Removal of anionic ions from single material solution by bauxite tailings modified with FeCl3·6H2O
来源期刊:中南大学学报(英文版)2008年第5期
论文作者:兰叶 王毓华 黄传兵
文章页码:656 - 656
Key words:bauxite tailings; adsorption; anionic ions; modification
Abstract: The adsorbabilities of the unmodified and modified bauxite tailings for Cr(Ⅵ), As(Ⅴ) and F(Ⅰ) ions were investigated. Batch experiments were carried out to determine the removal rate as a function of adsorbent dosage, solution pH value and shaking time. The results show that the maximum removal rates of Cr(Ⅵ), As(Ⅴ) and F(Ⅰ) are respectively 99%, 99% and 90% by using the modified bauxite tailings. The isoelectric point of the unmodified bauxite tailings is 3.6, and that of the modified bauxite tailings is 5.0, which shifts to lower pH values in Cr(Ⅵ) solution. This indicates a specific adsorption of the anionic species on the modified bauxite tailings. A new band of Cr2O72- appears in the FTIR, showing that Cr(Ⅵ) is adsorbed on the modified bauxite tailings in the form of chemistry adsorption. The adsorption data of Cr(Ⅵ) on the modified bauxite tailings are well described by Freundlich model. The investigations of kinetic models show that pseudo-second-order kinetic model provides the best correlation for the experimental data.
基金信息:the Major State Basic Research Development Program of China
J. Cent. South Univ. Technol. (2008) 15: 656-662
DOI: 10.1007/s11771-008-0122-8
![]()
LAN Ye(兰 叶)1, WANG Yu-hua(王毓华)1, HUANG Chuan-bing(黄传兵)2
(1. School of Resources Processing and Bioengineering, Central South University, Changsha 410083, China;
2. Institute of Process Engineering, Chinese Academy of Sciences, Beijing 100086, China)
Abstract: The adsorbabilities of the unmodified and modified bauxite tailings for Cr(Ⅵ), As(Ⅴ) and F(Ⅰ) ions were investigated. Batch experiments were carried out to determine the removal rate as a function of adsorbent dosage, solution pH value and shaking time. The results show that the maximum removal rates of Cr(Ⅵ), As(Ⅴ) and F(Ⅰ) are respectively 99%, 99% and 90% by using the modified bauxite tailings. The isoelectric point of the unmodified bauxite tailings is 3.6, and that of the modified bauxite tailings is 5.0, which shifts to lower pH values in Cr(Ⅵ) solution. This indicates a specific adsorption of the anionic species on the modified bauxite tailings. A new band of Cr2O72- appears in the FTIR, showing that Cr(Ⅵ) is adsorbed on the modified bauxite tailings in the form of chemistry adsorption. The adsorption data of Cr(Ⅵ) on the modified bauxite tailings are well described by Freundlich model. The investigations of kinetic models show that pseudo-second-order kinetic model provides the best correlation for the experimental data.
Key words: bauxite tailings; adsorption; anionic ions; modification
1 Introduction
The increasing level of hazardous substances in water represents a serious threat to human health, living resources and ecological systems. Hazardous species, such as Cr(Ⅵ), As(Ⅴ) and F(Ⅰ), are not biodegradable and tend to accumulate in living organisms, causing various diseases and disorders. The contamination of hazardous substances exists in aqueous waste streams of many industries, such as metal plating facilities and mining operations. The soils surrounding these factories are also polluted and make a risk of groundwater and surface water contamination[1].
Several treatment processes have been developed over the years to remove heavy metals from industrial wastewater, such as chemical precipitation, phytoextraction, reverse osmosis, electrodialysis, ion exchange and membrane filtration or adsorption[2]. However, most of these techniques have some disadvantages, such as complicated treatment process, high cost and energy. For adsorption treatment methods, the main disadvantage is the high price of the adsorbents, which increases the price of wastewater treatment. So, it is necessary to develop some adsorbents with low cost and high efficiency for hazardous substances.
