
Microstructure transformation of deformed AZ91D during isothermal holding
ZHANG You-fa(张友法)1, LIU Yong-bing(刘勇兵)2, CAO Zhan-yi(曹占义)2, ZHANG Liang(张 亮)2
1. Jiangsu Key Laboratory of Advanced Metallic Materials, School of Materials Science and Engineering,
Southeast University, Nanjing 211189, China;
2. Key Laboratory of Automobile Materials of Ministry of Education, School of Materials Science and Engineering,
Jilin University, Changchun 130022, China
Received 15 October 2008; accepted 24 December 2008
Abstract: In order to understand the thermodynamic properties of deformed AZ91D alloy during isothermal holding, the microstructure characteristics and transformation were investigated. The results present that deformation mainly concentrates on the edge of the chips and billets, especially at the interface of α/β. Microstructure transformation mechanism of deformed AZ91D during holding mainly includes recrystallization, spheroidization and Ostwald ripening. The mechanism was then thermodynamically analyzed. During the heating and isothermal holding process, recrystallization driven by residual energy within the deformed AZ91D alloy, spheroidization and Ostwald ripening induced by the reduction of interfacial energy, will inevitably and continuously occur with the extension of heating and holding.
Key words: AZ91D magnesium alloy; deformation; isothermal holding; microstructure transformation
1 Introduction
During the last three decades, the research on magnesium alloys, especially AZ91D, and their processing technologies became one of the hottest topics in the field of metallic materials and got a great of achievement[1]. As the latest-developed metal forming technology, semisolid forming or semisolid processing (SSF/SSP) in manufacturing the products of magnesium alloys has been getting more and more attention[2-3]. The research of SSF concentrates on the preparation of semisolid slurry or semisolid billet that should contain fine globular solid phase to ensure the ultimate mechanical properties[3-4]. A number of preparing technologies have been invented to produce fine semisolid microstructure[3]. According to the difference of preparing process, the technologies mainly fall into two categories. The first category is classified for the intense convection forced into the slurry, represented by thixomolding, rheocasting, mechanical or electro- magnetism stirring[5-7]. The second group covers the processes in which the alloys with a certain deformation are heated from a fully solid state to semisolid state without shearing, represented by strain induced melt activation(SIMA), and semisolid extrusion[8-9]. A lot of work on the processing conditions of SSF has also been made due to the predominant effect of processing conditions on microstructure development[5, 10]. However, the efforts did not take deep investigation on the whole stage of microstructure transformation from initial state to final semisolid state. Taking thixomolding as an example, the recrystallization mechanism of non-dendritic solid phase was supposed, but the microstructure characteristics of fragmented AZ91D chips and the thermodynamic analysis of subsequent microstructure change did not get much attention[11-12]. Therefore, this work aims to comprehensively understand microstructure transformation and thermodynamic property of deformed alloy during isothermal holding.
2 Experimental
AZ91D ingot was mechanically machined into chips or compressed into columnar billets. In this work, the compressed AZ91D billets and the commercial AZ91D chips for thixomolding were chosen to study the deformation behavior and microstructure development during isothermal holding process[8, 12]. The compression ratio of compressed AZ91D was defined by the relative reduction of the sample height. The eutectic and liquidus temperatures of AZ91D were 439 ℃ and 595 ℃, respectively, by the curve of differential scanning calorimetry(DSC)[5]. Under protective atmosphere of flowing mixed SF6 and CO2, isothermal holding was carried out in furnace with the heating rate of 5 ℃/min. When the sample was isothermally held for a certain time, it was taken out immediately for air quenching. Temperature changes in the sample were measured using a thermocouple mounted near the center of the sample. After being rough ground, fine ground and polished, and then chemically etched in 4% solution of nitric acid in ethanol, the cross section of the samples was observed by optical microscope of OLYMPUS-PMG3. The morphological characteristics of the machined surface of AZ91D chips were conducted by JSM-5310 SEM. Self-programmed software that can preferably analyze the size, fraction, roundness, distribution of the solid particles and their inter-connecting degree, was used for quantitative metallographic analysis of microstructure images[13].
3 Results
3.1 Morphology and microstructure of AZ91D chips
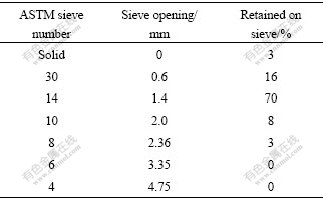
Fig.1(a) shows the grey eutectic and white α-Mg dendrite composed of the microstructure of as-cast AZ91D ingot. After being fragmented from the ingot, the chips display prism shape (Fig.1(b)) and its size is mainly about 1 mm×1 mm×5 mm as shown in Table 1.
