
Self-assembling organomodified Co/Al based
layered double hydroxides (LDH) via one-step route
WANG De-yi(王德义)1, 2, 3, A. LEUTERITZ3, U. WAGENKNECHT3, G. HEINRICH3
1. Center for Degradable and Flame-Retardant Polymeric Materials (ERCEPM-MoE),
College of Chemistry, Sichuan University Chengdu 610064, China;
2. State Key Laboratory of Polymer Materials Engineering, Sichuan University, Chengdu 610064, China;
3. Leibniz Institute of Polymer Research, Dresden D01069, Germany
Received 10 August 2009; accepted 15 September 2009
Abstract: The preparation of self-assembling organomodified Co/Al-layered double hydroxide (LDH) via one-step route was studied. A common surfactant, sodium dodecylbenzenesulfonate (DBS), was employed as an organic modifier. The behavior and structure of self-assembled intercalated organic Co/Al-LDH were investigated by FTIR, SEM, WAXS, element analysis and TGA. Based upon the WAXS results and calculation by Bragg equation, the interlayer distance (d value) for organic Co/Al-LDH is enlarged from 0.75 nm to 3.10 nm, showing that the self-assembling behavior has been carried out successfully. Considering the observation from SEM, the product shows the morphology of organic Co/Al-LDH of a layered structure. In addition, FTIR, element analysis and TGA analysis show that the modifier is intercalated into the gallery of the Co/Al-LDH. Since organic modification for nanofiller is deemed to be necessary before applying it into polymer, the successful preparation of organomodified Co/Al-LDH will be significantly beneficial to the preparation and investigation of novel polymer/LDH nanocomposite.
Key words: layered double hydroxide (LDH); self-assembling; Co/Al; nanostructure
1 Introduction
Layered double hydroxides (LDH) as a layered nanostructure emerged in recent years have drawn enormous attention among researchers on novel nanofiller for polymers due to their various unique properties not common in layered silicates[1]. LDH can be represented by the formula [M2+1-xM3+x(OH)2]An-x/n- yH2O, where M2+ and M3+ are divalent and trivalent metal cations, such as Mg2+ and Al3+, respectively, A is an anion, such as CO32-, Cl- and NO3-. LDHs can be described as host–guest materials consisting of positively charged metal hydroxide sheets with intercalated anions and water molecules[2]. In principal, the intercalated anions can be exchanged with wide variety of anionic species both of organic and inorganic, indicating that there is a wide field of potential applications of LDH materials, for example, as catalysts[3], vehicles for drug delivery and gene therapy[4], flame retardants[5], and (nano)filler for polymer (nano)composite materials[6-7].
Usually, LDH needs to be organomodified by some surfactants before employment as nanofiller in polymer to obtain a good dispersion in polymer matrices. To modify LDH, two methods are often used, which are ion-exchange method and regeneration method. The direct ion-exchange method is only of limited value as especially LDH with high crystallinity derived mainly in carbonate form is not successfully anion exchanged with organic anions[8]. For regeneration method, a specific drawback is the large amount of energy required during calcination and time necessary for restructuring and separation. Furthermore, most researches on LDH focus on the Mg/Al-LDH concerning preparation, modification, application, etc, while the research of novel kinds of LDH is few regardless of the preparation or properties.
Actually, self-assembly has been employed widely as a new approach in chemical synthesis, nanotechnology, polymer science, materials science, and engineering [9-13]. In addition, in-situ approaches to prepare polymer nanocomposites have been reported in our recent work [14]. In our latest study, the preparation of functional LDH via incorporation self-assembly with in-situ method was researched[15].
In this study, the preparation of self-assembling organomodified Co/Al-LDH via one-step route is reported. A common surfactant, sodium dodecyl- benzenesulfonate (DBS), is employed as an organic modifier. The schematic illumination of self-assembling organomodified Co/Al-LDH via one step route is shown in Fig.1. The behavior and structure of self-assembled intercalated organic Co/Al-LDH are investigated by FTIR, SEM, WAXS, element analysis and TGA, respectively.
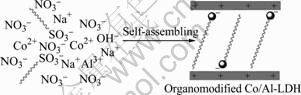
Fig.1 Schematic illumination of self-assembling organo- modified Co/Al- LDH
2 Experimental
2.1 Materials
The metal nitrate salts (Co(NO3)2 and Al(NO3)3), sodium dodecylbenzenesulfonate (DBS) used for self-assembly of organic Co/Al-LDH were obtained from Aldrich Chemical Company and used without further purification. Deionized water was used to dilute the solutions and wash the filtered precipitates.