Silicate minerals have great potential as inexpensive and efficient adsorbents due to their large quantities, good chemical and mechanical stability, large surface area and good structural properties. In recent years, great utilization of silicate minerals as adsorbents has led to much work on their adsorption properties for many kinds of hazardous substance[3-6].
Aluminosilicate-tailings are the main solid wastes generated during aluminum production from bauxite by a combined Bayer process and bauxite flotation method. It is reported that 0.2 t of aluminosilicate-tailings will be generated when 1 t of bauxite ore is treated by flotation. The main compositions of bauxite tailings are diaspore, kaolinlite, illite and pyrophyllite. The enormous quantity of tailings generated every year possess a very serious and alarming environmental problem, such as dust pollution caused by fine particles, land occupation and so on[7-8]. It would be advantageous, if possible, to utilize these tailings in other industrial fields. Although some investigations and trials on the utilization and minimization of tailings in producing cement, absorbent materials and building materials, namely bricks, ceramics, tiles, glazes and wood substitute for panels have been carried out for many years, no suitable utilization has yet been identified. In addition, there is no report on the use of bauxite tailings to treat waste water.
In this work, the preparation and the evaluation of new adsorption materials from bauxite tailings to adsorb hazardous substance ions in aqueous solution, were investigated, including the influence of adsorbent dosage, shaking time, and solution pH value on the adsorption of Cr(Ⅵ), As(Ⅴ) and F(Ⅰ) from aqueous solution by the unmodified and modified tailings. The adsorption mechanisms of Cr(Ⅵ) on the modified tailings were studied by zeta potential measurement and FTIR spectroscopy.
2 Experimental
2.1 Materials
The bauxite tailings samples were obtained from Zhongzhou Bauxite Flotation Plant, Henan, China. They were dried in electric oven at 85 ℃ for about 4 h and screened by a screen with sieve diameter of 0.325 mm. The particle size of tailings samples was determined by a laser granulometer named CILAS-1064, and the average particle size was about 25.33 μm. The samples were placed in a desiccator before modification experiments. The dried bauxite tailings samples were composed of 39.52% Al2O3, 28.89% SiO2, 3.12% TiO2, 0.46% MgO, 0.61% CaO, 7.31% total Fe, 0.13% S, 4.71% K2O and 0.82% Na2O. Diaspore, kaolinite, illite, anatase, hematite and quartz were determined as the component minerals in the bauxite tailings samples by an X-ray powder diffractometer (XRD, Shimadzu D/MAX-rA model).
2.2 Modifying tests
The bauxite tailings samples (20 g) were treated with 80 mL of 0.1 mol/L FeCl3·6H2O (analytical grade) in a 300-mL Erlenmeyer flask in a rotary orbital shaker for 12 h. Thereafter, the tailings samples were washed twice with distilled water in order to remove the excessive Fe(Ⅲ) ions till no iron ion can be determined in washed solution. After being filtrated and dried in an electric oven at 85 ℃ for approximately 4 h, the bauxite tailings samples were screened by a screen with sieve diameter of 0.325 mm and placed in a desiccator. The specific surface areas of the modified and the unmodified bauxite tailings were 22.14 m2/g and 12.57 m2/g, respectively, which were determined by N2-BET adsorption tests.
2.3 Adsorption tests
The solutions of single Cr(Ⅵ) (30 mg/L), As(Ⅴ) (1 mg/L) and F(Ⅰ) (40 mg/L) were prepared from a stock solution of 1 g/L each of salt using distilled water. The volume and shaking time were fixed at 100 mL and 2 h, respectively, while the amounts of both the modified and unmodified bauxite tailings were varied from 0.5 to 8.00 g. The adsorption batch tests were done at a shaking speed of (170±3) r/min and a temperature of (30±1) ℃. After filtration, the concentration of As(Ⅴ) ion was determined by inductively coupled plasma-mass spectro- metry (ICP-MC); Cr(Ⅵ) was determined by ultraviolet- visible spectrophotometry; and the concentration of F(Ⅰ) was determined by potentiometry.
