石灰存在条件下铁闪锌矿的碳热还原动力学
来源期刊:中国有色金属学报(英文版)2020年第8期
论文作者:Rafael PADILLA Juan Yamid CARVALLO Maria Cristina RUIZ
文章页码:2265 - 2273
关键词:动力学;铁闪锌矿;高铁闪锌矿;碳热还原;直接还原;ZnCaOS
Key words:kinetics; marmatite; high-iron sphalerite; carbothermic reduction; direct reduction; ZnCaOS
摘 要:采用热重法研究石灰存在条件下铁闪锌矿的直接碳热还原,以确定生产Zn(g)而没有SO2(g)污染的技术可行性。部分反应的样品X射线衍射分析表明,还原反应是通过形成ZnCaOS和Ca2Fe2O5中间产物进行的,最终产物为Zn(g)、固体Fe和CaS。温度对还原速率存在重要影响。在1100 °C下,对于300 mg (Zn,Fe)S:CaO:C比为1:1:1的样品,10 min左右可实现铁闪锌矿的完全转化。用ln(1-X)=-kt模型分析总还原反应的动力学,发现在1000~1150 °C、转化率不超过0.95时,该模型很好地描述试验数据,在此温度范围内确定的活化能为257 kJ/mol。研究结果证实该方法生产Zn(g)而不产生有害的SO2(g)排放的技术可行性。
Abstract: The direct carbothermic reduction of marmatite in the presence of lime was studied by thermogravimetric method to determine the technical feasibility to produce Zn(g) without polluting with SO2(g). X-ray diffraction analysis of partially reacted samples indicated that the reduction occurred through the formation of ZnCaOS and Ca2Fe2O5 as intermediate products to yield Zn(g), and solid Fe and CaS as the final products. Temperature had the major effect on the rate of reduction. Complete conversion of marmatite was obtained at 1100 °C in about 10 min using 300 mg samples with molar ratio of (Zn,Fe)S:CaO:C equal to 1:1:1. The kinetics of the overall reduction reaction was analyzed by the model ln(1-X)=-kt, which represented the data well up to a fractional conversion of 0.95 in the temperature range of 1000-1150 °C. The determined activation energy in this temperature range was 257 kJ/mol. The results demonstrated the technical feasibility to produce Zn(g) by this method without producing noxious SO2(g) emissions.
Trans. Nonferrous Met. Soc. China 30(2020) 2265-2273
Rafael PADILLA, Juan Yamid CARVALLO, Maria Cristina RUIZ
Metallurgical Engineering Department, University of Concepcion, Concepcion 4070371, Chile
Received 17 December 2019; accepted 13 April 2020
Abstract: The direct carbothermic reduction of marmatite in the presence of lime was studied by thermogravimetric method to determine the technical feasibility to produce Zn(g) without polluting with SO2(g). X-ray diffraction analysis of partially reacted samples indicated that the reduction occurred through the formation of ZnCaOS and Ca2Fe2O5 as intermediate products to yield Zn(g), and solid Fe and CaS as the final products. Temperature had the major effect on the rate of reduction. Complete conversion of marmatite was obtained at 1100 °C in about 10 min using 300 mg samples with molar ratio of (Zn,Fe)S:CaO:C equal to 1:1:1. The kinetics of the overall reduction reaction was analyzed by the model ln(1-X)=-kt, which represented the data well up to a fractional conversion of 0.95 in the temperature range of 1000-1150 °C. The determined activation energy in this temperature range was 257 kJ/mol. The results demonstrated the technical feasibility to produce Zn(g) by this method without producing noxious SO2(g) emissions.
Key words: kinetics; marmatite; high-iron sphalerite; carbothermic reduction; direct reduction; ZnCaOS
1 Introduction
Sphalerite is the most abundant zinc- containing mineral and thus, it is the main source for the production of metallic zinc. Natural sphalerites can contain several other elements substituting the Zn in their structure and the most frequent impurity is iron, which can be as high as 23 wt.% [1]. When the iron content of sphalerite is high, exceeding 6 wt.%, its color becomes black and it is commonly known as marmatite [2].
The technology for the production of zinc from sphalerite includes both hydrometallurgy and pyrometallurgy. The primary zinc production by the conventional pyrometallurgical treatment of roasting-sintering have received little attention and been gradually replaced by the hydrometallurgical treatment [3,4]. This is mainly due to the high operating and capital costs [5] and due to the increasingly stringent environmental regulations on SO2 gas emissions in the roasting–sintering operations. At present, more than 80% of the primary zinc is produced by roasting-leaching- electrowinning (RLE) or pressure leaching processes [6].