2.2 Self-assembly organomodified Co/Al-LDH
Self-assembly synthesis was carried out by the slow addition of a mixed nitrates (0.3 mol/L in total) solution with a molar ratio of Co to Al of 2 into a DBS stoichometric solution under forcefully magnetic stirring. During the synthesis the pH value was kept at 9±0.2 by adding suitable amounts of 1 mol/L NaOH solution, maintaining the reaction temperature at 60 ℃. After the addition of the mixed nitrades solution, the resulting slurry was continuously stirred at the same temperature for 0.5 h and then was allowed to age in heater at 70 ℃ for 24 h. The final products were filtered and washed several times with distilled water to remove unreacted surfactant until the pH value of the supernatant solution was neutral, and then dried in oven at 60 ℃ overnight.
2.3 Characterization
Wide angle X-ray scattering (WAXS) was performed using 2-circle diffractometer XRD 3003 θ/θ (GE Inspection Technologies /Seifert-FPM, Freiberg) with Cu Kα radiation (λ=0.154 nm) generated at 30 mA and 40 kV in the range of 2θ=0.5-25? using 0.05? as the step length.
The Fourier transform infrared spectra (FTIR) of the LDH materials were obtained using the BRUKER VERTEX 80V spectrometer over the wave number range of 4 000-400 cm-1. The powdered samples were mixed with KBr and pressed in the form of pellets for the measurement of FTIR analysis.
The scanning electron microscopy (SEM) (microscope model: LEO 435 VP, Carl Zeiss SMT) was used to study morphological features of the powdered samples.
The thermogravimetric analysis (TGA) was done using a TA Instruments TGA Q 5000 in the range between room temperature and 800 ℃ at a heating rate of 10 K/min in nitrogen atmosphere. The elemental analysis was carried out with CHNS analyzer (varioMICRO V1.5.7).
3 Results and discussion
3.1 WAXS and SEM analyses
The WAXS is one of the powerful technologies to characterize the layered structure of nano-materials. The WAXS pattern of organic Co/Al-LDH synthesized by self-assembly (shown in Fig.2(a)) reveals that the modifier anion can be efficiently intercalated within the Co/Al-LDH layers by this method. For the unmodified Co/Al-LDH, the first basal reflection (003) at 2θ=11.8? corresponds to an interlayer distance of 0.75 nm[16].
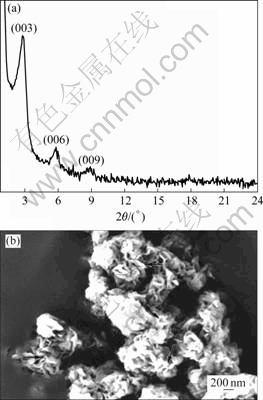
Fig.2 WAXS patterns (a) and SEM image (b) of Co/Al-DBS-LDH
The absence of any distinguishable reflection at this position in WAXS pattern of organomodified Co/Al-LDH indicates that no unmodified Co/Al-LDH phase is formed. It is found that three reflection peaks appear at 2θ=2.84?, 5.79?, 8.90? in the WAXS pattern, corresponding to the refections of (003), (006) and (009), respectively. Based on the calculation by Bragg equation, the basal reflections with d value of 3.10 nm for the organomodified Co/Al-LDH are obtained, suggesting that the modifier (DBS) has been intercalated successfully into the interlayer of Co/Al-LDH and thereby new series of intense basal reflection at lower 2θ instead. As a result of the introduction of DBS, the basal spacing of organic Co/Al-LDH increases from 0.75 to 3.10 nm, indicating that it is possible to prepare polymer nanocomposite.
The morphology of organomodified Co/Al-LDH is also investigated by SEM, as shown in Fig.2(b). From SEM image, a similar morphology is found for Co/Al-DBS-LDH and unmodified Co/Al-LDH with small differences due to the organic surfactant. The primary particles are plate-like without any defined shape and the edges are more irregular. This indicates that the modifier is not only intercalated into the interlayer of LDH, but also covers the outer surface of the plate-like LDH.