The experiments on each metal ion were performed to determine the adsorption equilibrium time from 0.1 to 3 h. Solution pH value was adjusted by HCl and NaOH solutions (0.1-1.0 mol/L).
The adsorption percentage was determined by employing the following expression:
![]() ×100% (1)
×100% (1)
where C is the adsorption percentage of anions on tailings; C0 and Ce are the initial and the equilibrium ion concentrations, respectively. All the adsorption experiments were carried out in triplicate and the average of at least three values was used through this study.
2.4 Zeta potential measurements
Zeta potentials were determined using Brookhaven Zeta plus zeta meter (USA). The mineral samples were ground in an agate mortar to less than 5 μm. The suspensions containing 0.05% (mass fraction) solids were conditioned in a beaker for 15 min and pH value was measured at 25 ℃.
2.5 Fourier transform infrared spectrum
The infrared spectra for powder samples of the unmodified and modified bauxite tailings were obtained. The model FTIR-750 infrared spectrophotometer (Nicolet Co, USA) was used, and the samples were air- dried at 25 ℃.
3 Results and discussion
3.1 Effect of adsorbent dosages
The adsorbent dosage is an important parameter to determine the adsorbability of adsorbent under a given initial condition. The influences of the modified and the unmodified bauxite tailings on the adsorptions are given in Fig.1. It can be seen that the adsorption percentage of Cr(Ⅵ) evidently increases with the increase of the dosage of the modified bauxite tailings, and changes little in the case of the unmodified bauxite tailings with low adsorption percentage. The adsorption percentages of As(Ⅴ) and F(Ⅰ) increase with the increase of the dosage of the modified and unmodified absorbents. The modified bauxite tailings adsorb more As(Ⅴ) and F(Ⅰ) than the unmodified tailings.
3.2 Effect of shaking time
Fig.2 presents the effect of shaking time on the adsorption of anions Cr(Ⅵ), As(Ⅴ) and F(Ⅰ), which indicates that the removal of anions by the modified bauxite tailings is improved with increasing shaking time. The adsorption reaches equilibrium in about 2 h on the modified tailings and the maximum adsorption percentages approximate to 100% for Cr(Ⅵ) and As(Ⅴ), and 80% for F(Ⅰ). Although the adsorption reaches equilibrium only in about 1 h on unmodified tailings, the adsorption percentage is low. The maximum adsorption percentages of unmodified tailings are about 30% for Cr(Ⅵ), 60% for As(Ⅴ) and F(Ⅰ).

Fig.1 Effect of tailings dosage on adsorption percentage of anionic ions (Solution: 100 mL; Ion concentration: 30 mg/L Cr(Ⅵ), 1 mg/L As(Ⅴ) and 40 mg/L F(Ⅰ); Shaking time: 2 h; Temperature: 303 K; pH: 5-6).
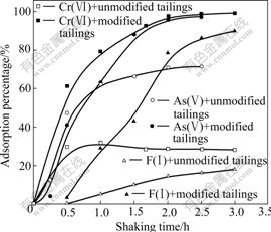
Fig.2 Adsorption percentage of anions as function of shaking time (Solution: 100 mL; Ion concentration: 30 mg/L Cr(Ⅵ), 1 mg/L As(Ⅴ) and 40 mg/L F(Ⅰ); Adsorbent dosage: 4 g Cr(Ⅵ), 1 g As(Ⅴ) and 4 g F(Ⅰ); Temperature: 303 K; pH: 5-6).
3.3 Effect of pH value
The influences of pH value on the adsorption of anions were also investigated. The results are shown in Fig.3.
