
Effect of temperature on oxidation behavior of
ternary Fe-15Cu-5Al alloy in pure oxygen
XIANG Jun-huai(向军淮)1, 2, ZHAO Qiong(赵琼)2, ZHANG Wen-hua(张文华)2
1. Jiangxi Key Laboratory of Surface Engineering, Jiangxi Science and Technology Normal University,
Nanchang 330013, China;
2. School of Materials Science and Engineering, Jiangxi Science and Technology Normal University,
Nanchang 330013, China;
Received 15 July 2007; accepted 10 September 2007
Abstract: The effect of temperature on oxidation behavior of ternary Fe-15Cu-5Al (mole fraction, %) alloy in pure oxygen was studied. Fe-15Cu-5Al alloy presents an irregular high-temperature oxidation behavior between 700-1 000 ℃, though the kinetic curve at each temperature can be approximately considered as being composed of two quasi-parabolic stages. At 700 ℃ the alloy forms bulky stratified scales. On the contrary, at 800 ℃ the alloy forms an external protective Al2O3 layer. The trend of decrease of oxidation rate does not continue at elevated temperatures. Due to the high stress-growth effect at 900 ℃, the thin Al2O3 layer cannot completely prevent further oxidation of the alloy underneath. When the temperature rises to 1 000 ℃, the high stress-growth effect is more obvious and the alloy forms overgrown large nodules. Compared with the Fe-10Al binary alloy, the presence of 15 % Cu tends to greatly increase the flaw of the formed Al2O3 layer at elevated temperatures, resulting in a peculiar oxidation pattern of Fe-15Cu-5Al alloy.
Key words: Fe-15Cu-5Al; oxidation behavior; stress-growth
1 Introduction
The advantage that aluminum imparts to the oxidation resistance of multi-component alloys at elevated temperature is well known and equally well documented[1-3]. Metallic materials for high temperature applications are often multiphases, mainly to improve their mechanical properties, such as strength and creep resistance[4]. During the last few years, the oxidation mechanism of complex alloys has been investigated frequently due to their practical importance[5-8]. However, effect of temperature on oxidation behavior of multi-component alloys has seldom been studied.
Fe-Cu-Al ternary alloy is considered typical model systems useful for the investigation of the oxidation mechanisms of ternary two-phase alloys[9-10]. In contrast with the common trend of forming protective alumina easily at elevated temperature for most alloys, Fe-15Cu-5Al ternary alloy shows an irregular oxidation behavior. In the present work, the effect of temperature on oxidation behavior of ternary Fe-15Cu-5Al was investigated to clarify the complex effect of the presence of a limited copper on the oxidation.
2 Experimental
Fe-15Cu-5Al alloy was prepared by repeated melting appropriate amounts of the three pure components(99.8%) under a Ti-gettered inert atmosphere using non-consumable tungsten electrodes. The alloy ingot was subsequently annealed at 900 ℃ for 24 h in pure argon atmosphere to remove residual mechanical stresses and to achieve a better equilibration of the alloy phases.
In agreement with the phase diagram of ternary Fe-Cu-Al system at 25 ℃(Fig.1)[11], at room temperature the alloy Fe-15Cu-5Al (nominal composition) is composed of a mixture of two phases, including a Cu-rich solid solution and a Fe-rich solid solution. The situation remains the same at 700-1 000 ℃.

Fig.1 Phase diagram of ternary Fe-Cu-Al system at 25 ℃
Samples with dimensions of 12 mm×6 mm×1.2 mm were oxidized at 700-1 000 ℃ under 1×105 Pa flowing pure O2. Continuous mass change measurements were carried out by a Cahn Versatherm TGA system for 24 h.
X-ray diffraction (XRD) analysis was performed on the resulting specimen without stripping the oxide film. The corroded specimens were mounted in a cold-setting epoxy resin for examining the cross-section by scanning electron microscopy (SEM). The distribution of the elements in the scales was determined by EDS.
