
Structure and hydrogen storage performance of LaNi4.25Al0.75 alloy
CAO Da-li1, CHEN De-min2, LIU Yi1, MA Lei1, L? Man-qi2, YANG Ke2
1. School of Materials Science and Engineering, Shenyang University of Chemical Technology,
Shenyang 110141, China;
2. Institute of Metal Research, Chinese Academy of Sciences, Shenyang 110016, China
Received 25 August 2009; accepted 22 April 2010
Abstract: Hydrogen storage properties of LaN4.25Al0.75 alloy were experimentally investigated by XRD, PC isotherm curves, hydrogen absorption kinetics curves, XPS and its particle diameter. The structure of unit cell of LaNi4.25Al0.75 alloy was deduced. The relationship between its structure and hydrogen storage performance of LaNi4.25Al0.75 alloy was analyzed. The results show that LaNi4.25Al0.75 alloy has rapid hydrogen absorption rate and good resistance to combustibility. It is also found that the function of the hydrogen absorption plateau pressure and temperature is ln peq=-4 820/T+12.46, and the hydrogen absorption rate of the alloy decreases with increasing the temperature.
Key words: hydrogen storage alloy; LaNi4.25Al0.75; microstructure; resistance to combustibility
1 Introduction
Hydrogen storage alloys have been widely studied for their application as energy storage media and battery electrodes[1-3]. Among these materials, LaNi5 alloy exhibits excellent hydrogen storage characteristics, such as high volumetric storage density, easy activation and moderate kinetics[2]. However, LaNi5 alloy has unsatisfactory properties, such as easy decay of storage capacity in cyclic absorption/ desorption process, easy pulverization, easy combustion after pulverization and higher plateau pressures [3].
It was found that the partial replacement of Ni in LaNi5 alloy by a small amount of Al could prominently increase its cycle life time, pulverization and modulate moderate plateau pressures without obvious decrease in hydrogen absorption capacity[1, 4-6], so LaNixAl5-x is considered to be very useful hydrogen absorption material for its moderate plateau pressure and resistance to impurities in hydrogen gas[1, 3-5]. For example, LaNi4.25Al0.75-tritide is used in the Savannah River Site (SRS) tritium processing to be the primary tritium storage medium because the material is easily activated, a delivery pressure of 200 kPa is easily achieved by moderate heating and it captures nearly the entire tritium[7]. So, much attention has been particularly focused on LaNi4.25Al0.75 compounds for their application in energy storage[1, 7]. However, there is less report available on the relationships between structure and hydrogen storage behavior of the LaNi4.25Al0.75 alloy.
In the present work, hydrogen storage properties of LaNi4.25Al0.75 alloy were systematically studied, the structure of four-unit cell of LaNi4.25Al0.75 alloy was deduced, and the relationship between structure and its hydrogen storage performance was analyzed.
2 Experimental
Stoichiometric LaNi4.25Al0.75 alloy was prepared in a high-frequency induction melting furnace using argon atmosphere protection (lanthanum 99.50%; nickel 99.95%; aluminum 99.70%, mass fraction). Those ingots were turned over and re-melt twice for homogeneity. Those ingots were then annealed at 1 323 K for 8 h in argon atmosphere in a sealed quartz tube. About 1 g single phase LaNi4.25Al0.75 with CaCu5-type hexagonal structure was individually placed in cells made of stainless steel to test hydrogen storage behavior.
The sample cell whose volume was precisely calibrated was then connected with testing apparatus which was set up with actuator-driven valves and needle valves. High-pressure transducers, vacuum transducers and thermocouple were attached to the system for pressure and temperature measurements. Oil bath and water bath were employed to keep temperature constant.
High-purity hydrogen (H2≥99.999%, mass fraction) was used to test activation and PC isotherms for absorption hydrogen as well as absorption kinetics curves.
The phase composition was identified by XRD (Rigaku, Japan). The size distribution of the particles after 25 hydrogen absorption/ desorption cycles was investigated with a Malvern mastersizer mirco laser particle sizer. Surface survey of sample was carried out on LAS-3000 surface analysis systems.