The direct carbothermic reduction of sphalerite in the presence of lime as a sulfur acceptor is a promising alternative method that addresses the drawbacks of the conventional pyrometallurgical treatment of zinc sulfide minerals.
The direct reduction of zinc sulfide in the presence of lime can be represented by the overall reaction:
ZnS(s)+CaO(s)+(1-z)C(s)=Zn(g)+CaS+(1-2z)CO(g)+zCO2(g) (1)
where z is the stoichiometric coefficient dependent on the thermodynamics and relative kinetics of the intermediate reactions. Thus, z can vary from 0 to 0.5 and the limiting values 0 and 0.5 correspond to the production of solely CO or CO2 gas product, respectively.
The main advantages of this direct reduction of zinc sulfide method is that the sulfur contained in ZnS is fixed as solid calcium sulfide; thus, the SO2 emissions are eliminated. Furthermore, in the conventional zinc smelting, the sintering operation is complex and hard to control, and the coke production operation is capital intensive [5]; therefore, in the direct reduction of ZnS, these operations would also be eliminated alleviating in this way the cost of producing zinc.
Thus, several investigators have studied the carbothermic reduction of various metal sulfides in the presence of lime [7-14]. In these studies, there was a general agreement that the carbothermic reduction of metal sulfides generally proceeded through the formation of metal oxide as an intermediate compound. Therefore, for the direct reduction of zinc sulfide, the reaction scheme considered by HUANG et al [15,16] and WU et al [17] were as follows:
ZnS+CaO=ZnO+CaO (2)
ZnO+CO=Zn(g)+CO2(g) (3)
C+CO2(g)=2CO(g) (4)
Assuming that the rate-limiting step was Reaction (2), HUANG et al [16] using an empirical rate equation reported an energy of activation of 311 kJ/mol for the temperature range of 1180-1353 °C. In addition to this sequence of reactions, ABRAMOWITZ and RAO [18] reported that the direct reduction of zinc sulfide by carbon and lime proceeded through the formation of COS as the intermediate gaseous compound and reported the activation energy of 210 kJ/mol in the temperature range of 900-1100 °C.
On the other hand, IGIEHON et al [19] reported limited data on the formation of a quaternary oxysulfide of zinc and calcium (ZnCaOS), which was formed from the reaction of mixtures of ZnS-CaO and ZnO-CaO. These investigators concluded that this quaternary oxysulfide appeared to be stable after 1 h at 900 °C and that this oxysulfide instead of ZnO could be part of the mechanism for the production of zinc in the carbothermic reduction of zinc sulfide in the presence of lime. Later, PETROVA et al [20] synthesized pure ZnCaOS by annealing an equimolar mixture of ZnS and CaO at 1000 °C in helium atmosphere for the determination of the crystal structure of this quaternary compound.
Therefore, considering the limited data available on the kinetics and the controversy on the mechanism of the carbothermic reduction of zinc sulfide in the presence of calcium oxide, in this research, a kinetics study on the reduction of high-iron sphalerite, marmatite, concentrate with carbon and lime was undertaken to determine the mechanism and the rate of the reduction reaction.
2 Experimental
2.1 Materials and sample preparation
Marmatite sample, composed of hand-picked mineral particles obtained from Tupiza, Bolivia, was used in this study. These large mineral particles were ground and subjected to size separation by sieving. The most abundant size fraction of 45-53 mm was used for the reduction experiments. The chemical analysis of this mono-sized fraction indicated that the zinc content was 49.54 wt.% and iron content was 12.84 wt.%. The corresponding empirical composition of the marmatite was calculated as Zn0.77Fe0.23S, and trace amounts of other phases such as Fe-Mn-O and Mg-Al-Si were identified in SEM-EDS analysis on single marmatite particles. The XRD analysis of the marmatite sample showed strong diffraction lines for (Zn,Fe)S, as shown in Fig. 1.
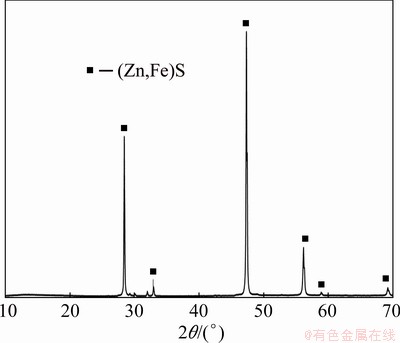
Fig. 1 XRD spectrum of marmatite sample, symbolized by (Zn,Fe)S, as prepared for reduction experiments
The reduction agent used in this study was activated carbon (Merck KGa A, Darmstad Germany) with 94% <75 mm, whose fixed carbon content was 86%. The calcium oxide used was of reagent grade with Ca content >60 wt.% (CaO content >95 wt.%).
