J. Cent. South Univ. Technol. (2011) 18: 68-72
DOI: 10.1007/s11771-011-0660-3
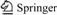
Improving extraction yield of humic substances from lignite with anthraquinone in alkaline solution
JAING Tao(姜涛), HAN Gui-hong(韩桂洪), ZHANG Yuan-bo(张元波), HUANG Yan-fang(黄艳芳),
LI Guang-hui(李光辉), GUO Yu-feng(郭宇峰), YANG Yong-bin(杨永斌)
School of Minerals Processing and Bioengineering, Central South University, Changsha 410083, China
? Central South University Press and Springer-Verlag Berlin Heidelberg 2011
Abstract: Based on the characteristics of the lignite sample, effects of assistant anthraquinone (AQ) on extraction yield of humic substances (HS) and the action mechanisms of AQ in alkaline condition were studied by Fouvier transform infrared (FT-IR) spectroscopy. The results indicate that assistant AQ can not only increase the extraction yield of HS but also reduce the alkali dosage (NaOH) as well as the extraction temperature and extraction time. Under the optimal conditions of alkali dosage of 9%, AQ dosage of 0.75%, extraction temperature of 80 ?C, extraction time of 30 min, stirring speed of 600 r/min and solid-to-liquid ratio of 1:3, the extraction yield of HS reaches 80.08%, which is increased by more than 20% compared with the conventional extraction. FT-IR spectra show that AQ is able to prevent dissolved HS from being destroyed into undissolved substance by alkali and HS obtained in the presence of AQ possesses more groups of —COOR and —COOH than that obtained without AQ.
Key words: humic substances; extraction; lignite; FTIR; anthraquinone
1 Introduction
Binders are essential in the production of iron ore pellets. One promising type of binder is based on humic substances (HS), which has been shown in laboratory studies to be suitable for producing iron ore pellets [1]. Lignite is used as a main source of the humic substances, and great reserves are found in China [1-2]. The humic substances are prepared by the extraction procedure of the original lignite sample. Therefore, humic substances would be much cheaper alternatives to the current organic binders.
In recent years, researchers have focused on the HS extraction from lignite by the primary three methods: alkaline extraction, acid extraction and microorganism fermentation. Compared with the alkaline extraction method, HS extracted by acid extraction method contains more impurities, and microorganism fermentation method has longer reaction period and lower extraction yield [3-5]. Therefore, the two methods are only limited to the laboratory stage.
At present, alkaline extraction for HS from lignite is widely used. But, during the extraction, there exists some problems to be resolved, including the low extraction yield, high extraction agent dosage and so on. Some researchers have used catalysts (such as nickel vitriol) or pretreating measures (including air oxidation, nitric acid oxidation and ultrasonic pretreatment) to improve the extraction process. However, the total extraction yield of HS is still very low (less than 60% usually) [6]. So far, there has been few reports on the extraction methods of HS from lignite with anthraquinone (AQ) as assistant in alkaline solution for improving the extraction yield.
In this work, the characteristics of the lignite sample are firstly studied by chemical analysis method and FT-IR spectroscopy. And the assistant AQ is used to improve the extraction process of HS from lignite. More attention is paid to the effect of AQ and its dosage, alkali dosage and other extraction parameters on yield of HS during the extraction procedure. The action mechanisms of AQ are analyzed by FT-IR spectra at the same time.
2 Experimental
2.1 Raw materials
2.1.1 Lignite
The lignite sample was taken from Liling in Hunan Province, China. At the beginning, the sample was homogenized and dried at room temperature for three days, then crushed to powers with the granularity below 1 mm.
As well-known, lignite contains organic and inorganic constituents simultaneously. The proximate analysis of lignite indicates that the sample consists of 39.43% fixed carbon, 34.79% volatile matter and 25.78% ash.
The FT-IR spectrum of the lignite is shown in Fig.1.
