Preparation and photocatalytic performance of ZnO/ZnGa2O4 composite microspheres
来源期刊:中南大学学报(英文版)2016年第12期
论文作者:阎建辉 张丽 戴超华 张秀秀 刘又年
文章页码:3092 - 3099
Key words:ZnO/ZnGa2O4; photocatalytic performance; microspheres; simulated sunlight
Abstract: ZnO/ZnGa2O4 composite microspheres with heterojunction were successfully synthesized by one-pot hydrothermal method. These samples were characterized by TG/DTA, XRD, TEM, HRTEM, UV-vis DRS, FL and BET techniques. The results indicated the as-prepared samples showed better degree of crystalline and large specific surface area. The photocatalytic activity was evaluated by degradation of methyl orange with the concentration of 50 mg/L under the irradiation of simulated sunlight. The effects of molar ratio of Zn to Ga and calcination temperature on the photocatalytic activity were investigated in detail. The results showed that the highest photocatalytic degradation efficiency was observed at the molar ratio of Zn to Ga of 1:0.5 in the starting materials and the calcination temperature of 400 °C. The maximum photocatalytic degradation rate of MO was 97.1% within 60 min under the simulated sunlight irradiation, which is greatly higher than that of ZnO and ZnGa2O4.
J. Cent. South Univ. (2016) 23: 3092-3099
DOI: 10.1007/s11771-016-3374-8

