy(SEM), energy dispersive X-ray spectroscopy (EDX) and X-ray diffractometry (XRD). As indicated by the results, both types of coatings possess high corrosion resistance, especially the nanostructured NiCrC coating. The enhanced grain boundary diffusion in the nanostructured coating not only promotes the formation of a denser Cr2O3 scale with a higher rate, but also helps to mitigate the Cr depletion at the metal/scale interface. The less porosity of the nanostructured coating is also thought to be beneficial to the anti-corrosion properties.
基金信息:the National High-tech Research and Development Program of China

TAO Kai(陶 凯)1, ZHOU Xiang-lin(周香林)1, CUI Hua(崔 华)2, ZHANG Ji-shan(张济山)1
1. State Key Laboratory for Advanced Metals and Materials, University of Science and Technology Beijing,Beijing 100083, China
2. School of Materials Science and Engineering, University of Science and Technology Beijing,Beijing 100083, China
Received 23 September 2008; accepted 14 January 2009
Abstract: The oxidation and hot corrosion behaviors of HVAF-sprayed conventional and nanostructured NiCrC coatings were studied. The oxidation experiment was conducted in air, and the hot corrosion was conducted in the Na2SO4-30%K2SO4 environment, in the temperature range of 550-750 ℃ for periods up to 160 h. The corrosion kinetics was tested with the thermogravimetric method. The corrosion products were characterized by scanning electron microscopy(SEM), energy dispersive X-ray spectroscopy (EDX) and X-ray diffractometry (XRD). As indicated by the results, both types of coatings possess high corrosion resistance, especially the nanostructured NiCrC coating. The enhanced grain boundary diffusion in the nanostructured coating not only promotes the formation of a denser Cr2O3 scale with a higher rate, but also helps to mitigate the Cr depletion at the metal/scale interface. The less porosity of the nanostructured coating is also thought to be beneficial to the anti-corrosion properties.
Key words: corrosion; nanocrystalline materials; coating; thermal spray; nickel-based alloys
1 Introduction
High-temperature corrosion and erosion of heat transfer pipes in coal-fired boilers, such as tubes for superheaters and waterwalls, are recognized as severe problems, resulting in tube thinning and premature failure[1-2]. Superalloys have been developed for high- temperature applications; however, these alloys are usually not able to meet the requirements in both the high-temperature strength and corrosion or erosion resistance simultaneously. One possible way to solve these problems is applying a thin layer of anti-wear and anti-corrosion coating with good conductivity, such as thermal sprayed nickel- or iron-based alloy coatings [3-4].
Nanostructured (or nanocrystalline) materials, usually characterized by a microstructural length scale in 1-200 nm, have received considerable interest for the superior properties to their conventional coarse-grained counterparts, such as increased hardness and strength, improved ductility, enhanced corrosion and wear resistance[5-6]. Cryomilling, the mechanical attrition of powders in a cryogenic medium, is a recently developed method to synthesize nanostructured metallic or cermet powders[5,7]. Nanostructured bulk and coating materials using the cryomilled powders as precursors have exhibited encouraging results in improving material properties[7-8]. The high velocity oxy/air-fuel (HVOF/ HVAF) process is one of the most popular thermal spraying technologies and has been widely adopted in many industries due to its flexibility, cost effectiveness and superior quality of the coatings produced[9]. The characteristics of ultra-high flying velocity and relatively low temperature for injected feedstock powders much favor depositing of nanostructured coatings[6, 10], as the nano-features of the materials could remain well after the spraying process. HVOF/HVAF sprayed nanostructured coatings have proved great success in improving the performances of existing material systems, and are believed to be one of the most possible ways for commercial application of nanomaterials.