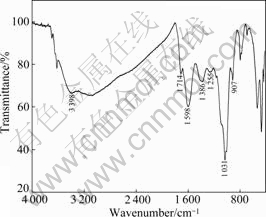
Fig.1 FT-IR spectrum of lignite sample
From Fig.1, by considering the high ash content in lignite, the absorption of some hetero-oxy compounds such as Si—O—Si (1 055-1 020 cm-1) or Si—O—C (1 110-1 080 cm-1) is also likely to occur within this range [7]. The absorption at 3 398 cm-1 is attributed to stretching of —OH groups. Within the wide frequency range from about 1 550 to 1 790 cm-1, peaks are principally assigned to protonate carboxylic (—COOH), carboxylate anion (—COO—) and ester carbonyl (—COOR) groups [8-9]. It is noteworthy that there are protonated groups (—COOH) and ester carbonyl groups (—COOR) in the lignite [8-11].
2.1.2 Extractant and assistant AQ
In this experiment, chemically pure sodium hydroxide (NaOH) was used as the extractant according to the former researches [1-2].
AQ stands for anthraquinone which is characterized by colorless or yellowish needle crystal. It is soluble in alcohol, ether and alkali solution. The FT-IR spectrum and structural formula of assistant AQ is shown in Fig.2, indicating that AQ consists of benzene rings and two carbonyl groups. Literatures have shown that AQ can maintain the stability of carbohydrate, and enhance the extraction yield and reaction rate in the chemical pulp production [12-13].
2.2 Methods
2.2.1 FT-IR study
The infrared spectra of lignite, assistant AQ and humic substances extracted from lignite were recorded
Fig.2 FT-IR spectrum of AQ
using a Nicolet Model Nexus670 series Fourier transform infrared spectrometer from Digilab operating in the range of 4 000–400 cm-1. The KBr pellet technique is adopted for recording the spectra. About 1 mg of the desired powder sample was thoroughly mixed with 100 mg of spectroscopic grade KBr and pressed into pellets for recording the spectra.
2.2.2 Extraction yield of humic substances
Extraction yield of humic substances is calculated by the following equation [5-6]:
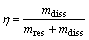 (1)
(1)
where h is the extraction yield, mdiss is the mass of the dissolved humic substances extracted from the 100 g lignite sample, and mres is the mass of the indiscerptible residues after extracting.
2.2.3 Experimental flow sheet and procedures
The experimental flow sheet is shown in Fig.3.
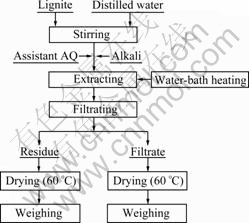
Fig.3 Experimental flow sheet of extraction of HS
As shown in Fig.3, the main testing procedures included extracting, filtrating, drying, and determining of extraction yield of HA. Firstly, 100 g of the lignite sample was mixed with distilled water designed by solid- to-liquid (S/L) ratio in an enamel cup, which was heated by the electronic constant-temperature water-bath heater. Secondly, certain percentages of alkali and assistant AQ were placed into the enamel cup. Under the conditions of designed extraction temperature, time, and stirring speed, HS was extracted from the lignite and transferred into the solution. After extracting, the residue and filtrate were separated by a vacuum suction filter. The filtrates and residue were dried at 60 °C to a constant mass. Finally, extraction yield of HS was calculated according to Eq.(1).
3 Results and discussion
3.1 Effects of solid-to-liquid (S/L) ratio and stirring speed
The former literature [14] has shown that S/L ratio and stirring speed have obvious effects on the extraction process of HS. And the experimental results are listed in Table 1. The other experimental conditions are as follows: alkali dosage of 12%, AQ dosage of 0.25%, extraction temperature of 100 ?C, and extraction time of 60 min.
Table 1 Effects of S/L ratio and stirring speed on extraction yield of HS
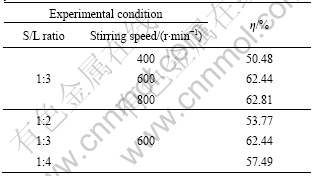
As shown in Table 1, the extraction yield of HS is obviously increased when the stirring speed ranges from 400 r/min to 600 r/min. But the extraction yields of HS is increased slightly when the stirring speed is increased from 600 r/min to 800 r/min. Considering that high stirring speed leads to energy consumption, stirring speed of 600 r/min is fixed in the later tests. It is also seen from Table 1 that, with the change of S/L ratio from 1:2 to 1:4, the variation trend of extraction yield of HS is sharply increased then slowed down. With the decrease of S/L ratio, the concentration of alkali solution is obviously reduced under the same alkali dosage. Generally speaking, the extraction yield of HS will reach the optimum value until the alkali concentration is up to a significant quantity.