ZHANG Li(张丽)1, DAI Chao-hua(戴超华)1, ZHANG Xiu-xiu(张秀秀)2,
LIU You-nian(刘又年)3, YAN Jian-hui(阎建辉)1, 2, 3
1. College of Chemistry and Chemical Engineering, Hunan Institute of Science and Technology,Yueyang 414006, China;
2. College of Chemical Engineering, Xiangtan University, Xiangtan 411105, China;
3. School of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China
 Central South University Press and Springer-Verlag Berlin Heidelberg 2016
Central South University Press and Springer-Verlag Berlin Heidelberg 2016
Abstract: ZnO/ZnGa2O4 composite microspheres with heterojunction were successfully synthesized by one-pot hydrothermal method. These samples were characterized by TG/DTA, XRD, TEM, HRTEM, UV-vis DRS, FL and BET techniques. The results indicated the as-prepared samples showed better degree of crystalline and large specific surface area. The photocatalytic activity was evaluated by degradation of methyl orange with the concentration of 50 mg/L under the irradiation of simulated sunlight. The effects of molar ratio of Zn to Ga and calcination temperature on the photocatalytic activity were investigated in detail. The results showed that the highest photocatalytic degradation efficiency was observed at the molar ratio of Zn to Ga of 1:0.5 in the starting materials and the calcination temperature of 400 °C. The maximum photocatalytic degradation rate of MO was 97.1% within 60 min under the simulated sunlight irradiation, which is greatly higher than that of ZnO and ZnGa2O4.
Key words: ZnO/ZnGa2O4; photocatalytic performance; microspheres; simulated sunlight
1 Introduction
Since FUJISHIMA and HONDA reported the evolution of oxygen and hydrogen from a TiO2 electrode under the irradiation of light in 1972 [1], TiO2, ZnO, and CdO have been widely used as photocatalysts for the photodegradation of organic or inorganic pollutants [2-4]. Especially, ZnO has been recognized as the excellent materials for photocatalytic process due to their high photosensitivity and nontoxic nature [5]. It was also reported that ZnO has been regard as an electron transport material due to its optical bandgap and its excellent electronic properties [6]. It is even more efficient than TiO2 in the photodegradation. However, the quick recombination of e- and h+ pairs is the major limitation in achieving high photocatalytic efficiency. Some reports point out that the composite between ZnO and another nonsensitive semiconductor (usually have very wide bandgap), e.g. In2O3 (3.6 eV) [7], SnO2 (3.8 eV) [8], and NiO (Eg 3.5 eV) [9] also has been proved to be an effective method to improve their photocatalytic activity. In particularly, the coupling of the bandgap structure of both ZnO and ZnAl2O4 phase in the ZnO/ZnAl2O4 nanocomposite ensured the efficient separation of photogenerated e- and h+ pairs and it might also even more realize the enhancement of photocatalytic activity from UV-light to visible light [10]. With a broad bandgap of 4.4 eV [11], spinel ZnGa2O4 has been attracting considerable attention since it can be used in many fields, such as fluorescent material [12], degradation of pollutants [13], photoreduction of CO2 [14]. In particular, the particle size and crystal morphology of samples prepared by hydrothermal method could be well controlled [15].
Herein, ZnO/ZnGa2O4 composite microspheres with heterojunction were prepared by one-pot hydrothermal method. The structure, composition, morphology, texture, UV-vis absorbing properties of the resulting composites were investigated by TEM, XRD, BET, and UV-vis spectra techniques. Subsequently, the photocatalytic activities of the ZnO/ZnGa2O4 composites were evaluated by the degradation of MO and a possible mechanism was discussed.
2 Experimental
2.1 Sample preparation
ZnO, ZnGa2O4 and ZnO/ZnGa2O4 samples were prepared by one-pot hydrothermal method [10]. In a typical synthetic produce, a mixture of Zn(NO3)2·6H2O and Ga(NO3)3·xH2O with a certain of Zn to Ga molar ratio (varying from 0.5 to 2 ) in the starting materials was dissolved into deionized water to form a solution with a total cationic concentration of 0.3 mol/L, and polyethylene glycol (PEG, as a dispersion agent, ensuring that the content of it in catalysts was 10%) was subsequently added with constant stirring to the above solution until complete dissolution. Then, aqueous ammonia was added into the mixture solution under magnetic stirring until the pH reached 8-10, respectively, and a certain amount of glucose ( as a template agent ) was dissolved in the above solution to ensure the ratio of glucose to metal ion to be 1:1. After mixing, the reaction solution was transferred into a 200 mL Teflon-lined autoclave. The autoclave was sealed and kept at 180 °C for 24 h, and then cooled to the room temperature naturally. After filtration and washing with distilled water and ethanol for several times, the obtained sample was dried in oven at 80 °C for 6 h. Finally, the catalysts were obtained after calcination at different temperatures (300 °C, 400 °C and 500 °C).
2.2 Sample characterization
The crystalline phase and the crystal size of the samples were identified by X-ray diffraction (XRD, Rigaku, Cu Kα, λ=0.15418 nm). The changes both weight and heat along with the increasing temperature were studied by Thermogravimetry-Differential Thermal Analysis (TG/DTA, Perkin Elmer, TAC 7/DX). Transmission electron microscopy (TEM) images were recorded on JEOL JEM-2010 transmission electron microscope. UV-vis diffused reflectance spectra of the samples were obtained using UV-vis spectrophotometer (UV-2550, Shimadzu, Japan). BaSO4 was used as a reflectance standard in the UV-vis diffused reflectance experiment. The electron-hole separation-recombination characteristics of the samples were determined with Fluorescence spectrophotometer (FL, LS-55, Perkin Elmer). Brunauer-Emment-Teller (BET) surface areas (SBET) were measured by the nitrogen absorption isotherms apparatus (ST-08 analyzer).
2.3 Measurement of photocatalytic activity
The photocatalytic reaction was carried out in homemade tube shaped quartz reactor including three layers connected with gas collecting devices, and water was used as the external circulation cooling and wind as the internal cooling. The reaction temperature was kept at (25±0.2) °C by controlling the external circulation water in the water jacket of the reactor during the entire experiment. The photocatalyst powder (0.3 g) was dispersed by a magnetic stirrer in a 600 mL MO solution with the concentration of 50 mg/L. A 150 W xenon lamp with λ=200-900 nm was used as the simulated sunlight source. The luminous intensity was measured at 100 mW/m2 by the auto-range ST-85 optical radiometer (Photoelectric Instrument Factory of Beijing Normal University). Prior to light illumination, the suspension was strongly magnetically stirred for 30 min in the dark for adsorption/desorption equilibrium. During irradiation, the catalyst was kept in suspension state by a magnetic stirrer. Samples for analysis were extracted through pipette every 10 min and centrifuged immediately. Absorbance of the suspension and initial solution was determined, respectively. A 752 UV-vis spectro- photometer was used at the maximum absorption wavelength (465 nm) of MO. Degradation rate are presented as C/C0, where C0 and C are the initial concentration of MO under adsorption equilibrium and the concentration of MO at different irradiation time, respectively. Each set of photocatalytic measurements was repeated three times, and the experimental error was found to be within ±5%.
3 Results and discussion
3.1 Characterization
3.1.1 XRD study
Figure 1 shows XRD patterns of ZnO/ZnGa2O4 samples prepared with different molar ratios of Zn to Ga at 400 °C for 4 h. Figure 1(a) shows the typical XRD pattern of the as-prepared ZnO. All diffraction peaks can be indexed to wurtzite structure of ZnO, which is in good agreement with the literature values (JCPDS Card, No. 36-1451). The sharp diffraction peaks indicate the good crystallinity of the synthesized ZnO. No peaks of other impurities are appeared in the patterns. Figures 1(b)–(d) shows that all the patterns of ZnO/ZnGa2O4 are very similar. The intensity of ZnO peaks decreases gradually with decreasing Zn to Ga. This result can be attributed to the fact that the amount of ZnO becomes lower than that of ZnGa2O4 and main diffraction peaks of ZnO are very close to those of ZnGa2O4 and covered by the diffraction peaks of ZnGa2O4. It is obvious that the main diffraction peaks of the (220), (311), (400), (422), (511) and (440) reflections are observed in Fig. 1(e), which can be indexed to spinel ZnGa2O4 (JCPDS Card No. 38-1240). The fact that no peak characteristic of any other phase or impurity is observed in the XRD pattern implies high purity of ZnGa2O4 crystalline phase synthesized in this work. This result is in agreement with the reported data [16-17].