High-chromium-content nickel-based alloy, as a widely used corrosion resistant coating material, possesses good ability against oxidation, sulfur induced corrosion and hot corrosion[11-12]. A continuous Cr2O3 scale forms on the surface of the alloy with high bond strength when chromium content reaches the critical value, which protects the alloy from deterioration. Besides, chromium has a strong ability to form chromium carbide, which could effectively strengthen the matrix. Then Ni-Cr-C alloys with high corrosion and wear resistance together will be obtained only if the Cr- to-C ratio is optimized. Accordingly, a high-chromium- content nickel-based alloy with addition of C element was selected in this study.
The conventional and nanostructured NiCrC coatings have been successfully prepared by HVAF technique, using the gas-atomized and cryomilled alloy powders, respectively, as the feedstock[13]. Preliminary results indicate that nanostructured NiCrC coating possesses more uniform and compact microstructure and better mechanical properties than its conventional counterpart[13], and also exhibits good thermal stability during long term heat treatment[14]. In this work, the oxidation and hot corrosion behaviors of HVAF-sprayed nanostructured NiCrC coating are investigated. Experiments are also performed on the conventional NiCrC coating as well as ASTM1020 steel (which is usually employed as boiler tube material) coupons for comparison, aiming to explore the potentiality of applying nanostructured coatings for boiler tubes protection in high temperature environment.
2 Experimental
Two types of NiCrC alloy powders, namely, as- atomized powder with a conventional coarse-grained structure and as-cryomilled powder with a nanocrystalline structure, were employed as HVAF feedstock. The former was prepared using gas atomization method with N2 as the atomizing gas. The latter was produced by ball milling a slurry of gas- atomized alloy powder in liquid nitrogen for 20 h. Specifications of the two types of powders are listed in Table 1.
Table 1 Specifications of as-atomized and as-cryomilled NiCrC alloy powders

An intelli-jet activated combustion high velocity air-fuel(AC-HVAF) spraying system of UniqueCoat technologies was used to deposit the conventional and nanostructured NiCrC coatings onto medium steel coupons under the same spray conditions, using propane as fuel gas and nitrogen as powder carrier gas. Description of the HVAF spraying process has been reported[13]. The thickness of the as-deposited coatings is about 0.5 mm.
Coating-only samples for the corrosion tests were prepared by cutting the substrate with a linear cutting machine. The thin layer of remaining substrate material was removed from the samples via grinding followed by immersing in a hot 10% nitric acid solution, where copper plates in contact with the samples were used to accelerate the steel dissolution[15]. Then coating-only samples with dimensions of 10 mm×10 mm×0.30 mm were obtained. ASTM1020 steel samples were cut into dimensions of 15 mm×10 mm×2 mm. Then all the specimens were mechanically polished with sand paper. The specimens for hot corrosion tests were additionally applied with a camel hairbrush on the preheated surface (150 ℃) to gain a uniform thickness of salt film with 3-4 mg/cm2 of Na2SO4-30% K2SO4 (in molar fraction). Oxidation and hot corrosion experiments were conducted at 550 ℃, 650 ℃ and 750 ℃ separately for 160 h in a muffle furnace in static air at atmospheric pressure. These temperatures were selected to provide the typical working temperature for boiler tubes. For each experiment, 5 coatings or 3 steel samples were put into a crucible as the measured object. All crucibles were previously heat treated at 900 ℃ for 4 h to avoid the influence of volatile matter. The mass change measurements were taken at an interval of 20 h using the electronic balance (METTLER TOLEDO XS105DU) with a sensitivity of 0.1 mg. In the hot corrosion experiment, the salt films were re-coated onto the samples every 20 h after the mass measurement. Besides, extra short-interval mass gains were determined for the coating samples with the electronic balance in the initial 20 h of hot corrosion experiments, which benefited the specification of corresponding corrosion kinetics.
Phase analysis of the corrosion products was performed by XRD in a RIGAKU DMAX-RB diffractometer using Cu Kα monochromatized radiation. Metallographic sections were prepared from the coating specimens by standard metallographic procedures. Characterization on the surface and cross-sections of the coatings was carried out using back scattered electron (BSE) scanning electron microscope(SEM) (ZEISS SUPRA 55) equipped with EDX.