The preparation of the sample mixtures included thorough mixing of predetermined amounts of marmatite, calcium oxide, and carbon in order to produce various samples of different molar ratios of (Zn,Fe)S:CaO:C.
2.2 Reduction experiments
Isothermal tests were performed in a custom- built thermogravimetric apparatus (TGA), which consisted essentially of a high temperature vertical furnace with a quartz reaction tube (45 mm in inner diameter), a microbalance, data acquisition system, and ancillary equipment for controlling the reaction environment.
A typical experiment was started by heating the furnace to the set temperature under a slow flow of nitrogen. When the temperature of the reaction tube was stabilized at the set temperature, about 300 mg of the mixture sample (Zn,Fe)S-CaO-C contained in a cylindrical alumina crucible (14 mm in inner diameter and 25 mm in height) was hanged very rapidly from the balance into the equitemperature zone of the reaction tube. This procedure took about 10-20 s approximately in most of the experiments. The mass of the sample was recorded continuously during the experiment for the analysis of the reduction reaction and the sample temperature was also recorded continuously by a chromel/alumel thermocouple placed just below the crucible. The extent of reaction was calculated from the mass loss of the sample caused by the reduction of the marmatite. The solid products of the reduction reaction were identified by X-ray diffraction spectroscopy (XRD) in some experiments.
3 Results
3.1 Preliminary experiments
Preliminary experiments were carried out using various nitrogen flow rates and homogeneous mixtures of (Zn,Fe)S-CaO, and (Zn,Fe)S-CaO-C. The purpose of these tests was to determine the appropriate range of experimental conditions to study the reduction reaction.
Experiments carried out with increasing nitrogen flow rates indicated that 0.6 L/min of nitrogen was sufficient to avoid the infiltration of oxygen into the reaction tube. Thus, all subsequent experiments were executed using 0.6 L/min nitrogen flow rate and 300 mg sample mixture. Few experiments were carried out using 1200 mg samples for the identification of the solid product species by XRD and/or chemical analysis. These preliminary experiments showed that in the upper section of the tube of the TGA setup, a small fraction of the gaseous Zn product in the temperature range of 950-1150 °C precipitated over the sample-holder chain. Consequently, for the calculation of the conversion of the marmatite, the mass loss was corrected accordingly considering the zinc precipitated in the chain.
Figure 2 shows the mass loss versus temperature data of a non-isothermal reduction experiment. This experiment was conducted under a heating rate of 30 °C/min using a sample with molar ratios of (Zn,Fe)S:CaO:C equal to 1:1:1. This experiment was aimed to find the temperature range where the carbothermic reduction of the marmatite occurred. As can be seen in Fig. 2, a substantial mass loss of the sample occurred in the temperature range of 900-1200 °C. This result established the temperature range of interest in this study.
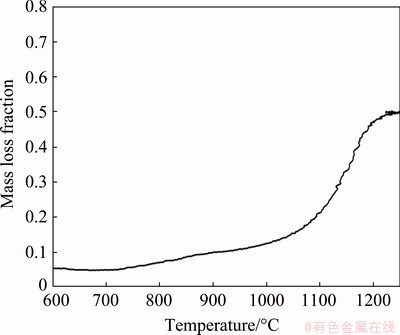
Fig. 2 Mass loss fraction of sample with molar ratio of (Zn,Fe)S:CaO:C equal to 1:1:1 during non-isothermal reduction experiment
Consequently, isothermal experiments were conducted in this temperature range and the results are shown in Fig. 3 with sample mass loss fraction as a function of time. These experiments were carried out with 300 mg of samples with molar ratio of (Zn,Fe)S: CaO:C equal to 1:1:1.
It can be seen in Fig. 3 that temperature has a significant effect on the mass loss of the sample. Increasing the temperature from 950 to 1150 °C resulted in a dramatic increase in the rate of mass loss. For example, at 1000 °C, complete conversion can be reached in about 75 min; while at 1150 °C, the same conversion can be reached in about 5 min.