It can be seen from Fig.3 that the adsorption percentages of Cr(Ⅵ), As(Ⅴ) and F(Ⅰ) on the modified tailings in solution decrease as the pH value increases. For Cr(Ⅵ), the adsorption percentage decreases from 99% to less than 80% when pH value increases from 2.1 to 9.7; for As(Ⅴ), the adsorption percentage increases
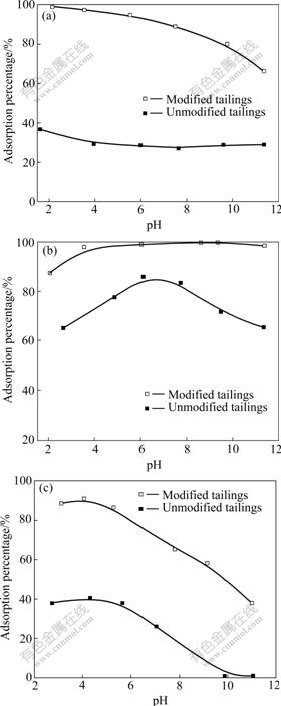
Fig.3 Effect of pH value on adsorption percentage of metal ions under conditions without adding tailings and with unmodified and modified tailings (Solution: 100 mL; Ion concentration: 30 mg/L Cr(Ⅵ), 1 mg/L As(Ⅴ) and 40 mg/L F(Ⅰ); Adsorbent dosage: 4 g Cr(Ⅵ), 1 g As(Ⅴ) and 4 g F(Ⅰ); shaking time: 2 h; temperature: 303 K): (a) Cr(Ⅵ); (b) As(Ⅴ); (c) F(Ⅰ)
from 90% to 99% when the pH value increases from 2.8 to 10.5; for F(Ⅰ), the adsorption percentage decreases from 90% to 38% when the pH value increases from 3.1 to 11.0. For the unmodified tailings, the adsorption perentages of Cr(Ⅵ) and F(Ⅰ) decrease slowly with increasing pH value. The As(Ⅴ) adsorption exhibits a better region around pH 6-8. The low adsorption percentage of Cr(Ⅵ), As(Ⅴ) and F(Ⅰ) at higher pH value may be due to the competitive adsorption of OH- on the available exchange sites of minerals. The oxides of aluminum, iron and silicon are present in varying amounts in tailings. The hydroxylated surfaces of oxides develop a charge on the surface in aqueous solution through amphoteric dissociation[9], as shown as follows:
![]() (2)
(2)
![]() (3)
(3)
where M stands for Al, Fe, Si, etc. These surfaces will be positively charged at lower pH value and will consequently be favourable for the adsorption of Cr(Ⅵ) and As(Ⅴ) in the anionic form of HCrO4- and HAsO42-.
3.4 Zeta potential measurement
The zeta potential—pH curves of the unmodified and the modified bauxite tailings in distilled water and in the presence of 30 mg/L Cr(Ⅵ) are presented in Fig.4. It can be seen that the zeta potentials in all suspensions increase with decreasing pH value. With the decrease of solution pH, the surface positive charges of the unmodified and the modified bauxite tailings increase and their surface negative charges decrease due to the protonation of surface hydroxyl groups on the unmodified and modified bauxite tailings. The changes in surface charge result in the changes of zeta potential in suspensions. Under acidic conditions, the unmodified bauxite tailings carry net negative surface charges with an isoelectric point(IEP) value of 3.6, but the modified bauxite tailings possess net positive surface charges when pH value is less than 5.0 (IEP equals 5.0). Bauxite tailings are the mixture of alumino-silicates. The broken bonds of the unmodified bauxite tailings surface are

Fig.4 Zeta potential vs pH value in different solutions (30 mg/L Cr(Ⅵ), solid concentration 0.05% (mass fraction), temperature 298 K)
bonds, causing absorption and electrostatic action with covalent Al—O bonds and Si—O bonds which are ≡Si—O-1, ≡Si—OH and ≡Al—O-1.25, ≡Si—O-1, ≡Si—OH and ≡Al—O-1.25 function cation like ferric ion[10].