3 Results
3.1 Oxidation and hot corrosion kinetics of HVAF- sprayed NiCrC coatings
Figs.1(a) and (b) show the mass gain per unit area of the ASTM1020 steel and HVAF-sprayed NiCrC coatings during oxidation at 550, 650 and 750 ℃, respectively, for 160 h in air. For the steel samples, the mass gain values kept increasing at a rapid speed during the entire test, and the oxidation rate increased intensely with the rising of testing temperature.

Fig.1 Mass gain vs oxidation time plots of tested samples in air at 550 ℃, 650 ℃ and 750 ℃: (a) ASTM1020 steel coupons; (b) HVAF-sprayed NiCrC coatings
However, the oxidation behaviors of the HVAF-sprayed NiCrC coatings differed much from those of the steel. The mass gains of the coating samples were about one order of magnitude less than those of the steel specimen. During the initial 20 h of oxidation, the mass gain values of both types of coatings increased rapidly at all the three tested temperatures. After then, the oxidation rate slowed down and the mass gain values quickly stepped into the equilibrium state. The time needed for the samples to reach the relevant equilibrium state at 550 ℃ was longer than that at the higher temperatures.
At the three temperatures, the nanostructured coating possessed the smallest oxidation rate. The mass gains of the nanostructured NiCrC coating were about 25% less than those of the conventional NiCrC coating. It should be noted that the mass gain values of the coatings did not always increase with the increase in oxidation temperature. The mass gains at 550 ℃ had the smallest value for both the coatings. However, the mass gain values of oxidation at 650 ℃ were clearly larger than those at 750 ℃ for both type of coatings, which was unlike the case happening in the steel samples. Similar situations were also reported in Ref.[16]. The protecting oxide scale of the HVAF-sprayed NiCrC coatings could form on the coating surface at a more rapid speed when the temperature increased, which prevents the further reaction with oxygen. Consequently, the phenomenon of smaller mass gains at higher oxidation temperature happened. As for the steel samples, the oxide scale was loose and porous, which could not protect the matrix from further oxidation effectively. Then, the oxidation became more serious with the increase of temperature, as indicated in the mass gain data.
Fig.2(a) and (b) show the mass gain vs corrosion time plots for the steel and coating samples during corrosion in Na2SO4-30%K2SO4 environment at 550 ℃, 650 ℃ and 750 ℃ separately for 160 h. For the steel samples, the shape of the plots was very similar to that in Fig.1(a), indicating that the hot corrosion kinetics of ASTM1020 steel had the similar characteristics to the oxidation process. The mass gains kept increasing rapidly during the entire corrosion process at all the tested temperatures. The corrosion rate went up markedly with the increase of the testing temperature. Compared with the thermogravimetric plot for the oxidation experiment, one prominent characteristic of the hot corrosion kinetics for ASTM1020 steel was the mass gain. The mass gain values in the hot corrosion experiment were about twice those for oxidation at the same temperature, which meant a different mechanism from the pure oxidation.