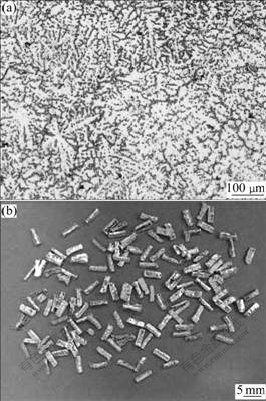
Fig.1 Microstructure of as-cast AZ91D ingot (a) and morphology of chipped feedstock (b)
Table 1 Distribution of chip sizes established by sieving method, ASTM E276-68
The machining surface of the chips displays obvious traces of chipping (Fig.2), and the interior microstructure of the chips is given in Fig.3. Deformed dendrite with bright color and band morphology presents the same deformation direction as machining (as the arrow in Fig.3(a)). In Fig.3(b), the fragment of β-Mg17Al12 for its low ductility is marked by arrow and the deformation of α/β interface is also clear for their different mechanical properties. Depending on the chip size, the distribution and morphology of β-Mg17Al12, deformation will be heavier and heavier when moving towards the surface of the chips, as shown in Fig.3(b). Although it is difficult to quantitatively measure the extent of deformation directly, it is obvious that the chips are thermodynamically instable because of the residual energy brought by fragmentation. The energy is going to activate the microstructure evolution with the increasing of temperature.
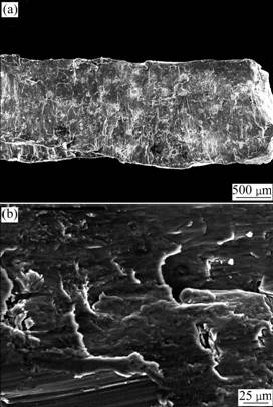
Fig.2 SEM micrographs of chipped surface: (a) Fragmented surface of chip; (b) Detailed deformation features on fragmented surface
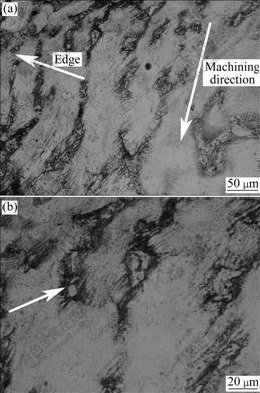
Fig.3 Interior microstructures of chip: (a) Deformation direction; (b) Deformation features
3.2 Microstructure evolution of AZ91D chips under isothermal holding at solid state
3.2.1 Effect of isothermal holding temperature
It should be pointed out that the solid state here means the temperature range before the melting of solid phase. Isothermal holding of the chips at solid state for this study was therefore carried out below the eutectic temperature of 439 ℃. Taking holding temperatures of 175 ℃ and 437 ℃, the chips were isothermally held for 5 min. The microstructure evolution is provided in Fig.4. It is indicated that the deformed characteristics including the deformed dendrite strip and fragment of β-Mg17Al12 still exist in the alloy after being treated at 175 ℃ for 5 min (Fig.4(a)). Compared with Fig.4(a), Fig.4(b) has a significant development in the disappearance of deformation characteristics and the occurrence of eutectic transformation distributed in the interface of α-Mg, especially in the triple position. Furthermore, at the same time, driven by the residual energy, the equiaxed grain nucleates and grows up to approximate 50 μm within the originally deformed dendrite by recrystallization, clearly displayed in Fig.4(c). However, the recrystallization is asymmetrical and incomplete in α-Mg matrix due to the difference of deformation extent within the chips (Fig.3) and short holding time.
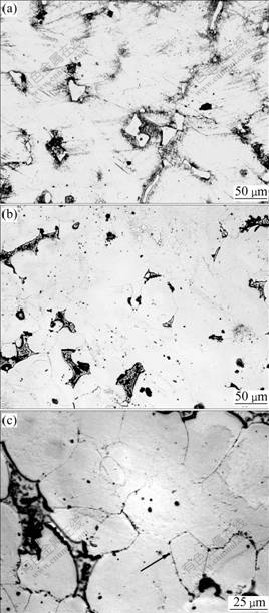
Fig.4 Microstructure evolution of chipped AZ91D held for 5 min at different temperatures: (a) 175 ℃; (b) 437 ℃; (c) Equiaxed grain at 437 ℃
3.2.2 Effect of isothermal holding time
During the experiments, the chips were isothermally held at 175 ℃ or 437 ℃ for 30 min or 60 min to investigate the influence of holding time on the microstructure (Fig.5).