The negative charges on the surface of both tailings increase in the presence of Cr(Ⅵ) in the pH range of 2-12. In the presence of Cr(Ⅵ), the IEP of the modified tailings changes from 5.08 to 3.26 and that of the unmodified tailings changes from 3.60 to 3.40, indicating the adsorption of Cr(Ⅵ) is in the form of HCrO4-.
3.5 FTIR spectrum
The infrared spectra of the samples were obtained by using a FTIR-750 model infrared (IR) spectrometer. As shown in Fig.5(a), the broad band observed at 3 620.19 cm-1 belongs to the O—H stretching vibration of the Si—OH groups and HO—H vibration of the water molecules adsorbed on the surface of the samples. The bands observed at 1 030.90, 751.63 and 696.64 cm-1 are caused by the Si—O—Si groups of the tetrahedral sheet and the deforming and bending modes of the Si—O bond. However, the modified tailings samples show a strong band of O—H—O at 3 431.40 cm-1, and this indicates that there is an adsorption of —OH on the surface of the tailings. Fig.5(b) shows that the modification of the bauxite tailings samples with FeCl3?6H2O tends to weaken the adsorption bands of Si—O and Al—O, respectively. This indicates that the modification of tailings may take place on the surface of the unmodified tailings and produce hydrated iron oxide[11].
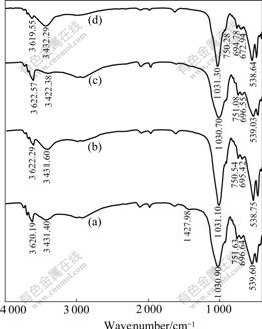
Fig.5 IR spectrum patterns of unmodified tailings (a), modified tailings (b), Cr(Ⅵ) on unmodified tailings (c) and on modified tailings (d)
Figs.5(c) and (d) present the results of adsorption of Cr(Ⅵ) on the modified and unmodified tailings, indicating that the bands of Fig.5(c) are generally similar to those of Fig.5(a). The strong band at 3 432.29 cm-1 and the weak bands of Si—O and Al—O can be found in Fig.5(d) in the presence of Cr(Ⅵ), and there is a new band at 672.94 cm-1 which is belongs to Cr2O72-. This suggests that Cr(Ⅵ) is adsorbed on the modified tailings surface in the form of chemistry adsorption. In Refs.[12-13], chromate can be adsorbed on the surface of hydrated iron oxide as follows:
![]() (4)
(4)
3.6 Adsorption isotherms
Langmuir and Freundlich isotherm models were applied to establishing the relationship between the adsorption density of Cr(VI) on tailings and its equilibrium concentrations in aqueous solution. The experimental data conform to the linear form of Langmuir model[14]:
![]() (5)
(5)
where Ce is equilibrium concentration of the ions (mg/L) and qe is the adsorption density of ions by per unit of tailings (mg/g); qm (mg/g) and KL (L/mg) are Langmuir constants which relate adsorption density and the energy of adsorption, respectively. qm and KL were evaluated from slope and intercept of the linear plot of Ce/qe vs Ce, respectively (see Fig.6(a)).
The adsorption equilibrium data were also applied to the Freundlich model in logarithmic form given as follows[15]:
![]() (6)
(6)
where KF (mg/g) and n are Freundlich constants that are related to adsorption density and adsorption intensity, respectively. KF and 1/n were determined from the intercept and the slope of linear plot of lg qe vs lg Ce, respectively (see Fig.6(b)).
The adsorption equations were obtained from experimental data by Eqns.(5) and (6). The isotherm constants and correlation coefficients were calculated from the linear Langmuir and Freundlich plots by plotting Ce/qe vs Ce and lg qe vs lg Ce (Fig.6). The results are represented in Table 1.