3 Results and discussion
3.1 Scaling kinetics
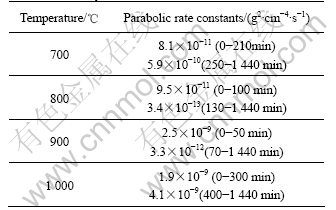
The kinetic curve and the corresponding parabolic plot for the oxidation of individual sample of Fe-15Cu-5Al alloy at 700-1 000 ℃ are shown in Figs.2 (a) and (b), respectively. Fe-15Cu-5Al presents an irregular oxidation behavior between 700-1 000 ℃, though the kinetic curve at each temperature can be approximately considered being composed of two quasi-parabolic stages. Approximate values of the mass-gain vs the square root of time, are listed in Table 1. On the whole, Fe-15Cu-5Al alloy oxidizes very slowly at 800 ℃, but very fast at 1 000 ℃. The alloy oxidizes much faster at 700 ℃ than at 800 ℃. Compared with the oxidation at 700 ℃, the alloy oxidizes faster at 900 ℃ in the first parabolic stage, but much slowly thereafter. In addition, at 900 ℃ the parabolic rate constant increases more than one order of magnitude than that at 800 ℃. On the whole, the scaling kinetics at 700-1 000 ℃ does not follow a regular pattern.
3.2 Scale microstructure and composition
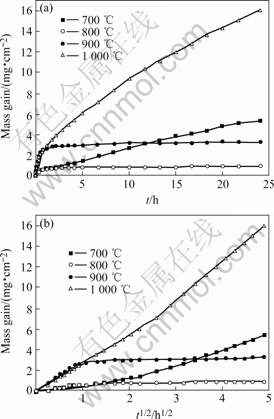
Fig.2 Oxidation kinetics of Fe-15Cu-5Al alloy under 0.1 MPa O2 at 700-1 000 ℃: (a) Normal plots; (b) Parabolic plots
Table 1 Approximate parabolic rate constants for oxidation of Fe-15Cu-5Al alloy at 700-1 000 ℃
Recently, the scaling behavior of Fe-15Cu-5Al alloy in pure O2 at different temperatures has been investigated in Refs.[9, 12-13]. The scales grown on Fe-15Cu-5Al alloy at 700-1 000 ℃ are shown in Fig.3, respectively. At 700 ℃ the alloy forms bulky stratified scales, which are composed of an outer layer of iron oxides and an inner layer containing a mixture of copper metal, iron and aluminium oxide[13]. On the contrary, at 800 ℃ the alloy forms an external protective Al2O3 layer[9]. At 900 ℃, the thin Al2O3 layer cannot completely prevent further oxidation of the alloy underneath and the alloy produces outer thin scales of iron oxides[12]. When the temperature rises up to 1 000 ℃, the alloy forms overgrown large nodules, resulting in serious corrosion of the alloy. In addition, microstructure and composition of the nodules are very similar to those of the scales formed at 700 ℃, with formation of external scales of iron oxides and internal alloy-consumption region characterized by the same spatial distribution of the copper islands.

Fig.3 Micrographs (SEM/BEI) of cross sections of Fe-15Cu-5Al alloy oxidized in 0.1 MPa O2 at different temperatures for 24 h: (a) 700 ℃; (b) 800 ℃; (c) 900 ℃; (d) 1 000 ℃
3.3 Discussion
The oxidation behavior of the binary Fe-Al alloys is briefly summarized before considering that of the ternary Fe-15Cu-5Al alloy. A series of tests were carried out to determine the effect of aluminum content and temperature on the oxidation behavior of Fe-Al alloys by SAEGUSA and LEE[14]. At any given temperature the oxidation of Fe-Al alloys decreases with increasing aluminum content. The Fe-1%Al(mass fraction) alloy shows increasing mass gain with increasing temperature, while the Fe-5%Al(mass fraction) alloy shows that the points of mass gain at 900 and 1 000 ℃ in the figure fall below those for 700 and 800 ℃[14]. As summarized by PRESCOTT and GRAHAM[15], the oxidation behavior of Fe-Al alloys is influenced by temperature, oxygen pressure and aluminum content. It has been proposed that for a protective behavior at least 8%Al(mass fraction) is necessary at 800 ℃[16], while 10%-12%Al(mass fraction) is required at 600 ℃[15]. At 1 000 ℃, 5%Al(mass fraction) is sufficient to form an external protective scale. Around 800 ℃, alloy with 2.5%Al(mass fraction) can form alumina scales which, however, are not fully protective since they may permit the development of nodular growths of bulky iron oxide, associated with a large increase in the oxidation rate[15].