Fig. 3 Effect of temperature on mass loss fraction of 300 mg initial sample mixture in carbothermic reduction of marmatite in presence of CaO
Additional isothermal experiments were also carried out by using 1200 mg mixture samples for different reaction times namely 8, 18 and 35 min for chemical analysis and for the identification of the reaction products by X-ray diffraction analysis. Figure 4 shows the diffractograms for the experiments carried out at 1100 °C with a sample with molar ratio of (Zn,Fe)S:CaO:C equal to 1:1:1. As expected, when using 1200 mg sample, the reaction rate becomes slower than that in the case of using 300 mg samples most likely due to external gas diffusion.
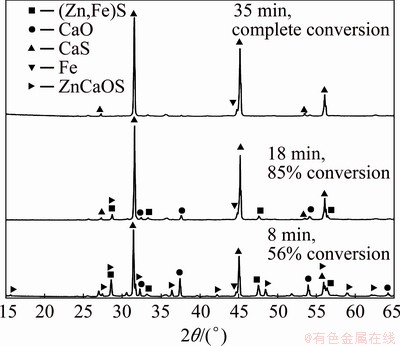
Fig. 4 XRD patterns for partial and complete reaction of 1200 mg sample with molar ratio of (Zn,Fe)S:CaO:C equal to 1:1:1, reacted at 1100 °C
We can see in Fig. 4 that at 8 min of reaction time, the identified species were CaS, ZnCaOS, metallic Fe, unreacted (Zn,Fe)S and CaO; whereas at 18 min, CaS, Fe and minor peaks of unreacted (Zn,Fe)S and CaO were still present. The absence of ZnCaOS can be noted at 18 min, which suggests that this compound is an intermediate product of the overall reaction. At the longer time of 35 min (complete conversion), the final solid products of the carbothermic reduction of marmatite in the presence of calcium oxide were only CaS and Fe. However, XRD results obtained from solids reacted at temperatures lower than 950 °C showed clearly the presence of Ca2Fe2O5 at 60 and 90 min of reaction in addition to the species identified at 1100 °C. These results are presented in Fig. 5.
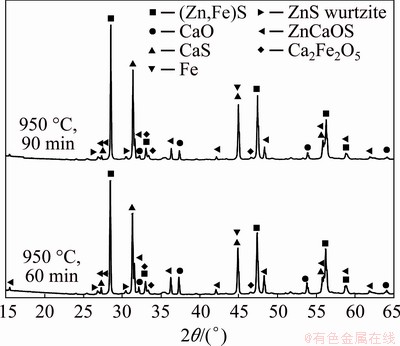
Fig. 5 XRD patterns of samples with molar ratio of (Zn,Fe)S:CaO:C equal to 1:0.76:0.73, reacted at 950 °C for different time, indicating presence of calcium-iron oxide
A few experiments were also carried out with mixtures of (Zn,Fe)S-CaO at various temperatures in oxygen-free nitrogen atmosphere to verify the formation of ZnCaOS and Ca2Fe2O5. The results showed negligible mass loss of the sample, indicating that no gaseous products were formed during the heating of these marmatite-lime mixtures. The solid samples reacted at 900 °C for 30 min and at 1000 °C for 87 min were also analyzed by XRD and the resulting diffraction patterns are shown in Fig. 6. Strong peaks for ZnCaOS, CaS, metallic Fe, Ca2Fe2O5, unreacted marmatite and CaO were identified definitely. At the higher temperature, the ZnCaOS peaks were the strongest, indicating that this quaternary compound is very stable and does not decompose even for the long time of 87 min.
Since the species, ZnCaOS and Ca2Fe2O5, were identified in conditions with carbon and without carbon, it is clear that the reduction of marmatite must proceed through the formation of these compounds as intermediate oxidized species of Zn and Fe, respectively. Conversely, the formation of ZnO was not observed in the XRD patterns in the temperature range of 950-1150 °C studied.
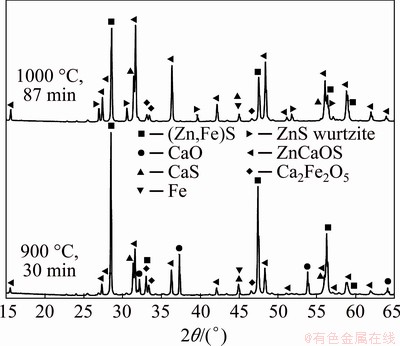
Fig. 6 XRD patterns for solid products using mixtures with molar ratio of (Zn,Fe)S:CaO equal to 1:0.76 at 950 °C, indicating presence of calcium-iron oxide
3.2 Reaction mechanism
Based on the results from the XRD analysis discussed above, the final solid products of the carbothermic reaction of marmatite in the presence of calcium oxide were CaS and Fe. Therefore, the overall reaction could be one of the following Reaction (5a) or (5b):
ZnxFe(1-x)S+CaO+C=xZn(g)+(1-x)Fe+CaS+CO(g) (5a)
ZnxFe(1-x)S+CaO+0.5C=xZn(g)+(1-x)Fe+CaS+0.5CO2(g) (5b)
where x=0.77 for the particular sample used in this research.