The adsorption patterns of the anions on tailings are well fitted with the Langmuir and Freundlich models because the values of R2 are equal to 0.975 5 and 0.992 9, respectively (Figs.6(a) and (b)). Freundlich model is more applicable than Langmuir model for the adsorption of Cr(Ⅵ) by the modified tailings. These results are in a good accordance with those results in Ref.[16].

Fig.6 Langmuir (a) and Freundlich (b) plots for adsorption of Cr(Ⅵ) on modified tailings
Table 1 Langmuir and Freundlich constants and correlation coefficients
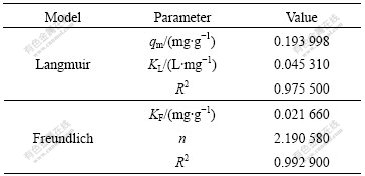
KF value of the Freundlich equation (Table 1) indicates that tailings have a very high adsorption capacity for Cr(Ⅵ) ions in aqueous solution. It has been reported that there is the beneficial adsorptions of metal ions when n is between 1 and 10[17]. Here, n≈2.19, it shows a good adsorption of Cr(Ⅵ).
3.7 Adsorption kinetics
The adsorption kinetics, demonstrating the solute uptake rate, is one of the most important characteristics, which represents the adsorption efficiency of the tailings. According to Fig.2, the adsorption rates of Cr(Ⅵ) increase dramatically in the first 2 h, and then reach equilibrium gradually at about 2 h. The adsorption rates of Cr(Ⅵ) on the modified tailings can be obtained by the pseudo-first-order rate equation of Lagergren (Eqn.(7)) and the pseudo-second-order rate equation (Eqn.(8)) based on the experimental data[18]:
![]() (7)
(7)
![]() (8)
(8)
where k1 is the Lagergren adsorption rate constant (h-1) and k2 is the pseudo-second-order adsorption rate constant (g/(mg?min)); qe and qt are the amounts of anions absorbed (mg/g) at equilibrium and time t, respectively. The plots of lg[qe/(qe-qt)] vs t and t/qt vs t are shown in Fig.7. It can be seen from Fig.7 that the pseudo-second-order kinetic model provides a good correlation for the adsorption of Cr(Ⅵ) on the modified

Fig.7 Pseudo-first-order (a) and pseudo-second-order (b) kinetic equation curves for adsorption of different concentrations of Cr(Ⅵ) by modified tailings at (303±1) K
tailings in contrast to the pseudo-first-order model. This result is in agreement with that in Ref.[19]. In addition, the correlation coefficient of the pseudo- second-order kinetic model is higher than that of the pseudo-first-order kinetic model (see Table 2).
Table 2 Kinetic parameters of Cr(Ⅵ) adsorbed on modified tailings

4 Conclusions
1) The pretreatment of bauxite tailings with FeCl3·6H2O (modified tailings) raises its isoelectric point (IEP) from 3.6 to 5.0. The maximum removal rates of the modified tailings for Cr(Ⅵ), As(Ⅴ) and F(Ⅰ) are 99%, 99% and 90%, respectively.
2) The IR spectra reveal that the presence of hydrated iron oxide on the modified tailings is perhaps responsible for the adsorption of Cr(Ⅵ) and produces a new band of Cr2O72- at 672.94 cm-1.
3) Freundlich model fits better than Langmuir model to the adsorption of Cr(Ⅵ) by modified tailings. The adsorption of Cr(Ⅵ) on the modified tailings is well expressed by the pseudo-second-order kinetic model.
References
[1] MYROSLAV S, BOGUSLAW B, ARTUR P T. Study of the selection mechanism of heavy metal (Pb2+, Cu2+, Ni2+, and Cd2+) adsorption on clinoptilolite [J]. Journal of Colloid and Interface Science, 2006, 304(1): 21-28.
[2] MOORE J W, RAMAMORTHY S. Heavy metals in natural waters [M]. New York: Springer Verlag, 1994.