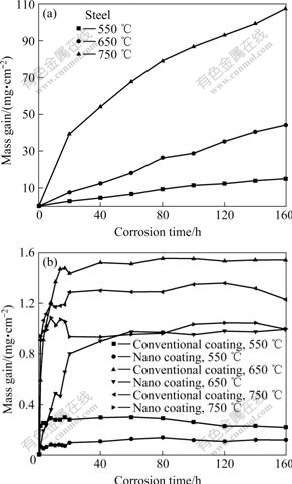
Fig.2 Mass gain vs. corrosion time plots of tested samples in Na2SO4-30% K2SO4 environment at 550 ℃, 650 ℃ and 750 ℃: (a) ASTM1020 steel coupons; (b) HVAF-sprayed NiCrC coatings
The hot corrosion kinetics of the HVAF-sprayed NiCrC coatings was very different from that of ASTM1020 steel. The mass gain values were about 1-2 orders of magnitude smaller than those of the steel samples. The corrosion rates of the two coatings exhibited a high value during the initial period of the test, and then went down quickly, which reflected that the mass gains stepped into a relevant equilibrium state. Like the case in the oxidation experiment, the mass gain values of the nanostructured coating were smaller than those of the conventional coating at all temperatures, which meant a better corrosion resistance for the nanostructured NiCrC coating. It was interesting to find that the mass gain of conventional NiCrC coating in the hot corrosion test at 650 ℃ was again larger than that at 750 ℃, and the mass gains of nanostructured coating at 650 ℃ and 750 ℃ were in the same level. Extra short-term interval mass measurement was taken to specify the corrosion kinetics in the initial 20 h of the corrosion process for both types of coatings, as plotted in Fig.2(b). The mass gains of the two coatings at 550 ℃ mounted up rapidly in the beginning. Only after 1 h or so, the corrosion process got to the equilibrium stage. When the temperature rose, the mass gains increased evidently. However, the period needed for the coatings to reach the equilibrium was longer at 650 ℃ than at 750 ℃, which was clearly illustrated in the plot. This indicated that a continuous dense oxide scale formed more quickly on the coating surface at 750 ℃ than at 650 ℃, which protected the coatings from further deterioration. Consequently, the mass gain had a smaller value at the higher temperature. This explanation was well consistent with that for the oxidation test.
3.2 XRD analysis of corrosion products
Fig.3 and Fig.4 show the XRD patterns of conventional and nanostructured NiCrC coatings in the as-deposited state and after hot corrosion at 550 ℃, 650 ℃ and 750 ℃, respectively, for 160 h. The matrix of the two NiCrC coatings was a supersaturated FCC NiCr solid solution, which had the strongest diffraction peaks among all the phases. The XRD patterns also contained a number of relatively weak peaks, which were identified as characteristic peaks from Cr7C3, Cr23C6 and Cr2O3. It should be mentioned that the as-deposited conventional and nanostructured NiCrC coatings consisted of NiCr solid solution, Cr7C3 and a very little amount of Cr2O3. And the coating composition could be simply expressed as 80%NiCr-20%Cr7C3 (mass fraction) according to the previous results[13]. After exposure to a high temperature for 160 h, part of the Cr7C3 phase evolved to Cr23C6 phase that was the most stable form of chromium carbide[17]. Another notable change was the formation of a large amount of Cr2O3 phase on the coating surface which was the only corrosion product detected by the XRD analysis for both types of NiCrC coatings under the present conditions. More characteristic diffraction peaks from the Cr2O3 phase appeared with a stronger intensity when the temperature increased. In the same corrosion environment, the relative diffraction peak intensity for the oxide of the nanostructured coating was weaker than that of the conventional coating, which usually meant that a thinner layer of oxide scale formed on the nanostructured NiCrC coating. This was in accordance with the corrosion kinetic results.

Fig.3 XRD patterns of conventional NiCrC coating in as- deposited state (a) and after corrosion in Na2SO4-30%K2SO4 environment at 550 ℃ (b), 650 ℃ (c) and 750 ℃ (d) for 160 h

Fig.4 XRD patterns of nanostructured NiCrC coating in as- deposited state (a) and after corrosion in Na2SO4-30%K2SO4 environment at 550 (b), 650 (c) and 750 ℃ (d) for 160 h
The XRD results of the conventional and nanostructured NiCrC coatings after oxidation at 550- 750 ℃ were very similar to those of the hot corrosion experiment. The four phases identified from the XRD peaks were also NiCr solid solution, Cr7C3, Cr23C6 and Cr2O3. With the increase of the oxidation temperature, the relative peak intensity of the oxide scale grew stronger.