3 Results and discussion
3.1 Crystal structure of LaNi4.25Al0.75 alloy
Fig. 1 shows XRD pattern of LaNi4.25Al0.75 alloy annealed, and the alloy is identified as single phase material with the CaCu5-type hexagonal structure.
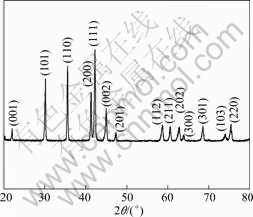
Fig.1 XRD pattern of LaNi4.25Al0.75 alloy
The lattice parameters and unit cell volumes of LaNi5[5] together with calculated values for the LaNi4.25Al0.75 samples are presented in Table 1. It can be seen from Table 1 that unit cell dimension and lattice parameters of LaNi4.25Al0.75 increase slightly compared with LaNi5.
Table 1 Unit cell volume and lattice constant of LaNi5 and LaNi425Al0.75

LaNi5-xAlx(x<1) alloy has CaCu5-type hexagonal structure. La atoms occupy the La (0, 0, 0) Wyckoff site, two nonequivalent Ni atoms occupy the 2c (2/3, 1/3, 0) and the 3g (0, 0.5, 0.5) Wyckoff sites, Al atoms prefer to occupy the 3g sites in LaNi5-xAlx[8]. This was verified by total energy calculations[9]. CHEN et al[10-11] insisted that Al atom substituting for Ni atoms was in the 3g (0.5a, 0.5a, 0.75c) site in double unit cell of LaNi5Al0.5 and Al atoms were nonadjacent in LaNi5-xAlx unit cell. So, Al atoms are not neighbor in LaNi4.25Al0.75 unit cell either, and maybe occupy in the 3g (0.5a, 0.5a, -0.5a, 0.5c), 3g (0.5a, 0.5a, -0.5a, 1.5c) and 3g (-0.5a, 0.5a, 0.5a, 1.5c) or 3g (-0.5a, 0.5a, 0.5a, 0.5c) sites in two neighbor HCP cells. As shown in Fig.2, the structure is composed of 4 La, 17 Ni and 3 Al atoms, i.e., 4La+17Ni+3Al= 4(LaNi4.25Al0.75 unit cells)or 3LaNi4Al and 1 LaNi5 unit cells.
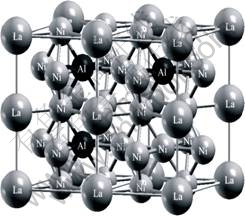
Fig.2 Structure of four-unit cell of LaNi4.25Al0.75
Table 2 lists atomic radii of La, Ni, Al and H[12], and interstice radii of polyhedron in LaNi5 unit cell are presented in Table 3[13], those data indicate that all of interstice radii of polyhedron in LaNi5 are clearly larger than the atomic radius difference of Ni and Al (0.018 nm), so 0.75 mol Al substituting Ni in LaNi5 unit cell cannot change crystal structure, and unit cell dimension and lattice parameters of LaNi4.25Al0.75 increase slightly compared with LaNi5, as seen in Table 1. The expansion is heterogeneous due to one without Al atom, the other three with Al atom in the four unit cells; meanwhile, the expansion in c-axis is larger than that in a-axis, indicating a lattice distortion in LaNi4.25Al0.75 crystal.
Table 2 Atomic radii of elements

Table 3 Interstice radii of polyhedron in LaNi5 unit cell (nm)

3.2 PCT curves
Fig.3 shows the hydrogen absorption PC isotherm(PCT) curves of LaNi4.25Al0.75 alloy after initial activation at 333, 353, 373 and 423 K. Hydrogen storage capacities of the alloy systematically decrease and absorption hydrogen plateau pressures significantly increase with increasing temperature. The function of plateau pressure (peq) and temperature (T) is ln peq=-4 820/T+12.46. The molecular formula of saturated hydride at 353 K is LaNi4.25Al0.75H4.2. Compared with LaNi5H6[3], saturated hydrogen absorption capacity of LaNi4.25Al0.75 decreases by nearly 30% and plateau pressures is significantly lower.