3.2 FTIR analysis
The FTIR spectra of the unmodified Co/Al-LDH and organomodified Co/Al-LDH are shown in Fig.3. First of all, the broad band in the range of 3 200-3 700 cm-1 originates from the O-H stretching of the metal hydroxide layer and interlayer water molecules of Co/Al-LDH; and the bending vibration of the interlayer H2O is also reflected in the broad bands around 1 627 cm-1. In comparison with that of unmodified Co/Al-LDH, the FTIR spectrum of the organomodified Co/Al-LDH reveals the presence of DBS anion in the materials. The characteristic vibration bands are detected for the SO3- group (symmetric stretching at 1 033 cm-1 and asymmetric at 1 186 cm-1), the benzene group (C-C stretching at 1 462 cm-1, C-H in plane bending at 1 001 and 1 136 cm-1) and alkyl group (asymmetric stretching of CH3 and CH2 at 2 963 and 2 925 cm-1, respectively; symmetric stretching of CH3 and CH2 at 2 872 and 2 851 cm-1, respectively). The bands recorded below 800 cm-1, especially the sharp and strong characteristic band around 426 cm-1 arise due to the vibration of metal-oxygen bond in the brucite-like lattice. It is noted that the characteristic vibration of NO3- at 1 380 cm-1 is absent in organomodified Co/Al-LDH, illuminating that a high degree of intercalation of DBS anion into the interlayer of Co/Al-LDH has been formed. These results are consistent with the results of WAXS.
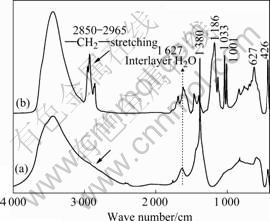
Fig.3 FTIR spectra of Co/Al-DBS-LDH (a) and Co/Al-LDH (b)
3.3 TGA and elemental analyses
The elemental analysis of unmodified Co/Al-LDH and organic Co/Al-LDH obtained by the one-step method along with their thermograms is shown in Fig.4. The results exhibit a very high intercalation of the DBS anions within Co/Al-LDH layers. The contents of C, H and S in organic Co/Al-DBS-LDH strongly prove that self assembling intercalation behavior is successful via one step route, compared with that of unmodified Co/Al-LDH.
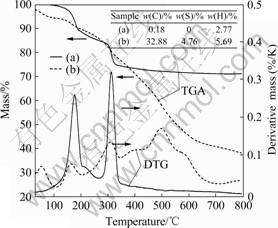
Fig.4 TGA and element analysis of Co/Al-LDH (a) and Co/Al-DBS-LDH (b) prepared via one-step method
The thermograms (TGA and DTG) of unmodified Co/Al-LDH presented in Fig.4 show two main steps in the thermal decomposition. The first decomposition step occurs at 120-200 ℃ with the decomposition peak around 180 ℃ corresponding to the loss of interlayer water molecules of Co/Al-LDH; the high temperature decomposition of unmodified Co/Al-LDH takes place with the decomposition peak around 310 ℃ which is relevant with the decomposition of metal hydroxide layer. In contrast, the thermal decomposition behaviors of organic Co/Al-LDH presents very complicated, showing multi-stages decomposition behavior. Besides the two same decomposition steps as those of the unmodified Co/Al-LDH discussed above, the mass loss below 100 ℃ is attributed to the water molecules adsorbed on the non-gallery surfaces of Co/Al-LDH, while the decomposition process above 400 ℃ could be the decomposition of DBS intercalated in the interlayer. The extent of intercalated DBS in the Co/Al-LDH is also reflected in the residue obtained, as Co/Al-DBS-LDH contains a high amount of intercalated DBS yielding a low amount of residue (38%) at 800 ℃ compared with the high amount of residue (71%) for unmodified Co/Al- LDH at the same temperature. This result is in accordance with the elemental analysis (the insert in Fig.4) and this is another evidence for the successful self assembling of organomodified Co/Al-LDH via one-step route.
4 Conclusions
1) One method of self assembly of Co/Al-LDH and surfactant modifier to prepare intercalated organomodified Co/Al-LDH is described. The common surfactant, sodium dodecylbenzene- sulfonate (DBS), was employed as an organic modifier. The intercalation behavior and structure of synthesized organomodified Co/Al-LDH were characterized by FTIR, SEM, WAXS, TGA and elemental analysis, and compared with pristine Co/Al-LDH.
2) The presence of modifiers in the interlayer of organomodified Co/Al-LDH was identified by means of FTIR analysis and elemental analysis, demonstrating that self-assembly was performed successfully. The WAXS experiments revealed that the interlayer distance was enlarged from 0.75 nm in unmodified LDH to 3.10 nm in organomodified Co/Al-LDH.
3) The morphological analysis showed a typical layered structure of LDH. In the meantime, the surface morphology in organic modified LDH indicated that the modifier was not only intercalated into the interlayer of LDH, but also covered the outer surface of the plate-like LDH. This facial approach for the preparation of organomodified Co/Al-LDH will be significantly beneficial to the preparation and investigation of novel polymer/LDH nanocomposite in the future.