3.3 Surface morphology of NiCrC coatings after oxidation and hot corrosion process
Figs.5(a)-(f) display the surface morphologies of HVAF-sprayed conventional and nanostructured NiCrC coating after oxidation at 550, 650 and 750 ℃, respectively, for 160 h. The corrosion products on the coating surface were subjected to EDX analysis, and the results indicated that all the oxidation products were in the form of chromium oxide. This in turn proved the XRD results. In the case of oxidation at 550 ℃, finely granular oxide particles formed homogeneously on the top of conventional NiCrC coating, seen in Fig.5(a). Meanwhile, the product which was uniformly distributed on the surface of the nanostructured coating exhibited different morphology. It was difficult to get a clear vision of the corrosion product from Fig.5(b), while the SEM images of the InLens mode showed that the tiny oxide particles were mainly in the flaky shape. With the increase of temperature up to 750 ℃, the oxide particles got increasingly larger in size for both types of coatings. The conventional NiCrC coating kept its oxide particles in granular shape, as depicted in Figs.5(c) and (e). However, some oxide particles grew to a much larger size at 750 ℃, which led to an uneven morphology of the corrosion product. Similar to its conventional counterpart, the nanostructured coating kept its corrosion product mainly in the flaky shape, as shown in Figs.5(d) and (f). Nevertheless, a small amount of granular oxide particles appeared when oxidation was proceeded at 750 ℃, as shown in Fig.5(f).

Fig.5 SEM images showing surface morphologies of conventional and nanostructured NiCrC coatings after oxidation at different temperatures for 160 h: (a) Conventional coating, 550 ℃; (b) Nanostructured coating, 550 ℃; (c) conventional coating, 650 ℃; (d) Nanostructured coating, 650 ℃; (e) Conventional coating, 750 ℃; (f) Nanostructured coating, 750 ℃
Figs.6(a)-(f) depict the surface morphologies of the conventional and nanostructured NiCrC coating after corrosion in Na2SO4-30% K2SO4 environment at 550 ℃, 650 ℃ and 750 ℃, respectively, for 160 h. Like the case in the oxidation condition, the corrosion products on the coating surface were all in the form of chromium oxide based on the EDX results. At this time, the corrosion products of the two types of coatings exhibited very similar morphology, both existing in the form of flake- like particles in the temperature range of 550-750 ℃. When performing corrosion at 550 ℃ and 650 ℃, it was hard to tell the two coatings just from their surface morphology, no matter in particle shape or size. However, the oxide particles of the conventional coating were evidently bigger than those of the nanostructured coating at 750 ℃. For the nanostructured coating, although the corrosion product was similar to the oxidation experiment in composition and shape, the oxide particles formed during the hot corrosion possessed larger size than those for the oxidation process.

Fig.6 SEM images showing surface morphologies of conventional and nanostructured NiCrC coatings after corrosion in Na2SO4- 30%K2SO4 environment at different temperatures for 160 h: (a) Conventional coating, 550 ℃; (b) Nanostructured coating, 550 ℃; (c) Conventional coating, 650 ℃; (d) Nanostructured coating, 650 ℃; (e) Conventional coating, 750 ℃; (f) Nanostructured coating, 750 ℃
3.4 Cross-sectional analysis of NiCrC coatings after oxidation and hot corrosion process
To better simulate the practical conditions of boiler tubes, the as-deposited coating samples without surface grinding were also subjected to hot corrosion test. Fig.7 and Fig.8 show the cross-sectional BSE images of such samples from the conventional and nanostructured NiCrC coatings after corrosion in Na2SO4-30%K2SO4 environment at 650 ℃ for 100 h, together with the corresponding elemental X-ray maps. For the conventional coating, a continuous scale formed on the coating surface, which had a relatively uniform thickness at different positions. According to the element distribution mapping results from EDX, this scale mainly comprised elements of chromium and oxygen, whereas the coating matrix consisted mainly of nickel and chromium. Beneath the scale, there was a stripe region, where the chromium content decreased and the nickel content increased. This was considered a chromium depletion zone, which resulted from the diffusion of Cr for the selective oxidation on the surface. For the nanostructured coating, the thickness of the oxide scale formed on top of the coating varied notably at various positions. The mean thickness value was smaller than that of the conventional coating, which was consistent with the thermogravimetric results. Similar to the case of its conventional counterpart, the scale was mainly composed of chromium and oxygen. And there was also a thin layer that should be the chromium depletion region. However, the boundaries of the region were not as clear as those in the conventional coating. It seemed that the chromium depletion zone in the nanostructured coating was not as notable as that in the conventional one.