Fig. 1 XRD patterns of ZnO/ZnGa2O4 with different molar ratios of Zn to Ga:
Figure 2 indicates XRD patterns of ZnO/ZnGa2O4 samples prepared with the starting Zn to Ga molar ratio of 1:0.5 calcined at different temperatures. We can clearly see that all the patterns of samples are very similar. The reflections at 2θ=31.8°, 34.4°, 36.2°, 47.6°, and 56.6° can be indexed to hexagonal wurtzite structure ZnO (JCPDS 36-1451), whereas the main diffraction peaks of the (220), (311), (400) can be indexed to ZnGa2O4. The sample shows a mixed phase of ZnO/ZnGa2O4. However, the peak intensities of samples are found to enhance with the increase of calcination temperature. When the temperature is increased to 500 °C, the peaks become sharp. The difference may be attributed to the sintering of the oxides and the degree of crystallinity.

Fig. 2 XRD patterns of ZnO/ZnGa2O4 samples calcined at different temperatures:
3.1.2 TG-DTA analysis
Figure 3 shows the TG-DTA curves of ZnO/ZnGa2O4 sample with the starting Zn to Ga molar ratio of 1:0.5. From the curves, we can see that the total mass loss occurs in the temperature range of 25-550 °C. The first mass loss about 6% before 110 °C is mainly attributed to the evaporation of absorbed water. The second mass loss about 2% occurs between 110 °C to 300 °C, which is due to the loss of the crystal water of the catalysis. The third mass loss at 300-550 °C is due to the decomposition of nitrate, oxidation of PEG, carbonization of organics, dehydration of hydroxide, and the combination of oxide. Meanwhile, the corresponding endothermic peak is observed from the thermo- gravimetric curve. The mass loss becomes almost constant when the temperature is more than 550 °C. The corresponding endothermic peak is perhaps owing to the forming of oxide.

Fig. 3 TG-DTA curves for precursor for ZnO/ZnGa2O4 sample
3.1.3 TEM and HRTEM images
Figure 4 shows the TEM and HRTEM images of ZnO/ZnGa2O4 sample with the starting Zn to Al molar ratio of 1:0.5 calcined at 400 °C. Figure 4(a) shows that the sample is irregularly spherical and its particle size is relatively identical. The particle size of sample is 10-20 nm. More detailed morphology about the ZnO/ZnGa2O4 composites is indicated by HRTEM (Fig. 4(b)). HRTEM image reveals that both ZnO and ZnGa2O4 form a heterojunction nanostructure proven by well-defined lattice fringes: the spacing of 0.26 nm represents the lattice-resolved (001) crystalline plane of ZnO phase, and the spacing values of 0.253 nm correspond to the (311) facets of ZnGa2O4 phase, which are in good agreement with the report in Refs.[18-19]. EDS (Fig. 4(c)) measurement also indicates that the sample is composed of only Zn, Ga, O in addition to the C peaks from the carbon support.
3.1.4 UV-vis diffuse reflectance spectra
The diffuse reflectance spectrum of the synthesized ZnO/ZnGa2O4 sample with the starting Zn to Ga molar ratio of 1:0.5 calcined at 400 °C is presented in Fig. 5. For comparison, the spectra of pure ZnO and ZnGa2O4 synthesized are also plotted. The UV-vis diffusion reflectance spectrum (Fig. 5(c)) of as-obtained ZnO/ZnGa2O4 displays a significant increase in the absorption at wavelength ≤400 nm compared with ZnGa2O4 (Fig. 5(b)). Furthermore, it can be seen that ZnO/ZnGa2O4 sample shows a certain absorption in the visible light region (the wavelength ranging from 400 to 800 nm), while almost no absorption can be observed for pure ZnO (Fig. 5(a)). This experimental result can be attributed to the coupling interaction between the ZnO and ZnGa2O4 phase, the optimizing content of ZnO and the form of heterojunction structure, which leads to the enhancement of utilization of light and photocatalytic activity [20-21].