For a sample with molar ratio of (Zn,Fe)S: CaO:C of 1:1:1, the theoretical maximum mass loss fraction due to the release of CO(g) and Zn(g) according to Reaction (5a) is 0.48 while for the release of CO2(g) according to Reaction (5b) is only 0.44, since half of the carbon would remain in the solid phase. Considering the zinc condensed in the sample-holder chain, the corrected experimental value of the mass loss fraction for complete conversion of this sample was 0.47, which is very close to the theoretical value for CO(g) production. Furthermore, the theoretical maximum mass loss fraction for a sample with the molar ratio of (Zn,Fe)S:CaO:C equal to 1:1:0.5 according to Reaction (5b) is 0.46 while the experimental maximum mass loss fraction was 0.3, which is far below the theoretical value of 0.46. Thus, the DTG data in both cases indicated that Reaction (5a) producing CO is the predominant reaction in the carbothermic reduction of marmatite in the presence of lime.
Hence, the reaction mechanism would involve the following steps:
(1) Formation of the intermediate Zn and Fe oxidized compounds:
ZnxFe(1-x)S+1/3(5-2x)CaO=xZnCaOS+(1-x)CaS+1/3(1-x)Fe2Ca2O5+1/3(1-x)Fe (6)
(2) Reduction of the oxidized compounds:
xZnCaOS+xCO(g)=xZn+xCaS+xCO2(g) (7)
1/3(1-x)Fe2Ca2O5+(1-x)CO(g)=2/3(1-x)Fe+2/3(1-x)CaO+(1-x)CO2(g) (8)
(3) Oxidation of the carbon (Boudouard reaction):
C+CO2(g)=2CO(g) (9)
Combining Reactions (6)-(9), we obtain the overall reduction Reaction (5a). This sequence of reactions is in agreement with the XRD results discussed above.
3.3 Reaction conversion
The overall conversion in the carbothermic reduction of marmatite in the presence of lime can be best visualized in terms of the fraction of Zn volatilized as the reduction progresses. Thus, the conversion, X, was defined as
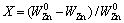 (10)
(10)
where 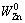 is the mass of the initial Zn in the sample and WZn is the mass of Zn in the sample at time t. Therefore, X can be calculated from the mass loss of the sample using the following equation:
is the mass of the initial Zn in the sample and WZn is the mass of Zn in the sample at time t. Therefore, X can be calculated from the mass loss of the sample using the following equation:
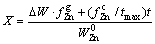 (11)
(11)
where DW is the mass loss of the sample at time t, 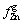 is the fraction of zinc in the gas phase,
is the fraction of zinc in the gas phase, 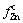 is the fraction of Zn precipitated in the sample chain, and tmax is the time for complete conversion.
is the fraction of Zn precipitated in the sample chain, and tmax is the time for complete conversion.
To verify the accuracy of Eq. (11), the fraction of Zn volatilized was also calculated by chemical analysis of the zinc content of the solid product obtained for samples reacted for different time and these values were compared to the values obtained by Eq. (11). The chemical analysis and XRD analysis required about 800 mg of reacted solids, thus, three experiments using 1200 mg mixed sample were run in the TGA apparatus at 1100 °C to obtain the necessary data for 8, 18 and 35 min of reaction time. The chemical analysis of the reacted solids was done by digestion of the solids and atomic absorption spectroscopy of the solutions. The results are shown in Fig. 7, where it can be seen an excellent agreement between the Zn volatilization as calculated from chemical analysis (Points A, B and C) and the Zn volatilization calculated from the experimental mass loss data. Consequently, these results not only demonstrate the validity of the experimental method used here, but also confirm the formation of CO as the reduction product in the carbothermic reduction of marmatite in the presence of lime. Therefore, for convenience, the effect of variables on the reduction reaction studied here was analyzed in terms of fraction of zinc volatilized as calculated from thermogravimetric data using Eq. (11).