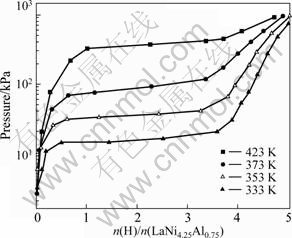
Fig. 3 Hydrogen absorption PC isotherms curves of LaNi4.25Al0.75 alloy after different initial activation temperatures
Lattice constants of LaNi425Al0.75 increase slightly compared with LaNi5, the differences of a-axis and c-axis of the two alloys are 0.002 nm and 0.007 nm, respectively, and the increased amounts of a-axis and c-axis are less than the difference of atomic radii of Al and Ni (0.018 nm), as shown in Table 1 and Table 2. So, the substitution of 0.75 mol Al for Ni induces lattice distortion in LaNi4.25Al0.75 and causes interstice radii of polyhedron decrease. Some interstice radii of polyhedron in LaNi4.25Al 0.75 alloy decrease so large that it is clearly shorter than hydrogen atomic radius. These interstitial sites (especially some tetrahedral and octahedral interstices) cannot accommodate hydrogen atoms, so hydrogen storage capacity of LaNi4.25Al0.75 alloy decreases compared with LaNi5. GAO et al[8] found that the Fermi energies sharply increased in the order from LaNi5 to LaNi4.5Al0.5 and LaNi4Al with the content of aluminum increasing. But electronegativities of these elements are H 2.1, Al 1.5, Ni 1.9 [11], the affinity of Al and H is stronger than that of Ni and H. So the hydrides of LaNi4.25Al0.75 is stable and its plateau pressures decrease compared with LaNi5.
3.3 Hydrogen absorption kinetics curves
Hydrogen absorption kinetics curves of LaNi4.25Al0.75 alloy after 25 circles at 1 020 kPa (initial pressure of storage cell) are shown in Fig.4. 1 mol LaNi4.25Al0.75 can absorb over 4 mol H2 in 60 s at 333 K and 373 K, but only absorb 3 mol H2 in 100 s at 423 K, and hydrogen absorption rate decreases with increasing temperature.
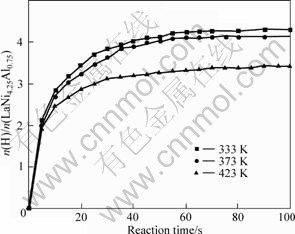
Fig.4 Hydrogen absorption kinetics curves of LaNi4.25Al0.75 alloy at different temperatures
Ni in LaNi5-xAlx alloys can catalyze the reaction of alloy and H[4], so hydrogen absorption rates of these alloys are very rapid. More cracks and fissures are formed both on the surface and inside of the samples with the Al addition, which can shorten the hydrogen atom diffusion distance and provide more fresh surfaces for hydrogen atom association[13], so the alloy has rapid hydrogen absorption rate.
Fig.5 shows van’t Hoff plot of LaNi4.25Al0.75 hydrogen absorption. The calculated enthalpy change and entropy change of LaNi4.25Al 0.75 hydride formation are (-41.4±2.04) kJ/mol H2 and (-106.0±2.57) J/(K?mol) H2, and Gibbs free energy changes are all negative from 333 to 423 K. So, LaNi4.25Al0.75 alloy absorption hydrogen is exothermic reaction, hydrogen absorption rate decreases with increasing temperature.
3.4 Spontaneous combustion resistance performance
LaNi5 hydride is easy to combust spontaneously in atmosphere gas in 10 circles, but LaNi4.25Al0.75 hydride cannot combust spontaneously in atmosphere gas after 25 circles.
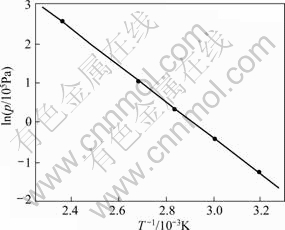
Fig.5 van’t Hoff plots of LaNi4.25Al0.75 absorption hydrogen
FENG et al[6] investigated LaNi5 and LaNi4.7Al0.3 during absorption/desorption cycling. They found that alloys with Al substituting exhibited excellent resistance to oxygen impurities due to the formation of Al2O3, which restricted the surface absorption of oxygen and prevented degradation; and Al element retarded the decomposition by restricting the exchange between La and Ni atoms in the neighboring sites. GIZA et al[13] insisted that Al accumulated on the grain boundaries and associated with segregated La to form porous oxide layer
which protected the material from further corrosion and oxidation.