Fig. 4 TEM image (a), HRTEM image (b) and EDS spectrum (c) of ZnO/ZnGa2O4 sample
3.1.5 Fluorescence analysis
To investigate the recombination rate of the photogenerated electron–hole pairs, the fluorescence emission spectra of ZnO/ZnGa2O4 and ZnGa2O4 are shown in Fig. 6. Compared with ZnGa2O4 (Fig. 6(a)), the relatively low FL intensity of ZnO/ZnGa2O4 (Fig. 6(b)) suggests that it has the low recombination rate of electron–hole pairs [22-23], which results in the highest photocatalytic activity, as shown in Fig. 8.

Fig. 5 UV-vis diffusion reflectance spectra of ZnO (a), ZnGa2O4 (b) and ZnO/ZnGa2O4 (c) samples

Fig. 6 Fluorescence spectra of ZnGa2O4 (a) and ZnO/ZnGa2O4 (b) samples
3.1.6 BET surface area
Table 1 gives the BET surface area, pore volume, and average pore size of ZnO/ZnGa2O4 samples prepared with the starting Zn to Ga molar ratio of 1:0.5 calcined at different temperatures. With the increase of calcination temperature, the BET surface area decreases from 90.87 m2/g to 61.60 m2/g, while the pore size increases from 12.54 nm to 18.9 nm. The difference may be concluded that increasing the calcination temperature will lead to the aggregation of crystalline particles, thereby decreasing the surface area and increasing the pore size [24]. Although the sample calcined at 300 °C has a large BET surface area and strong adsorption, but weak crystallinity, which may result in relatively low photocatalytic activity. In addition to the specific surface area, the pore size distribution has also significant influence on the adsorption because it may affect the mass transport [25].
Figure 7 shows the N2 adsorption–desorption isotherm and the corresponding pore-size distribution curve for the ZnO/ZnGa2O4 samples prepared with the starting Zn to Ga molar ratio of 1:0.5 calcined at different temperatures. Figure 7(a) shows that all the samples display the type IV isotherms with H3 type hysteresis according to Brunauer–Deming–Deming– Teller (BDDT) classification with two capillary condensation steps, implying bimodal pore size distributions mainly in the mesoporous regions [26]. With calcination temperatures increasing, the shapes of nitrogen adsorption and desorption isotherms underwent obvious changes, implying a significant variation of pore structures. Firstly, the isotherms corresponding to the samples calcined at 400 °C and 500 °C (compared with that calcined at 300 °C) show higher absorption at high relative pressures (0.8 0<1), indicating the formation of larger inter-aggregated pore between the secondary aggregated particles [27]. When calcination temperature was further decreased to 300 °C, the hysteresis loop shifted to relatively lower pressure region (p/p0<0.8), implying the formation of finer intra- aggregated pore within the primary agglomerated particles [28], as further confirmed in Fig. 7(b). This may be due to the collapse of interaggregated mesopores and the aggregation at high calcination temperature, which results in small surface area. Higher specific surface area benefits the adsorption of reagent on the surface of photocatalysts and enhancement of photocatalytic efficiency. Meanwhile, the slope of isotherms decreased with increasing calcination temperature, which can be due to the weak interaction between adsorbent and adsorbate at a high calcination temperature [29].
Table 1 BET surface areas and pore-structure data of ZnO/ZnGa2O4 samples


Fig. 7 Nitrogen adsorption–desorption isotherms (a) and pore size distribution curves (b) of ZnO/ZnGa2O4 samples
3.2 Photocatalytic activity
3.2.1 Effect of molar ratio of Zn to Ga on photocatalytic activity
Figure 8 displays the effect of molar ratio of Zn to Ga in the starting material on the decolorization of MO. For comparison, under dark conditions without light irradiation, the concentration of MO changes a little within 30 min in the presence of different samples. However, compared with pure ZnGa2O4 and ZnO, all the ZnO/ZnGa2O4 composites have a higher photocatalytic activity under simulated sunlight irradiation, indicating the content of ZnO also shows a significant effect on the photocatalytic activity of the final ZnO/ZnGa2O4 composites. When the molar ratio of reaches 1:0.5, the adsorption rate achieves 30.5% in the first 30 min under dark condition, and the highest degradation rate of MO reaches 97.1% within 60 min under simulated sunlight irradiation. While for other samples with Zn to Ga molar ratio of 1:1 and 1:1.5, the degradation rate of MO is 51.3% and 83.4%, respectively. This phenomenon is interpreted by two reasons. Firstly, the effective heterojunction with forming good transfer channels to separate electrons and holes could be formed at the interface between ZnO and ZnGa2O4 as the introduction of an appropriate amount of ZnO into the ZnGa2O4. The efficient separation of the photogenerated e- and h+ pairs is regarded as the key factor for the high photocatalytic activities. However, when the content of ZnO increases or decreases to a certain extent, ZnO will reduce the catalytic efficiency of ZnO/ZnGa2O4, which is attributed to complete or sparse coverage of ZnO on the ZnGa2O4 [30]. It displays low photocatalytic activity of ZnO or ZnGa2O4 itself, which can only use about 3%–5% of UV light in sunlight. Secondly, the high photocatalytic activity of sample is attributed to higher specific surface area (Table 1). A larger surface area provides more surface active sites for the adsorption of reactants molecules, making the photocatalytic process more efficient [31].