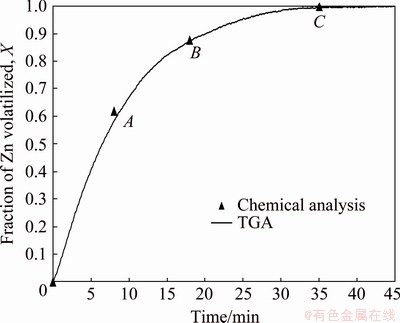
Fig. 7 Volatilization of zinc during carbothermic reduction of sphalerite in presence of calcium oxide at 1100 °C using 1200 mg sample with molar ratio of (Zn,Fe)S:CaO:C equal to 1:1:1
3.4 Effect of amount of CaO on reduction rate
The influence of the calcium oxide on the reduction of the marmatite was investigated at 1100 °C. The results are shown in Fig. 8, where the results for the direct reduction of the marmatite with carbon and without calcium oxide are shown for comparison purposes.
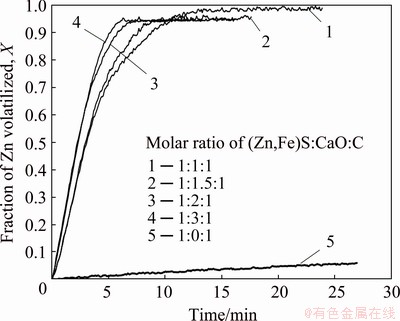
Fig. 8 Influence of amount of calcium oxide on zinc volatilization rate at 1100 °C
It can be seen that the conversion corresponding to the direct reduction of marmatite in the absence of CaO is very low. This result is in accordance with thermodynamic predictions reported for other direct reduction of pure sulfides with carbon in the absence of CaO [7-14]. It is very clear in Fig. 8 that there is a large increase in the rate of zinc volatilization when using CaO; this fact definitely indicates the exceptional capacity of CaO to improve the rate of the direct reduction of marmatite with carbon. Additionally, it is also clear that in this system, an increase in the amount of CaO affects little the rate of reduction and thus, in practice, an excess of CaO over the stoichiometric amount may not be necessary.
3.5 Effect of amount of carbon on reduction rate
The data concerning the effect of carbon on the zinc volatilization is shown in Fig. 9. It is shown that the use of excessive carbon has very little influence on the volatilization rate. An increase in the amount of carbon increases the surface area available for the Boudouard reaction, and thus, it should increase the rate of the overall reaction if the oxidation of carbon, Reaction (9) controls the overall rate of reduction. This suggests that in this reaction system the oxidation of carbon is not rate-controlling. Instead, the reaction might be controlled by the rate of formation of the oxidized species of zinc or iron or the reduction of those oxidized species by CO(g).
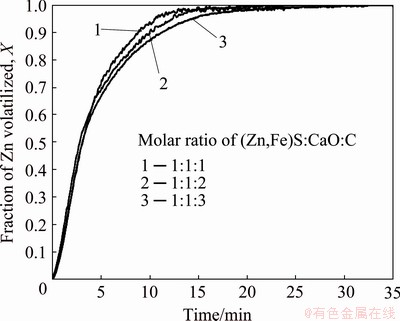
Fig. 9 Effect of excessive carbon on rate of zinc volatilized at 1100 °C
3.6 Effect of temperature on reduction rate
Zinc volatilization data are presented in Fig. 10 for experiments carried out for sample mixtures with molar ratio of (Zn,Fe)S:CaO:C equal to 1:1:1 at temperatures in the range of 1000-1150 °C. We can see that temperature has a significant effect on the reaction rate and, this large effect of temperature on the rate of reaction suggests that the reduction of marmatite with carbon may be controlled by chemical reaction.
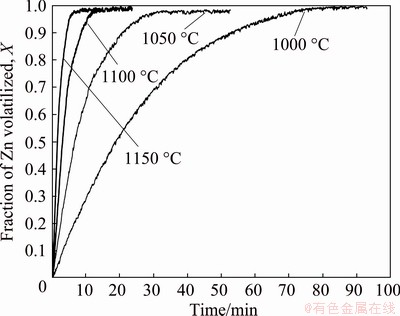
Fig. 10 Temperature dependence of reaction rate of carbothermic reduction of marmatite
3.7 Reaction kinetics
The rate of the overall carbothermic reduction of marmatite in the presence of lime can be analyzed by using a simple model, which assumes a chemical reaction control. Therefore, the following first order kinetics model with respect to the fractional zinc volatilized has been found to fit the experimental data well:
ln(1-X)=-kt (12)
where k is the apparent rate constant. This model has also been used in the carbothermic reduction of other metal sulfides and oxides [10,11,13].