X-ray photoelectron spectroscopy(XPS) of LaNi4.25Al0.75Hx hydride is shown in Fig.6. There are La2O3 and La(OH)3, no Al2O3 on LaNi4.25Al0.75Hx hydride surface.
Al atoms are surrounded by La and Ni atoms in LaNi4.25Al0.75 unit cell in Fig.2. Oxygen atom radius (0.73 nm) is significantly larger than interstice radius of tetrahedron or hexahedron in LaNi4.25Al0.75 unit cell, so Al atoms cannot be oxidized if LaNi4.25Al0.75 unit cell is not destroyed. In addition the electronegativities are La 1.0, Al 1.5, Ni 1.9 and H 2.1[12], respectively, so lanthanum atoms are firstly oxidized when LaNi4.25Al0.75Hx connects with atmosphere gas. If Al2O3 is formed when LaNi4.25Al0.75 hydride connects with atmosphere gas, but it may be reduced by lanthanum atoms in neighbor sites, so Al2O3 is not found on the surface of LaNi4.25Al0.75Hx.
The particle diameter distribution plots of LaNi5 and LaNi4.25Al0.75 alloys after 25 circles are shown in Fig.7. It can be seen that the particle diameters of LaNi5 spread at a wider range of 5-60 ?m and focus in 8-40 ?m. Particle diameters of LaNi4.25Al0.75 spread 5-100 ?m and focus in 20-70 ?m. It is also found that LaNi5 and LaNi4.25Al0.75 alloys easily pulverize after initial activation and hydrogen absorption, but LaNi4.25Al0.75

Fig.6 XPS of LaNi4.25Al0.75Hx

Fig.7 Plots of particle diameter vs volume fraction
alloy has better pulverization-resistant performance than LaNi5 alloy.
CHENG et al[1] found that particle size of LaNi4.25Al0.75 alloy after 1 000 cycles was narrower (0.36-103 ?m) compared with that after initial activation (0.78-301 ?m). With the increase of volume fraction, mean diameter decreases from 34.81 to 11.34 ?m, and the alloy was in homogeneous single phase with CaCu5 structure and almost kept this structure even after 1 000 cycles.
Compared with LaNi5, hydrogen storage capacity of LaNi4.25Al0.75 alloy decreases, so the lattice stress of LaNi4.25Al0.75 alloy decreases during the absorption/desorption hydrogen. On the other hand, the atomic radius difference between Ni and Al is 0.018 nm which is prominently larger than the expansion amount of a-axis (0.002 nm) and c-axis (0.007 nm), so there must exist compressive pre-stress in LaNi4.25Al 0.75 alloy, which can counteract partial stress from absorption/ desorption hydrogen. So, LaNi4.25Al0.75 alloy has an excellent pulverization-resistant property.
KIYONORI and KAZUHZRO[14] subjected LaNi5 and LaNi4.7Al0.3 up to 2 000 absorption/desorption cycles at 293 K in super-high pure (99.99999%) hydrogen, and found that partial substitution of Ni by Al can reduce lattice stress which is caused during the cycle of absorption/desorption hydrogen. So Al substitution could improve the cycle stability.
4 Conclusions
1) The crystal structure of LaNi4.25Al0.75 alloy is CaCu5 structure.
2) Hydrogen storage capacities of LaNi4.25Al0.75 systematically decrease and absorption hydrogen plateau pressures significantly increase with increasing temperatures.
3) The hydrogen absorption rate of the LaNi4.25Al0.75 alloy is very fast, and there is slight decrease with the increase of temperature.
4) LaNi4.25Al0.75 hydride cannot combust spontaneously in atmosphere gas after 25 cycles.
References
[1] CHENG H H, YANG H G, LI S L. Effect of hydrogen absorption/desorption cycling on hydrogen storage performance of LaNi4.25 Al0.75 [J]. J Alloys Compounds, 2006, 453(4): 448-452.
[2] LIU Y F, PAN H G, GAO M X, ZHU Y F, LEI Y Q. Hydrogen storage and electrochemical properties of the La0.7Mg0.3Ni3.825-xCo0.675Mnx hydrogen storage electrode alloys [J]. J Alloys Comp, 2004, 365: 246-252.