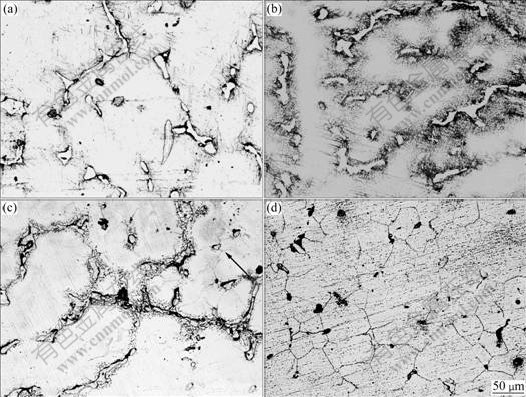
Fig.5 Microstructure evolution of chipped AZ91D held at solid state for different times: (a) 175 ℃, 30 min; (b) 175 ℃, 60 min; (c) 437 ℃, 30 min; (d) 437 ℃, 60 min
Similar to Fig.4(a), the microstructure does not have much change with the time at 175 ℃ (Figs.5(a) and (b)), while recrystallization results in the appearance of equiaxed grain in size of about 50 μm at 437 ℃, and the number of grain increases with the extension of holding time. Eutectic melting begins and expands in the interface of α-Mg under the effect of Al diffusion (Fig.5(c)), and Ostwald ripening and solution subsequently happen to make the alloy into the solution of α-Mg (Fig.5(d)).
3.3 Microstructure evolution of AZ91D chips under isothermal holding at semisolid state
3.3.1 Effect of isothermal holding temperature
According the DSC curve of AZ91D chips, the AZ91D chips were held at the temperatures of 560, 570, 580 and 590 ℃ respectively at semisolid state. After being held for 5 min, the chips have apparent transformation in microstructure (Fig.6).
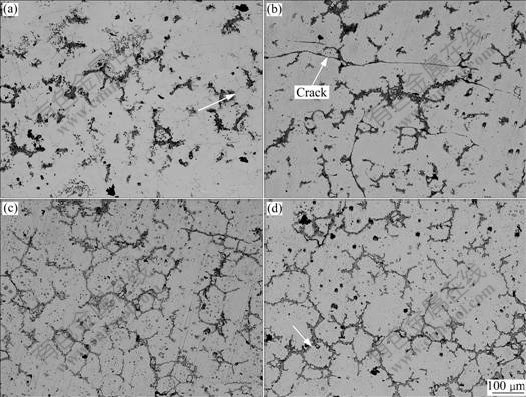
Fig.6 Microstructure development of AZ91D chips held at semisolid state for 5 min: (a) 560 ℃; (b) 570 ℃; (c) 580 ℃; (d) 590 ℃
In the general view of Fig.6, recrystallization occurs in the beginning of holding, leading to the formation of equiaxed grain (marked by arrow in Fig.6(a)). Similar to the results of Fig.4, only partial microstructure shows the developing characteristics and the recrystallization in Fig.6(a) is incomplete. With the increase of holding temperature, eutectic and edge of α-Mg start to melt, accelerated by the diffusion of Al (Fig.6(b)). The equiaxed grain boundary is continuously soaked by the increased liquid phase till liquid convergence detaches solid phase, as revealed in Figs.6(c) and (d). Subsequently, due to the effect of curvature over-heat, the solid phase becomes rounder and rounder, named spheroidization. Meanwhile, some liquid islands induced by chemical segregation of Al appear within the solid phase. Through a series of transformation depicted above, spherical solid particles are obtained in the alloy, marked by arrow in Fig.6(d).
3.3.2 Effect of isothermal holding time
AZ91D chips were isothermally held at 570 ℃ for 15, 30 and 60 min and the microstructure evolution is shown in Fig.7.