Fig.7 BSE images of cross-section of conventional NiCrC coating after corrosion in Na2SO4-30%K2SO4 environment at 650 ℃ for 100 h with corresponding elemental X-ray maps

Fig.8 BSE images of cross-section of nanostructured NiCrC coating after corrosion in Na2SO4-30%K2SO4 environment at 650 ℃ for 100 h with corresponding elemental X-ray maps
4 Discussion
4.1 Oxidation and hot corrosion mechanisms of HVAF-sprayed NiCrC coatings
As aforementioned, NiCrC coating as a high- chromium-content nickel-based alloy possesses good oxidation and hot corrosion resistance. When being exposed to the oxidizing environment, selective oxidation occurs if the chromium content of a nickel based alloy reaches a certain level. A single-phase scale of Cr2O3 will form on the material surface to protect the matrix against further deterioration. The present study of the oxidation of NiCrC coatings in air seems to be a good demonstration of this theory.
Metals and alloys sometimes experience accelerated oxidation when their surfaces are covered by a thin film of fused salt in an oxidizing atmosphere at elevated temperatures. This mode of attack is called “hot corrosion”, where a porous non-protective oxide scale is formed on the surface and sulfide in the substrate. Hot corrosion is a serious problem in power generation equipment, gas turbines for ships and aircrafts and other energy conversion and chemical process systems, and should be either totally prevented or detected at an early stage to avoid catastrophic failure. HVOF/HVAF- sprayed coatings can play a vital role in the protection of engineering materials from the hot corrosion[9]. Since the tested temperatures in this work were all lower than the melting points of Na2SO4 (884 ℃) and the mixture of Na2SO4-30%K2SO4 (817 ℃)[18], the corrosion should belong to the type of low temperature hot corrosion. The eutectoid of Na2SO4+ Na2O (or K2SO4+K2O) was likely to form in this case [19]. For instance, the action of Fe+Na2SO4→FeS+Fe2O3/Fe3O4/FeO+Na2O happened to the ASTM1020 steel samples, where a eutectoid mixture of Na2SO4+ Na2O formed[19-20]. This salt mixture had a low melting point of 550 ℃, which was expected to exist in liquid form during the experiments. According to the theory of Rapp-Goto, the ferric oxide dissolved in the interfacial region of oxide-fused salt where the oxygen ion had a high activity. Then, the precipitation of ferric oxide occurred on the fused salt/gas interface with a porous structure, where the activity of oxygen ion fell to a low level. Consequently, the accelerated oxidation happened to the steel material in the Na2SO4-30%K2SO4 environment. However, when it came to the two types of NiCrC coatings, the preferential oxidation of chromium happened. The continuous dense scale of Cr2O3 effectively prevented the further contact of the molten salt with the coating material, thus the accelerated corrosion could be avoided. Although no obvious sulfide was observed in the corrosion product and the coating matrix (Fig.7 and Fig.8), the sulfate film covering the coating surface did promote the corrosion process at 650 and 750 ℃, which resulted in more mass gains in the hot corrosion experiment than in the oxidation test.