From the results, the optimal S/L ratio and stirring speed are chosen as 1:3 and 600 r/min, respectively.
3.2 Effects of AQ dosage
Fig.4 illustrates the effect of AQ dosage on HS extraction yield. The experimental conditions are fixed as follows: alkali dosage of 12%, extraction temperature of 100 ?C, time of 60 min, S/L ratio of 1:3 and stirring speed of 600 r/min.
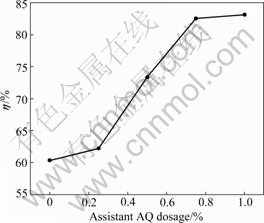
Fig.4 Effect of AQ dosage on extraction yield of HS
As shown from Fig.4, assistant AQ significantly improves the extraction yield of HS. With the variation of AQ dosage from 0 to 1.0%, the extraction yield presents a rising trend. As far as the extraction yield is concerned, it almost reaches the utmost value of 82.75% if AQ dosage is up to 0.75%.
The structural differences between the HS-A, HS-A extracted with 0.75% AQ dosage and HS-B, HS-B extracted without AQ are studied by FT-IR spectroscopy. As displayed in Fig.5, differences in structural signatures are observed between HS-A and HS-B. The relative peak intensities, reflecting the relative amount of each functional group, are different for HS-A and HS-B. The spectra show that the bands are centered between 1 700 cm-1 and 800 cm-1, but the bands at 1 695 cm-1 and 1 275 cm-1 for HS-A are stronger than those for HS-B. On the HS-B spectrum, the peak at 1 344 cm-1 for HS-A undergoes a shift towards higher wavenumber at
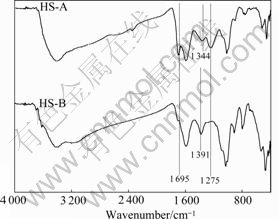
Fig.5 FT-IR spectra of HS
1 391 cm-1 [15-16]. Fig.5 also shows that the most discriminating variables for the two HS are FT-IR functional groups of O—H (H-bonded COOH) and —COOR. In comparison with HS-B, HS-A has more groups of —COOR and —COOH. This gives forcible evidence that assistant AQ is capable of preventing HS from being destroyed by NaOH, which increases the extraction yield of HS.
3.3 Effects of alkali dosage
Under the conditions of extraction temperature of 100 ?C, extraction time of 60 min, S/L ratio of 1:3 and stirring speed of 600 r/min, effects of alkali dosage on extraction yield of HS are studied and the results are plotted in Fig.6.
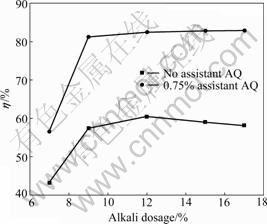
Fig.6 Effects of alkali dosage on extraction yield of HS
Fig.6 indicates that the extraction yield of HS without AQ is obviously increased when the alkali dosage ranges from 7% to 12%. When the alkali dosage is 12%, the extraction yield of HS almost reaches the maximum of 60.38%. While the alkali dosage further increases to 15% or 17%, the extraction yield starts to decrease. The changing rule of extraction yield is the same as that presented in Ref.[14]. The main reason is that the dissolvable carbohydrates in the lignite are easily destroyed and changed into indissoluble substances with higher sodium hydroxide dosage, resulting in the decrease of extraction yield of HS [15, 17].
In the presence of 0.75% assistant AQ during alkaline extraction, the extraction yield of HS always presents an ascending trend with the increase of alkali dosage between 7% and 17%, sharply increases when the alkali dosage is enhanced from 7% to 9%, while the extraction yield is improved only a little along with a further rising of alkali dosage. When using assistant AQ in the alkaline extraction, the dissolvable carbohydrates in HS are prevented from being destroyed by alkali to some degree, even if the dosage of sodium hydroxide is high.