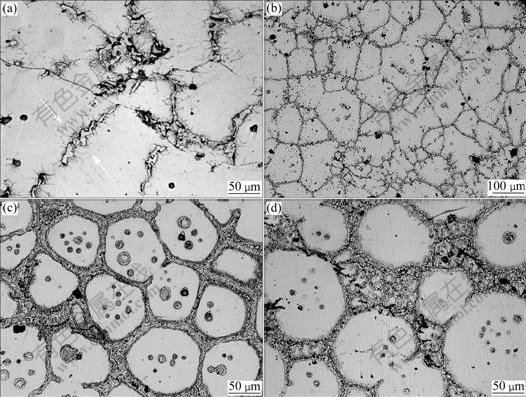
Fig.7 Microstructure development of AZ91D chips held at 570 ℃ for different times: (a) 0 min; (b) 15 min; (c) 30 min; (d) 60 min
The nucleating and growing up of equiaxed grain in deformed alloy at first, mainly concentrate on the edge of dendrite, especially near the fragment of β-Mg17Al12 (marked by arrows in Fig.7(a)) as residual energy induced by heavy deformation, is rich in these positions. With the extension of holding time, the main developing process, similar to the results of Fig.6, includes eutectic melting, solute diffusion, melting at the edge of α-Mg, spheroidization, liquid immergence and convergence, solid detachness and precipitation of liquid island (Fig.s7(b) and (c)). However, different from Fig. 6, phase transformation can be sufficiently achieved in Fig.7, so the equilibrium of the alloy can be reached for enough holding time (Fig.7(c)). Another difference is the occurrence of Ostwald ripening which leads to the disappearance of small solid particle and the growth of big solid particle (Fig.7(d)). In the end, the typical microstructure is attained, including liquid phase, liquid islands and fine round solid particle.
Therefore, the microstructure development of AZ91D chips during isothermal holding at semisolid state is concluded as follows: deformed dendrite→recrystallization→eutectic melting, solute diffusion→melting at the edge of α-Mg, spheroidization→liquid phase immergence and convergence, precipitation of liquid island→solid detachness→spherical solid particle.
3.4 Microstructure transformation of compressed AZ91D during isothermal holding
The compression of AZ91D billets with different ratios had been previously carried out to investigate microstructure modification in our group[8]. The deformation characteristics in the compressed billets with ratio of 27.3% or 36.8%, characterized by band morphology of α-Mg and fragment of β (Fig.8), are similar to the deformation features in Fig.3. It is therefore believed that the extent of deformation in AZ91D chips is much more than its critical deformation ratio of 2%-3%[14].
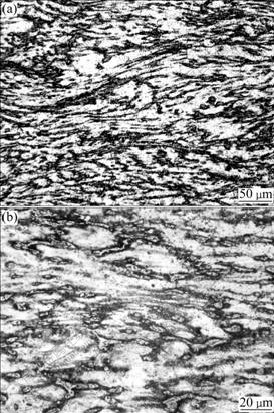
Fig.8 Microstructure in central position of AZ91D billet under compression ratio of 36.8%: (a) Microstructure in deformed area; (b) Detailed deformation features
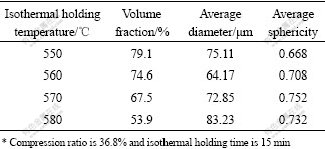
The microstructure evolution of compressed AZ91D during isothermal holding at semisolid state had also been investigated[8]. For the sake of concentrating on the effect of holding temperature and time on the microstructure, microstructure development was just investigated in the central position of compressed AZ91D to reduce or even eliminate the influence of asymmetrical deformation. The characteristic parameters of solid particles after being held at different time and temperatures were quantitatively analyzed and listed in Table 2 and Table 3.
From Table 2, the volume fraction of solid particles decreases to a stable value with the extension of holding time, but the particle has a minimum average size after being held for 15 min and then gradually grows up accompanied by a globular morphology. Based on the data in Table 3, the content of solid particles decreases all the time when increasing holding temperature, while the changes of size and morphology are similar to the corresponding values in Table 2. From Table 2 and Table 3, it is concluded that solid particles can obtain the best state characterized by small size and spherical morphology after being held at 560 ℃ for 15 min.
Table 2 Quantitative analysis of solid particles within compressed AZ91D after being held for different time
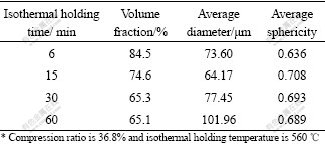
Table 3 Quantitative analysis of solid particles within compressed AZ91D after being held at different temperatures
4 Discussion
In order to provide an indirect insight into the formation of thixotropic structure that cannot be observed directly during the SSF/SSP processes with intensive convection, this presentation takes the study in microstructure development of AZ91D chips and compressed AZ91D billets during isothermal holding.