4.2 Corrosion resistance of conventional and nanostructured NiCrC coatings
Both the HVAF-sprayed conventional and nanostructured NiCrC coatings possess good properties against the oxidation and hot corrosion attacks. Compared with its conventional counterpart, nanostructured NiCrC coating exhibits better performance, which is believed to result from the faster formation of Cr2O3 scale with a denser structure. According to our previous results, the as-deposited nanostructured NiCrC coating had a homogeneous nanocrystalline microstructure with an average grain size of 40 nm[13]. Even after thermal exposure at 650 ℃ for over 100 h, the nanostructured coating still kept its mean grain size less than 100 nm and seemed to be stable since then[14]. Consequently, a large number of grain boundaries in the nanostructured coating served as “short circuit” channels for Cr diffusion[21-22]. The enhanced grain boundary diffusion not only provided the formation of a denser oxide scale with a higher rate, but also helped to mitigate the Cr depletion at the metal/scale interface. This was well proved by our experimental results.
Besides the grain size effect, the porosity also influences the corrosion behavior of thermal spray coatings, as pores are the preferential corrosion paths, from where the corrosive species can penetrate through the coatings to reach the substrate. Dense coatings usually provide better corrosion resistance than porous coatings[2]. Porosity measurement was made for the NiCrC coatings by analyzing more than ten corresponding metallographic images with the software ImageTool. The results were 0.40% and 0.09% for the conventional and nanostructured NiCrC coatings, respectively. Both the coatings possessed low porosity, especially the nanostructured one, which exhibited a very compact structure and was expected to benefit the mechanical and anti-corrosion properties of the coating.
5 Conclusions
1) Both HVAF-sprayed conventional and nanostructured NiCrC coatings possess good properties against oxidation and hot corrosion, especially the nanostructured one. The main corrosion product is Cr2O3 for both coatings under either the oxidation in air or hot corrosion covered by a salt film of Na2SO4-30%K2SO4 in the temperature range of 550-750 ℃. Selective oxidation occurs during the corrosion process, with a continuous dense Cr2O3 scale forming on the coating surface to protect the coating matrix from further deterioration.
2) The better corrosion resistance of nanostructured NiCrC coating is primarily attributed to its nanocrystalline characteristic. The enhanced grain boundary diffusion in the nanostructured coating not only provides the formation of a denser oxide scale with a higher rate, but also helps to mitigate the Cr depletion at the metal/scale interface. The less porosity of nanostructured NiCrC coating also benefits its anti- corrosion properties.
References
[1] STRINGER J. Coatings in the electricity supply industry: Past, present, and opportunities for the future [J]. Surface and Coatings Technology, 1998, 108/109: 1-9.
[2] SIDHU T S, PRAKASH S, AGRAWAL R D. Characterisations of HVOF sprayed NiCrBSi coatings on Ni- and Fe-based superalloys and evaluation of cyclic oxidation behaviour of some Ni-based superalloys in molten salt environment [J]. Thin Solid Films, 2006, 515: 95-105.
[3] BRANAGAN D J, BREITSAMETER M, MEACHAM B E, BELASHCHENKO V. High-performance nanoscale composite coatings for boiler applications [J]. Journal of Thermal Spray Technology, 2005, 14: 196-204.
[4] SIDHU T S, PRAKASH S, AGRAWAL R D. Hot corrosion performance of a NiCr coated Ni-based alloy [J]. Scripta Materialia, 2006, 55: 179-182.
[5] HE J, SCHOENUNG J M. Nanostructured coatings [J]. Mater Sci Eng A, 2002, 336: 274-319.
[6] GROSDIDIER T, TIDU A, LIAO H L. Nanocrystalline Fe-40Al coating processed by thermal spraying of milled powder [J]. Scripta Materialia, 2001, 44: 387-393.
[7] WITKIN D B, LAVERNIA E J. Synthesis and mechanical behavior of nanostructured materials via cryomilling [J]. Progress in Materials Science, 2006, 51: 1-60.