[3] WAN Wei-hua, TANG You-gen, LU Zhou-guang. Modification of LaNiAl hydrogen storage alloys [J]. Journal Center South University: Science and Technology, 2007, 38(7): 107-111. ( in Chinese)
[4] ZHANG W, CIMATO J, GOUDY A J. The hydriding and dehydriding kinetics of some LaNi5-xAlx alloys [J]. J Alloys and Compounds 1993, 201(7): 175-181.
[5] MONMA S, USUI S, MASUDA Y. Studies on the electronic structures of LaNi5 and LaNi5H0.2 by means of the Dv-Xa method [J]. Journal of Alloys and Compounds, 2006, 426: 295-303.
[6] FENG F, HAN J W, GENG M M, NORTHWOOD D O. Hydrogen desorption kinetics of LaNi4.7Al0.3 metal hydride electrode using potentiostatic measurements [J]. Solar Energy Mater Solar Cells, 2000, 62(1-2): 51-56
[7] KIRK L, SHANAHAN J, HOLDER S. Tritium aging effects in LaNi4.25Al0.75 [J]. Journal of Alloys and Compounds, 2003, 1: 000-006.
[8] GAO Tao, QI Xin-hua, CHEN Bo. Alloying effects on electronic structures of LaNi5-xAlx [J]. The Chinese Journal of Nonferrous Metals, 2005, 15(7): 1092-1099. ( in Chinese)
[9] ZHANG R J, WANG Y M, LU M Q, XU D S, YANG K. First-principles study on the crystal, electronic structure and stability of LaNi5-xAlx (x=0, 0.25, 0.5, 0.75 and 1) [J]. Acta Materialia, 2005, 53: 3445-3452.
[10] CHEN D, GAO T, LI G X. Electronic structure and site occupation for the intermediate phase of LaNi4.5Al0.5Hy [J]. Solid State Communications, 2007, 141(7): 378-383.
[11] CHEN D, ZHOU L H, YU B H, WANG C L, GAO T, ZHANG D L. Density functional theory study on solid solution phase of LaNi4.5Al0.5 hydrogen storage alloys [J]. The Chinese Journal of Nonferrous Metals. 2007, 17(7): 1160-1165. (in Chinese)
[12] EODORE L B, EUGENE L H Jr, BRUCE E B. Chemistry [M]. New Jersey: Prentice Hall Upper Saddle River, 1997.
[13] GIZA K, WASIECZKO W I, PAVLYUK V V. Hydrogen absorption and corrosion resistance of LaNi4.8Al0.2 and LaNi4.8Al0.1 Li0.1 alloys [J]. J Alloys Compounds, 2007, 429: 352-357.
[14] KIYONORI S, KAZUHIRO I. Effect of hydrogen absorption/ desorption cycling on hydrogen storage performance ofLaNi5 and LaNi4.7Al0.3 [J]. Materials Transactions, JIM, 2000, 40(5): 581-589.
LaNi4.25 Al0.75 合金的结构与储氢性能
曹大力1, 陈德敏2, 刘 艺1, 马 雷1, 吕曼祺2, 杨 柯2
1. 沈阳化工学院 材料科学与工程学院,沈阳 110141;
2. 中国科学研究院 金属研究所,沈阳 110016
摘 要:采用XRD、吸氢PCT曲线、吸氢动力学曲线、XPS和粒径分析等测试方法,研究LaNi4.25 Al0.75合金的结构和储氢性能;推导LaNi4.25 Al0.75合金的四晶胞结构,分析LaNi4.25 Al0.75合金的结构与性能的内在联系。结果表明:LaNi4.25 Al0.75合金具有快速吸氢性能和较好的抗燃烧能力,其吸氢平台压和温度之间的函数关系式为lnpeq=-4 820/T+12.46,合金的吸氢速率随着温度的升高而降低。
关键词:储氢合金;LaNi4.25 Al0.75;微观结构;抗燃烧性
(Edited by LI Xiang-qun)
Foundation item: Project (50276063) supported by the National Natural Science Foundation of China
Corresponding author: CHEN De-min; Tel: +86-24-83978928; E-mail:demin.chen.1@126.com
DOI: 10.1016/S1003-6326(11)60745-0