It is well-known that two conditions in thermodynamics and dynamics determine the occurrence of recrystallization. Resulted from chipping or compression, both the chips and billets have residual energy, which makes the deformed AZ91D alloy thermodynamically unstable. And increasing temperature during the holding stage of deformed alloy accelerates atomic diffusion. Therefore, the deformed α-Mg matrix in the chips can inevitably recrystallize if held above recrystallization temperature, since the deformation in some positions of the chips was heavy enough (Section 3.4 of this study). According to some data, AZ91D alloy has already recrystallized at a temperature as low as 175 ℃. There are also results showing the stability of severely deformed AZ91D with submicron or nanosize grains up to 425 ℃[12].
Driven by the residual energy, recrystallization is described by the following equation[15]:
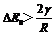 (1)
(1)
where ΔEs is the residual energy per volume; γ is the interface energy per area and R is the radius of recrystallized grain. This equation also shows the critical size of equiaxed grain is 2γ/ΔEs. Some recrystallized grain boundary is marked by arrows in Fig.4(c), Fig.5(c), Fig.6(a) and Fig.7(a). It is clear that the diameter of equiaxed grain in those experiments is about 50 μm and the value of γ for pure magnesium is 0.115 J/m2[14]. Then in terms of the above equation, ΔEs must be higher than 9.2×103 J/m3 so that recrystallization can happen when the alloy is heated up to recrystallization temperature.
After the formation of equiaxed grain within deformed α-Mg matrix, eutectic melting and solute diffusion, affected by solution coefficient of Al in Mg, start to happen[14]:
DAl(Mg)/(m2?s-1)=1.2×10-3exp[-143 000/(RT)] (2)
where R is the gas constant. DAl(Mg) increases with temperature increasing, such as 1.29×10-12 m2/s at 560℃ and 2.09×10-12 m2/s at 580 ℃. Due to solute diffusion, some positions of α-Mg matrix may be rich in Al. Then the melting point is reduced accordingly. The alloy at this position begins to melt and stays inside the solid particle as liquid islands with the improvement of temperature. The island size is influenced by solute diffusion that is controlled by holding temperature and time.
Meanwhile, spheroidization and Ostwald ripening, also controlled by solute diffusion, are accelerated with temperature increasing, which makes solid particles develop from equiaxed grain to big size and round shape, as shown in Table 2 and Table 3. Theoretically, assuming residency time of AZ91D within the holding furnace of 5 min, the root mean square displacement equals 20 μm at 560 ℃ and 25 μm at 580 ℃. Thus, the processing conditions in this study allow for enough diffusion and equal distribution of Al in α-Mg during the holding of the chips or the billets since the equiaxed grains are about 50 μm. Apparently, spheriodization and Ostwald ripening will inevitably happen in the present experiment.
It is supposed that there are two curvatures in a particle, R the radius of bulgy position and R0 the main radius (R<R0). Then the variation of Gibbs free energy induced by different curvature at the constant temperature is[15]
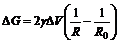 (3)
(3)
Thus, ΔG>0 for R<R0, which means the position with small curvature radius has high free energy. Accordingly, the melting point is reduced at the position and the reduction of ΔT can be calculated by the following equation[15]:
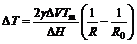 (4)
(4)
where Tm is the melting point of the solid particle when the interface is plane. It is seen that ΔT increases with decreasing the curvature radius.
Since there are just solid and liquid phases existing within AZ91D slurry in semisolid state, the equilibrium concentration of solid phase can be given by Thomson-Freudlich equation[15]:
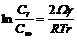 (5)
(5)
where Ω is Moore volume; C∞ is a constant and Cr is the concentration of solid phase with the radius of r. It can be deduced that small solid particles have high concentration while the concentration of big solid particles is low, called as Ostwald ripening. Therefore, the average particle size will increase with increasing both the time and temperature of isothermal holding, as indicated in Table 2 and Table 3, and there is the following equation [15]:
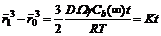 (6)
(6)
where K is the ripening rate influenced by temperature. Thus, it seems that large particles grow up at the expense of small ones by element diffusion.
5 Conclusions
1) Asymmetrical deformation is clear in mechanically chipped or compressed AZ91D alloy. Heavy deformation is concentrated at the edge of chips and billets, especially at the interface of α/β. Residual deformation energy makes the alloy thermodynamically unstable and leads the microstructure to transform in the beginning of isothermal holding.