Under the condition of alkali dosage of 9%, the extraction yield of HS gets to 81.24%. While under the optimal condition of alkali dosage of 12%, the extraction yield of HS without assistant AQ is no more than 60.38%. The results show that using 0.75% AQ as assistant can reduce alkali dosage (NaOH) by 3% at least.
3.4 Effects of extraction temperature
Under the conditions of alkali dosage of 9%, assistant AQ dosage of 0.75%, extraction time of 60 min, S/L ratio of 1:3, and stirring speed of 600 r/min, the effects of extraction temperature on extraction yield of HS are tested and displayed in Fig.7.
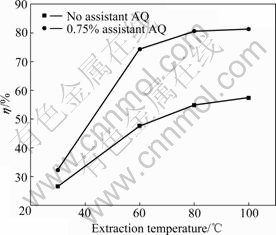
Fig.7 Effects of extraction temperature on extraction yield of HS
From Fig.7, it can be seen that the extraction yield of HS presents an ascending tendency with the increase of extraction temperature. Under the condition of assistant AQ of 0.75%, an extraction yield above 74.32% can be obtained when the temperature is higher than 60 ?C. At 80 ?C and 100 ?C, the extraction yield is almost the same. At 80 ?C, an extraction yield of 80.56% can be obtained in the presence of 0.75% AQ.
Fig.7 also shows that using 0.75% AQ as assistant can reduce the extraction temperature by 20 ?C, compared with the conventional extraction temperature of 100 ?C without assistant AQ.
3.5 Effects of extraction time
Fig.8 illustrates how extraction time affects the extraction yield under the conditions that alkali dosage is 9%, assistant AQ dosage is 0.75%, extraction temperature is 80 ?C, S/L ratio is 1:3 and stirring speed is 600 r/min.
As shown in Fig.8, with prolonging the extraction time from 10 min to 30 min, the extraction yield is increased from 30.76% to 80.08%. However, extraction time has little impact on the extraction yield if it is further increased to 60 min.
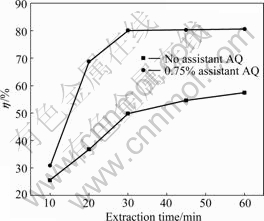
Fig.8 Effects of extraction time on extraction yield of HS
The results also imply that assistant AQ can decrease the extraction time by about 30 min, compared with the conventional optimal extraction time of 60 min.
4 Conclusions
1) FT-IR spectra indicate that the lignite sample mainly consists of —OH (3 398 cm-1) groups and —COOH, —COO— and —COOR (1 550-1 790 cm-1) groups, as well as some hetero-oxy compounds, such as Si—O—Si (1 055-1 020 cm-1) or Si—O—C (1 110- 1 080 cm-1).
2) Anthraquinone (AQ) is used to improve the extraction process of HS from lignite for the first time. Determination of FT-IR spectra shows that HS-A obtained in the presence of 0.75% AQ has more groups of —COOR and —COOH than HS-B obtained without AQ, and assistant AQ can prevent dissolved HS from being destroyed into undissolved substance by NaOH, which increases the extraction yield of HS.
3) Assistant anthraquinone (AQ) has positive influence on the extraction yield of HS. Compared with the conventional extraction conditions without AQ, the extraction yield of HS is increased by more than 20% by using AQ. However, the extraction temperature is decreased by 20 ?C, the alkali dosage (NaOH) by 3% at least, and the extraction time is reduced to 30 min.
4) When using anthraquinone (AQ) as assistant, optimal experimental parameters are obtained as follows: alkali dosage of 9%, AQ dosage of 0.75%, extraction temperature of 80 ?C, extraction time of 30 min, S/L ratio of 1:3 and stirring speed of 600 r/min. Under these conditions, the extraction yield of HS is 80.08%.