[3] BOWMAN R S, HAGGERTY G M, HUDLESTON R G, NEEL D, FLYNN M M. Sorption of nonpolar organic compounds, inorganic cations and inorganic oxyanions by surfactant-modified zeolite [C]// Washington: America Chemical Society, 1995: 54-64.
[4] PRADHAN J, DAS S N, THAKUR R S. Adsorption of hexavalent chromium from aqueous solution by using activated red mud [J]. Journal of Colloid and Interface Science, 1999, 217(1): 137-139.
[5] OSVALDO K J, LEANDRO V, ALVES G. Adsorption of heavy metal ion from aqueous single metal solution by chemically modified sugarcane bagasse [J]. Bioresource Technology, 2007, 98(6): 1291- 1297.
[6] ZHAO Xiao-rong, DU Dong-yun, LU Xiao-hua. Research on the adsorptive properties of rectorite to methylene blue [J]. Ion Exchange and Adsorption, 2003, 19(4): 337-342. (in Chinese)
[7] LIU Wei-ping, YUAN Jian-xiong. The application of tailings in the silicate material [J]. China Mining, 2004, 13(11): 16-18. (in Chinese)
[8] WANG Jian-li, WANG Huai-de, HUANG Jian. Study of making absorption water compound materials with the gangue from bauxite benefication [J]. Light Metal, 2004(3): 7-9. (in Chinese)
[9] WANG Dian-zuo, HU Yue-hua. Solution chemistry of flotation [M]. Changsha: Hunan Science and Technology Press, 1998: 35-38. (in Chinese)
[10] JIA Mu-xin. Study on surface properties of silicate minerals and their adsorption characteristics of metal ions [D]. Shenyang: Northeastern University, 2001. (in Chinese)
[11] HESHAM A S, PHILIP R W. An XPS study of the adsorption of chromate on goethite [J]. Applied Surface Science, 1997, 108(3): 371-377.
[12] SUN Zhen-ya, ZHU Chun-shui, CHEN He-sheng, GONG Wei-qi. A comparative study of the adsorption of chromium of five different types of FeOOH [J]. Acta Petrologica et Mineralogica, 2003, 22(4): 352-354. (in Chinese)
[13] WEERASOORIYA R, TOBSCHALL H J. Mechanistic modeling of chromate adsorption onto goethite [J]. Colloids and Surface A: Physicochemical and Engineering Aspects, 2000, 162(1/3): 167-175.
[14] MARKKU L, MATTI L. Determination of adsorption isotherms with quartz crystal microbalance in liquid phase [J]. Journal of Colloid and Interface Science, 1988, 125(2): 610-614.
[15] AHMET S, MUSTAFA T, MUSTAFA S. Adsorption of Pb(II) and Cr(III) from aqueous solution on Celtek clay [J]. Journal of Hazardous Materials, 2007, 144(1/2): 41-46.
[16] SAAD A K, RIAZ R, KHAN M A. Adsorption of chromium (III), chromium (VI) and silver (I) on bentonite [J]. Water Management, 1995, 15(4): 271-282.
[17] TAHIR S S, NASEEM R. Removal of Cr(III) from tannery wastewater by adsorption onto bentonite clay [J]. Separation Purification Technology, 2007, 53(3): 312-321.
[18] HUANG Y H, HSUEH C L, HUANG C P. Adsorption thermodynamic and kinetic studies of Pb(II) removal from water onto a versatile Al2O3-supported iron oxide [J]. Separation Purification Technology, 2006, 55(1): 23-29.
[19] BRIGATTI M F, LUGLI C, POPPI L. Kinetics of heavy metal removal and recovery in sepiolite [J]. Applied Clay Science, 2000, 16(1/2): 45-57.
Foundation item: Project(2005CB623701) supported by the Major State Basic Research Development Program of China
Received date: 2008-01-12; Accepted date: 2008-03-06
Corresponding author: WANG Yu-hua, Professor, PhD; Tel: +86-731-8830545; E-mail: wangyh@mail.csu.edu.cn