2) According to microstructure development of AZ91D chips during isothermal holding at solid and semisolid state, the microstructure transformation during the holding is as follows: deformed alloy→recrystallization→eutectic melting and solute diffusion→melting of α-Mg interface, spheroidization, precipitation of liquid pool→liquid immergence and convergence→solid detachness→Ostwald ripening→ unmelted solid particles.
3) By comparing with the results of compressed AZ91D, the deformation of AZ91D chips is heavy enough to result in recrystallization since heating temperature in the furnace is higher than recrystallization temperature. Recrystallization inevitably occurs during isothermal holding, driven by the residual energy stored in the chips.
4) Microstructure transformation of the deformed alloys proves that spheroidization and Ostwald ripening, driven by reduction of interfacial energy, also inevitably happen with the development of isothermal holding.
References
[1] MORDIKE M L, EBERT T. Magnesium properties—applications—potential [J]. Mater Sci Eng A, 2001, 302: 37-45.
[2] SPENCER D B, MEHRABIAN R, FLEMINGS M C. Rheological behavior of Sn-15pct Pb in the crystallization range [J]. Metall Trans, 1972, 3(7): 1925-1932.
[3] FAN Z. Semisolid metal processing [J]. Int Mater Rev, 2002, 47(2): 49-85.
[4] ZHANG Y F, LIU Y B, CAO Z Y, ZHANG Q Q, ZHANG L. Mechanical properties of thixomolded AZ91D magnesium alloy [J]. J Mater Process Tech, 2009, 29: 1375-1384.
[5] ZHANG Y F, LIU Y B, ZHANG Q Q, CAO Z Y, CUI X P, WANG Y. Microstructural evolution of thixomolded AZ91D magnesium alloy with process parameters variation [J]. Mater Sci Eng A, 2007, 444: 251-256.
[6] FAN Z, LIU G, WANG Y. Microstructure and mechanical properties of rheo-diecast AZ91D magnesium alloy [J]. J Mater Sci, 2006, 41(12): 3631-3644.
[7] ZHANG X L, LI T J, TENG H T, XIE S S, JIN J Z. Semisolid processing AZ91 magnesium alloy by electromagnetic stirring after near-liquidus isothermal heat treatment [J]. Mater Sci Eng A, 2008, 475(1/2): 194-201.
[8] ZHANG L, LIU Y B, CAO Z Y, ZHANG Y F, ZHANG Q Q. Effects of isothermal process parameters on the microstructure of semisolid AZ91D alloy produced by SIMA [J]. J Mater Process Tech, 2009, 209(2): 792-797.
[9] CZERWINSKI F. Semisolid extrusion molding of Mg-9%Al-1%Zn alloys [J]. J Mater Sci, 2004, 39(2): 463-468.
[10] ZHANG Y F, LIU Y B, CUI X P, CAO Z Y. Filling analysis for thixomolded process of AZ91D magnesium alloy [J]. Transactions of Nonferrous Metals Society of China, 2006, 16(s): s117-s121.
[11] CZERWINSKI F. On the generation of thixotropic structures during melting of Mg-9%Al-1%Zn alloy [J]. Acta Mater, 2002, 50: 3265-3281.
[12] CZERWINSKI F. The microstructural development of Mg-9 Pct Al- 1 Pct Zn alloy during shot molding [J]. Metall Mater Trans A, 2002, 33: 2963-2972.
[13] ZHANG Y F, LI S L, CAO Z Y, ZHANG Q Q, WANG Y. Quantitative analysis of the microstructure of semi-solid magnesium alloy [J]. Special Casting & Nonferrous Alloys, 2007, 27(5): 369-372. (in Chinese)
[14] CZERWINSKI F. Magnesium injection molding [M]. New York: Springer, 2008: 19-20.
[15] XIAO J M, ZHU F W. Materials energetics [M]. Shanghai: Shanghai Science and Technology Press, 1999: 410-417. (in Chinese)
Foundation item: Projects(2006BA104B04-1, 2006BAE04B07-3) supported by the National Science and Technology Supporting Program of China
Corresponding author: ZHANG You-fa; Tel: +86-25-52090691; E-mail: yfzhang@seu.edu.cn
DOI: 10.1016/S1003-6326(09)60090-X
(Edited by YUAN Sai-qian)