Compared with the oxidation behavior of Fe-Al alloys at 700-1 000 ℃, Fe-15Cu-5Al alloy presents a rather irregular behavior. The effect of temperature on oxidation behavior of ternary Fe-15Cu-5Al alloy is not similar to that of the corresponding Fe-Al alloys.
Mass gain of oxidation of Fe-15Cu-5Al alloy at 700-1 000 ℃ are compared with those of Fe-10Al (Table 2). At 700-800 ℃ Fe-10Al alloy presents increasing mass gain with increasing temperature since the protective Al2O3 layer is not fully formed. The protective Al2O3 layer tends to form fully at higher temperatures, especially when the temperature rises up to 900 ℃ or even 1 000 ℃. Therefore, Fe-10Al alloy presents decreasing mass gain with increasing temperature when the temperature is above 800 ℃.
The effect of temperature on oxidation behavior of Fe-15Cu-5Al alloy is more complex than that of Fe-10Al alloy. The presence of 5 % Al is not enough to form external Al2O3 layer on Fe-15Cu-5Al alloy at 700 ℃. Therefore, at 700 ℃ Fe-15Cu-5Al alloy corrodes much more quickly than Fe-10Al alloy, which forms mixtures of Fe2O3 and Al2O3. When the temperature rises up to 800 ℃, Fe-15Cu-5Al alloy shows fully protective behavior, with formation of scales mainly composed of Al2O3, since the diffusion of aluminium is much more rapid in this case. The presence of 5%Al significantly reduces the oxidation rate of this alloy with respect to a binary Fe-Cu alloy of similar composition[9]. Mass gain of oxidation of Fe-15Cu-5Al alloy at 800 ℃ for 24 h is even much smaller than that of Fe-10Al alloy, whose Al content is, however, much higher. Therefore, the addition of 15% Cu must be able to reduce the critical aluminium content needed to form alumina scales with respect to binary Fe-Al alloys. This is mainly a result of the effects of the presence of copper on the solubility and diffusivity of oxygen in the two-phase Fe+Cu matrix of the region of internal oxidation, of the decrease of the growth rate of the external scales with respect to the oxidation of pure iron and of an increase of the effective diffusion coefficient of aluminum in the ternary-alloy matrix[9]. When the temperature rises to 900 ℃, the oxidation rate of Fe-15Cu-5Al alloy does not decrease further as expected on the case of Fe-10Al alloy. On the contrary, at 900 ℃ the parabolic rate constant increases more than one order of magnitude. It is reasonable to deduce that with the increase of the temperature the addition of 15%Cu tends to increase greatly the flaw of the formed Al2O3 layer, which may experience great growth stress at high temperatures. In this case, the alloy may permit the development of bulky iron oxide, associated with a large increase in the oxidation rate. This situation is more obvious at 1 000 ℃, since Fe-15Cu-5Al alloy forms overgrown large nodules, resulting in serious corrosion. In contrast with Fe-15Cu-5Al alloy, oxidation of Fe-10Al alloy at 1 000 ℃ produces exclusive Al2O3 protective layer and the oxidation rate is very low.
Table 2 Mass gains of oxidation of Fe-15Cu-5Al alloy and Fe-10Al (Fe-5%Al, mass fraction) after at 700-1 000 ℃ for 24 h

4 Conclusions
The effect of temperature on oxidation behavior of Fe-15Cu-5Al alloy does not follow the regular pattern of Fe-10Al alloy. There are two opposite aspects of the effect of temperature: when it increases, the protective Al2O3 layer tends to form easily since the diffusion of aluminium is more rapid; however, at the same time the Al2O3 layer suffers much higher growth stress at elevated temperatures, resulting in the break of the protective behavior. For the oxidation of Fe-15Cu-5Al alloy, at 700-800 ℃ the former factor predominates, while at 900-1 000 ℃ the latter one predominates. Since for the corresponding binary Fe-Al alloy the former factor always predominates, the presence of 15%Cu in Fe-15Cu-5Al is assumed to greatly increase the flaw of the formed Al2O3 layer at elevated temperatures. When the temperature rises up to 900-1 000 ℃, the protective Al2O3 layer tends to suffer break, resulting in a much higher oxidation rate.