References
[1] LIU Guo-gen. Study on HA-type binders for direct reduction of cold bond pellets and its raw material characteristics [D]. Changsha: Central South University, 1997. (in Chinese)
[2] CHEN Bing-bing, CHI Hai. Talking about processing and utilization of brown coal [J]. Coal Technology,2005, 24(11): 113-114. (in Chinese)
[3] YUAN Zhong-wei, SUN Li-ping, LU Yuan, WANG Jian-wei. Study on the content of humic acid extracting from grass peat [J]. Municipal Engineering Technology, 2006, 26(2): 154-156. (in Chinese)
[4] BE?ATRICE A. A comparative study on the chemical composition of humic acids from forest soil, agricultural soil and lignite deposit bound lipid, carbohydrate and amino acid distributions [J]. Gendarme, 2006, 130: 77-96.
[5] ZOU Jing, WANG Fang-hui, ZHU Hong, WEI Yong-sheng. Study of the extraction of humic acid from weathered coal [J]. Chemical Industry Times, 2006, 20(6):10-12. (in Chinese)
[6] GAO Deng-zheng, WANG Li, LIU Li-hua, LU Jing-ping. Technologic experiment study of humic acid extracted from wali lignite [J]. Journal of Shandong University of Science and Technology: Natural Science, 2005, 40(3): 40-42. (in Chinese)
[7] PEURAVUORI J, ZBANKOVA P, PIHLAJA K. Aspects of structural features in lignite and lignite humic acids [J]. Fuel Processing Technology, 2006, 87: 829-839.
[8] CONTE P, PICCOLO A. Conformational arrangement of dissolved humic substances: Influence of solution composition on association of humic molecules [J]. Environmental Science and Technology, 1999, 33: 1682-1690.
[9] LIU Guo-gen, QIU Guan-zhou, HU Yue-hua. Study on infrared spectra of coals [J]. Journal of Central South University: Natural Science Edition, 1999, 30(40): 371-373. (in Chinese)
[10] IBARRA J V, MIRANDA J L. Detection of weathering in stockpiled coals by Fourier transform infrared spectroscopy [J]. Vibrational Spectroscopy, 1996, 10: 311-318.
[11] IBARRA J V, MU?OZ E, MOLINER R. FTIR study of the evolution of coal structure during the coalification process [J]. Organic Geochemistry, 1996, 24: 725-735.
[12] DIMMEL D R, SHEPARD D. Studies on the mechanism of action of anthraquinone as a pulping catalyst [C]// Canadian Wood Chemistry Symposium. Niagara Falls, 1982, 29-32.
[13] DIMMEL D R. Pulping with anthraquinone: Fundamental chemistry [C]// TAPPI Pulping Conference Proceedings. Nashville, 1996: 53- 58.
[14] LIU Guo-gen, QIU Guan-zhou, XU Jing-cang. A study of technological parameters of extract in preparation process of HA series pellet binder [J]. Sintering and Pelletizing, 1998, 23: 22-24. (in Chinese)
[15] LANG Cong-shan, DANG Zhi, LAU Cong-qiang. Structure characterization of soil humic acids and adsorption equilibria on phenanthrene [J]. Chinese Journal of Analytical Chemistry, 2006, 34(3): 288-292. (in Chinese)
[16] LIU Guo-gen, QIU Guan-zhou, XU Jing-cang. Study on spectroscopy of HA type binder [J]. Journal of Central South University: Natural Science Edition, 2003, 34(3): 238-241. (in Chinese)
[17] TATZBER M, STEMMER M. FTIR-spectroscopic characterization of humic acids and humin fractions obtained by advanced NaOH, Na4P2O7, and Na2CO3 extraction procedures [J]. J Plant Nutr Soil Sci, 2007, 70: 522-529.
(Edited by YANG Bing)
Foundation item: Project(50725416) supported by the National Science Fund for Distinguished Young Scholars in China; Project(50804059) supported by the National Natural Science Foundation of China; Project(2008BAB32B06) supported by the Key Program in National Science and Technology Pillar Program during the 11th Five-year Plan Period of China; Project(200805331080) supported by Specialized Research Fund for the Doctoral Program of Higher Education of China
Received date: 2010-01-20; Accepted date: 2010-04-13
Corresponding author: ZHANG Yuan-bo, Associate Professor, PhD; Tel: +86-731-88877214; E-mail: zybcsu@126.com