According to this proposed model, the rate constant can be determined from the experimental data by plotting ln(1-X) versus time. Thus, the experimental data shown in Fig. 10 were fitted to Eq. (12) and the result is shown in Fig. 11. As can be seen, the data fitted the first order model well up to the conversion value of 0.95.

Fig. 11 Kinetics of carbothermic reduction of marmatite in presence of lime
From the slopes of the straight lines of Fig. 11, the kinetic constants were obtained and used to draw an Arrhenius plot, which is depicted in Fig. 12. From the slope of the straight line, an activation energy value of 257 kJ/mol was calculated in the temperature range of 1000-1150 °C. There is not reported information on the activation energy on the carbothermic reduction of marmatite for comparison purposes. However, for the reduction of
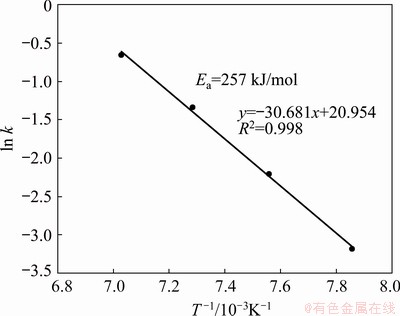
Fig. 12 Arrhenius plot for carbothermic reduction of marmatite in presence of lime in temperature range of 1000-1150 °C
synthetic reagent grade ZnS, HUANG et al [16] reported 311 kJ/mol while ABRAMOWITZ and RAO [18] reported 210 kJ/mol. Therefore, direct comparison of the activation energy determined in this investigation with literature values is difficult. Nevertheless, the activation value of 257 kJ/mol determined for the marmatite falls in the range determined for pure ZnS.
4 Conclusions
(1) X-ray diffraction analysis of reaction products showed evidence on the formation of ZnCaOS, Ca2Fe2O5, Fe and CaS during the isothermal reduction of the marmatite.
(2) Based on these findings, it is concluded that the marmatite reduction with carbon and lime probably proceeds through the formation of ZnCaOS and Ca2Fe2O5 as intermediate products, and subsequent reduction of these Zn and Fe oxidized compounds by CO to yield Zn(g), solid Fe and CaS as final products.
(3) Complete conversion of marmatite can be obtained at 1100 °C in less than 10 min for molar ratios of (Zn,Fe)S:CaO:C equal to 1:1:1.
(4) The first order kinetic model ln(1-X)=-kt represents well the experimental data for fractional conversion up to 0.95 in the temperature range of 1000-1150 °C.
(5) The determined activation energy in this temperature range was 257 kJ/mol.
(6) These results demonstrated the technical feasibility to produce Zn(g) from marmatite by this method without producing noxious SO2(g) emissions.
Acknowledgments
The program 100 Becas de la Soberanía Científica y Tecnológica, Bolivian Ministry of Education is acknowledged for the financial support of Juan Yamid CARVALLO during his graduate studies. The Department of Metallurgy and Materials of the Universidad Mayor y Pontificia de San Andres, Bolivia, is also acknowledged for providing initial XRD analysis of the marmatite sample.
References
[1] Deng J, Lai H, Chen M, Glen M, Wen S, Zhao B, Liu M. Effect of iron concentration on the crystallization and electronic structure of sphalerite/marmatite: A DFT study [J]. Minerals Engineering, 2019, 136: 168-174.
[2] Meng X, Zhao H, Sun M, Zhang Y, Zhang Y, LU X, Kim H, Vainshtein M, Wang S, Qiua G. The role of cupric ions in the oxidative dissolution process of marmatite: A dependence on Cu2+ concentration [J]. Science of the Total Environment, 2019, 675: 213-223.
[3] Ballarini J C, de Oliveira Polli L, Santos Miranda T L, Machado Zica de Castro R, Salum A. Importance of roasted sulphide concentrates characterization in the hydrometallurgical extraction of zinc [J]. Minerals Engineering, 2008, 21: 100-110.
[4] Kim B S, Jeong S B, Kim Y H, Kim H S. Oxidative roasting of low grade zinc sulfide concentrate from Gagok mine in Korea [J], Materials Transactions, 2010, 51(8): 1481-1485.
[5] SOHN H Y, OLIVAS-MARTINEZ M. Lead and Zinc Production [M]//SEETHARAMAN S. Treatise of Process Metallurgy Vol. 3, Chapter 23. Elsevier Ltd., 2014.
[6] Zhao H, Gan X, Wang J, Tao L, Qin W, Loui G. Stepwise bioleaching of Cu-Zn mixed ores with comprehensive utilization of silver-bearing solid waste through a new technique process [J]. Hydrometallurgy, 2017, 171: 374-386.