References
[1] COSTA F R, GRENZR M, WAGENKNECHT U, HEINRICH G. Layered double hydroxide based polymer nanocomposites [J]. Advances in Polymer Science, 2008, 210: 101-168.
[2] YOU Y W, ZHAO H T, VANCE G F. Hybrid organic-inorganic derivatives of layered double hydroxides and dodecyl- benzenesulfonate: Preparation and adsorption characteristics [J]. J Mater Chem, 2002, 12 (4): 907-912.
[3] LI F, DUAN X. Applications of layered double hydroxides [J]. Structure Bond, 2006, 119: 193-223.
[4] DEL HOYO C. Layered double hydroxides and human health: An overview [J]. Appl Clay Sci, 2007, 36: 103-121.
[5] COSTA F R, WAGENKNECHT U, HEINRICH G. LDPE/Mg-Al layered double hydroxide nanocomposite: Thermal and flammability properties [J]. Polym Degrad Stab, 2007, 92: 1813-1823.
[6] COSTA F R, ABDEL-GOAD M, WAGENKNECHT U, HEINRICH G. Nanocomposites based on polyethylene and Mg-Al layered double hydroxide (I): Synthesis and characterization [J]. Polymer, 2005, 46: 4447-4453.
[7] ZHAO C X, LIU Y, WANG D Y, WANG D L, WANG Y Z. Synergistic effect of layered double hydroxide (LDH) on thermal and flame-retardant properties of poly (vinyl alcohol) [J]. Polym Degrad Stab, 2008, 93: 1323-1331.
[8] NOBUO I, TAKAYOSHI S. Deintercalation of carbonate ions and anion exchange of an Al-rich MgAl-LDH (layered double hydroxide) [J]. Appl Clay Sci, 2008, 42(1/2): 246-251.
[9] SUN Y Y, LIU Y Q, ZHAO G Z, ZHANG Q J. Assembly of silver nanoparticles into hollow spheres using Eu(III) compound based on trifluorothenoyl-acetone [J]. Nanoscale Res Lett, 2008, 3: 82-86.
[10] YUAN Y J, PYATENKO A T, SUZUKI M. Self-assembly of micelles into designed networks [J]. Nanoscale Res Lett, 2007, 2: 119-122.
[11] SUBRAMANI K, KHRAISAT A, GEORGE A. Self-assembly of proteins and peptides and their applications in bionanotechnology [J]. Current Nanoscience, 2008, 4(2): 201-207.
[12] ZHOU J L, WEI C J, TONG H X, CHUN X G, CHEN Q Y. Self-assembly synthesis, crystal structure and nonlinear optical properties of cluster compound containing PPh2Py ligand [J]. Trans Nonferrous Met Soc China, 2008, 18(3): 738-742.
[13] ZHENG M, WANG B. One-step synthesis of antimony-doped tin dioxide nanocrystallites and their property [J]. Trans Nonferrous Met Soc China, 2009, 19(2): 404-409.
[14] WANG D Y, SONG Y P, WANG J S, GE X G, WANG Y Z, STEC A A, HULL T R. Double in-situ approach for the preparation of polymer nanocomposite with multi-functionality [J]. Nanoscale Research Letter, 2009, 4: 303-306.
[15] WANG D Y, COSTA F R, VYALIKH A, LEUTERITZ A, SCHELER U, JEHNICHEN D, WAGENKNECHT U, HAUSSLER L, HEINRICH G. One-step synthesis of organic LDH and its comparison with regeneration and anion exchange method [J]. Chem Mater, 2009, 21(19): 4490-4497.
[16] LIU Z P, MA R Z, OSADA M, IYI N, EBINA Y, TAKADA K, SASAKI T. Synthesis, anion exchange, and delamination of Co-Al layered double hydroxide: Assembly of the exfoliated nanosheet/ polyanion composite films and magneto-optical studies [J]. J Am Chem Soc, 2006, 128: 4872-4880.
Foundation item: Project(50703026) supported by the National Natural Science Foundation of China; project(F/4285-1) supported by International Foundation for Science (IFS); project(20080440182, 200902615) supported by China Postdoctoral Science Foundation; project supported by Guest-Scientist Research Fellowship granted by Leibniz Institute of Polymer Research Dresden, Germany
Corresponding author: WANG De-yi; Tel/Fax: +86-28-85410755; E-mail: deyiwang@scu.edu.cn
DOI: 10.1016/S1003-6326(09)60055-8
(Edited by YUAN Sai-qian)