References
[1] CHEN G F, LOU H Y. The effect of nanocrystallization on the oxidation resistance of Ni-5Cr-5Al alloy[J]. Scripta Materialia, 1999, 41: 883-887.
[2] LIU Z Y, Gao W, Li M S. Cyclic oxidation of sputter-deposited nanocrystalline Fe-Cr-Ni-Al alloy coatings[J]. Oxid Met, 1999, 51: 403-419.
[3] GLEEON B, CHEUNG W H, YOUNG D J. Cyclic oxidation of behavior of two-phase Ni-Cr-Al alloys at 1 000 ℃[J]. Corros Sci, 1993, 35: 923-929.
[4] SIMS C T, STOLOFF N S, HAGEL W C. Superalloys II[M]. New York: Wiley, 1987.
[5] WANG S Y, GESMUNDO F, WU W T, NIU Y. A non-classical type of third-element effect in the oxidation of Cu-xCr-2Al alloys at 1 173K[J]. Scripta Materialia, 2006, 54: 1563-1568.
[6] WU Y, NIU Y. Effect of silicon additions on the oxidation of a Ni-6%Al alloy at 1 273K[J]. Scripta Materialia, 2005, 53: 1247-1252.
[7] ZHANG Z G, GESMUNDO F, HOU P Y, NIU Y. Criteria for the formation of protective Al2O3 scales on Fe-Al and Fe-Cr-Al alloys[J]. Corros Sci, 2006, 48: 741-765.
[8] CAO Z Q, SHEN Y, WANG C J, LIU W H. Oxidation of a quaternary three-phase Cu-20Ni-20Cr-5Fe alloy at 700-900 ℃ in 1atm of pure O2[J]. Corros Sci, 2007, 49: 2450-2460.
[9] XIANG J H, NIU Y, GESMUNDO F. The oxidation of two ternary Fe-Cu-5%Al alloys in 1atm of pure O2 at 800 ℃[J]. Oxid Met, 2004, 61: 403-423.
[10] XIANG J H, NIU Y, GESMUNDO F. The oxidation of two ternary Fe-Cu-10%Al alloys in 1atm of pure O2 at 800-900 ℃[J]. Corros Sci, 2005, 47: 1493-1505.
[11] VILLARS P, PRINEC A, OKAMOTO H. Handbook of ternary alloy phase diagrams[M]. Materials Park, USA: ASM International, 1997.
[12] XIANG Jun-huai, NIU Yan, DUAN Xian-zhi. Oxidation behavior of ternary Fe-15Cu-5Al and Fe-85Cu-5Al alloys in pure oxygen at 900 ℃[J]. Trans Nonferrous Met Soc China, 2006, 16: s2038-s2041.(in Chinese)
[13] XIANG Jun-huai, NIU Yan, WU Wei-tao. Oxidation of two ternary Fe-Cu-5Al alloys in 1×105 Pa pure oxygen at 700 ℃[J]. Trans Nonferrous Met Soc China, 2006, 16: s829-s833. (in Chinese)
[14] SAEGUSA F, LEE L. Oxidation of iron-aluminum alloys in the range 500-1 000 ℃[J]. Corrosion, 1966, 22: 168-177.
[15] PRESCOTT R, GRAHAM M J. The oxidation of iron-aluminium alloys[J]. Oxid Met, 1992, 38: 73-87.
[16] TOMASZEWICZ P, WALLWORK G R. Observation of nodule growth during the oxidation of pure binary iron-aluminum alloys[J]. Rev High Temp Mater, 1978, 4: 165-185.
Foundation item: Project ([2005]224) supported by Science and Technology Research Item of Jiangxi Provincial Department of Science and Technology
Corresponding author: XIANG Jun-huai; Tel: +86-791-3831266; E-mail: xiangjunhuai@163.com
(Edited by CHEN Wei-ping)