[7] Igiehon U O, Heathcote S, Terry B S, Grieveson P. Carbothermic reduction of lead sulphide in the presence of lime [J]. Trans Inst Min Metall Sect C, 1992, 101: 159-163.
[8] Igiehon U O, Terry B S, Grieveson P. Carbothermic reduction of antimony sulphide [J]. Trans Inst Min Metall Sect C, 1992, 101: 144-154.
[9] Prasad P M, Mankhand T R, Suryaprakash P. Lime-scavenged reduction of molybdenite [J]. Minerals Engineering, 1993, 6(8-10): 857-871.
[10] Padilla R, Ruiz M C, Sohn Y H. Reduction of molybdenite with carbon in the presence of lime [J]. Metallurgical and Materials Transactions B, 1997, 28: 265-274.
[11] Padilla R, Ruiz M C. Kinetics of the lime enhanced reduction of Cu2S with carbon [J]. Canadian Metallurgical Quarterly, 2001, 40(2): 169-178.
[12] Hara Y S R. Mineral sulphide-lime reactions and effect of CaO/C mole ratio during carbothermic reduction of complex mineral sulphides [J]. International Journal of Minerals, Metallurgy and Materials, 2014, 21(1): 1-11.
[13] Padilla R, Chambi L C, Ruiz M C. Antimony production by carbothermic reduction of stibnite in the presence of lime [J]. Journal of Mining and Metallurgy, Sect B-Metall, 2014, 50(1): 5-13.
[14] Amini A, Maeda T, Ohno K, Kunitomo K. Carbothermic reduction behavior of FeS in the presence of CaO during microwave irradiation [J]. ISIJ International, 2019, 59(4): 672-678.
[15] HUANG C H, LIN C I, CHEN H K. Carbothermic reduction of zinc sulfide in the presence of calcium oxide [J]. Journal of Material Science, 2005, 40: 4299-4306.
[16] HUANG C H, LIN C I, CHENH K. Kinetics of the carbothermic reduction of zinc sulfide in the presence of calcium oxide [J]. Journal of the Chinese Institute of Chemical Engineers, 2007, 38: 143-149.
[17] WU C M, LIN C I, CHEN H K. Carbothermic reduction of zinc sulfide in the presence of calcium carbonate [J]. Metallurgical and Materials Transactions B, 2006, 37(3): 339-347.
[18] ABRAMOWITZ H, RAO Y K. Direct reduction of zinc sulfide by carbon and lime [J]. Trans Inst Min Metall Sec C, 1978, 87: 180-188.
[19] IGIEHON U O, TERRY B S, GRIEVESON P. Formation of ZnCaOS during heat-treatment in Zn-Ca-O-S system and its role in carbothermic reduction of zinc-sulfide in presence of lime [J]. Trans Inst Min Metall Sec C, 1992, 101: 155-158.
[20] PETROVA S A, MAR’EVICH V P, ZAKHAROV R G, SELIVANOV E N, CHUMAREV V M, UDOEVA L Y. Crystal structure of zinc calcium oxysulfide [J]. Doklady Chemistry, 2003, 393(1-2): 255-258.
Rafael PADILLA, Juan Yamid CARVALLO, Maria Cristina RUIZ
Metallurgical Engineering Department, University of Concepcion, Concepcion 4070371, Chile
摘 要:采用热重法研究石灰存在条件下铁闪锌矿的直接碳热还原,以确定生产Zn(g)而没有SO2(g)污染的技术可行性。部分反应的样品X射线衍射分析表明,还原反应是通过形成ZnCaOS和Ca2Fe2O5中间产物进行的,最终产物为Zn(g)、固体Fe和CaS。温度对还原速率存在重要影响。在1100 °C下,对于300 mg (Zn,Fe)S:CaO:C比为1:1:1的样品,10 min左右可实现铁闪锌矿的完全转化。用ln(1-X)=-kt模型分析总还原反应的动力学,发现在1000~1150 °C、转化率不超过0.95时,该模型很好地描述试验数据,在此温度范围内确定的活化能为257 kJ/mol。研究结果证实该方法生产Zn(g)而不产生有害的SO2(g)排放的技术可行性。
关键词:动力学;铁闪锌矿;高铁闪锌矿;碳热还原;直接还原;ZnCaOS
(Edited by Bing YANG)
Corresponding author: Rafael PADILLA; E-mail: rpadilla@udec.cl
DOI: 10.1016/S1003-6326(20)65377-8