[8] HE J, SCHOENUNG J. Nanocrystalline Ni coatings strengthened with ultrafine particles [J]. Metallurgical and Materials Transactions A, 2003, 34: 673-683.
[9] SIDHU T S, AGRAWAL R D, PRAKASH S. Hot corrosion of some superalloys and role of high-velocity oxy-fuel spray coatings-a review [J]. Surface and Coatings Technology, 2005, 198: 441-446.
[10] HE J, ICE M, LAVERNIA E, DALLEK S. Synthesis of nanostructured WC-12%Co coating using mechanical milling and high velocity oxygen fuel thermal spraying [J]. Metallurgical and Materials Transactions A, 2000, 31: 541-553.
[11] ZHAO S, XIE X, SMITH G D, PATEL S J. The corrosion of INCONEL alloy 740 in simulated environments for pulverized coal-fired boiler [J]. Materials Chemistry and Physics, 2005, 90: 275-281.
[12] WANG B, GONG J, WANG A Y, SUN C, HUANG R F, WEN L S. Oxidation behaviour of NiCrAlY coatings on Ni-based superalloy [J]. Surface and Coatings Technology, 2002, 149: 70-75.
[13] TAO Kai, ZHANG Jie, CUI Hua, ZHOU Xiang-lin, ZHANG Ji-shan. Fabrication of conventional and nanostructured NiCrC coatings via HVAF technique [J]. Trans Nonferrous Met Soc China, 2008, 18(2): 262-269.
[14] CUI Hua, TAO Kai, ZHOU Xiang-lin, ZHANG Ji-shan. Thermal stability of nanostructured NiCrC coating prepared by HVAF spraying of cryomilled powders [J]. Rare Metals, 2008, 27: 1-7.
[15] MATTHEWS S, HYLAND M, JAMES B. Microhardness variation in relation to carbide development in heat treated Cr3C2-NiCr thermal spray coatings [J]. Acta Materialia, 2003, 51: 4267-4277.
[16] DING Zhang-xiong, TU Guo-fu. The hot corrosion performance of NiCr-Cr3C2 cermet coating to boiler tube [J]. Journal of Wuhan University of Technology, 2004, 19: 52-54.
[17] LAI G Y. Factors affecting the performances of sprayed chromium carbide coatings for gas-cooled reactor heat exchangers [J]. Thin Solid Films, 1979, 64: 271-280.
[18] LONG Shi-zong, CHEN Yong-zhong, CHENG Bin, SHU Wei, XIONG Rong-jun. Temperature range of eutectic mixture formation for KCl-K2SO4-Na2SO4 system [J]. Journal of the Chinese Ceramic Society, 2006, 34: 504-506. (in Chinese)
[19] XU Bin-shi, MA Shi-ning, LI Chang-qing, LI Xiao-gang, LIU Qian. Study of hot corrosion resistance properties of several kinds of arc spraying coatings [J]. China Surface Engineering, 1998, 11: 14-18. (in Chinese)
[20] MA Shi-ning, LIU Qian, LI Chang-qing. Study of hot corrosion resistance mechanisms of arc spraying coatings [J]. Acta Metallurgica Sinica (English Letters), 1999, 12: 1007-1013.
[21] PENG X, YAN J, ZHOU Y, WANG F. Effect of grain refinement on the resistance of 304 stainless steel to breakaway oxidation in wet air [J]. Acta Materialia, 2005, 53: 5079-5088.
[22] CHEN G, LOU H. Oxidation kinetics of sputtered Ni-5Cr-5Al nanocrystalline coating at 900 ℃ and 1 000 ℃ [J]. Nanostructured Materials, 1999, 11: 637-641.
Foundation item: Project(2002AA331080) supported by the National High-tech Research and Development Program of China
Corresponding author: TAO Kai; Tel: +86-10-62332244; E-mail: kaitao321@yahoo.cn
DOI: 10.1016/S1003-6326(08)60421-5