Fig. 8 Photocatalytic activities of ZnO/ZnGa2O4 with different starting Zn to Ga molar ratios:
3.2.2 Effect of calcination temperature on photocatalytic activity
Figure 9 shows the effect of calcination temperature on the photocatalytic activity over the ZnO/ZnGa2O4 composite microspheres. From Fig. 9, it can be observed that the photocatalytic activity increases gradually with the increasing calcination temperature, arrived at the highest on the ZnO/ZnGa2O4 sample calcination at 400 °C. If the calcination temperature further increases, the photocatalytic activity decreases. It is well known that the particle size and crystallinity were both important factors for the photocatalytic activity [32]. The crystallinity of the samples will be enhanced with increasing temperature (as shown in Fig. 2). The enhancement of crystallinity will decrease the number of lattice defects and facilitate the electron transport to active sites [33]. Although the sample calcined at 300 °C possesses both high specific surface area and more adsorption sites, making it an effective adsorbent, its photocatalytic activity is lower, which may be ascribed to the relatively poor crystallinity. The sample calcined at 400 °C shows a better crystalline quality. The photocatalytic activity is enhanced with the temperature increasing from 300 °C to 400 °C. The efficiency of MO degradation arrives about 97.1% in 60 min. However, from another point of view, lack of lattice defects would be unfavorable to the photocatalytic process. Thus, although the crystallinity of the sample obtained at 500 °C is the highest among the samples, its photocatalytic activity is lower than that of the sample prepared at 400 °C. The high photocatalytic activity of sample calcined at 400 °C is also attributed to the higher specific surface area than that of the sample calcined at 500 °C (shown in Table 1).

Fig. 9 Photocatalytic activities of ZnO/ZnGa2O4 at different calcination temperatures:
3.3 Mechanism analysis of photocatalytic reaction
The probable mechanism of photocatalytic degradation on ZnO/ZnGa2O4 could be suggested as follows in Scheme 1:both ZnO and ZnGa2O4 generated e- and h+ pairs under the simulated light irradiation. For ZnGa2O4 catalysis, its valence band (VB) (3.13 V) [34] is more positive than that of E(HO·/OH-) (2.38 V) [35] and the conduction band (CB) (-1.27 V) [34] is more negative than that of E(O2/O2·-) (-0.33 V) [35], so HO· radicals could be formed on ZnGa2O4 [36]. For ZnO, although EVB (2.89 V) [10] is more positive than that of E(HO·/OH-) (2.38 V), ECB (-0.31 V) [10] is not more negative than E(O2/O2·-) (-0.33 V). Consequently, HO· can be generated only on the VB. It is worth mentioning that the degradation of MO could be attributed to the reaction with HO· [37]. On the other hand, when ZnO and ZnGa2O4 are coupled together, photons may be absorbed in both ZnO and ZnGa2O4 and form the e- and h+ pairs. Such process is energetically favorable and the photogenerated e- and h+ pairs can be efficiently separated, which is regarded as the key factor for the enhancement of photocatalytic activities of the ZnO/ZnGa2O4 composites.
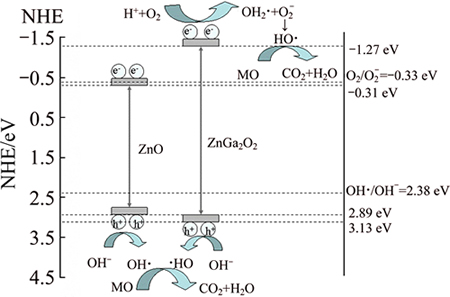
Scheme 1 Schematic diagram for degradation of MO with ZnO/ZnGa2O4 composite photocatalyst
4 Conclusions
ZnO/ZnGa2O4 composite microspheres have been successfully synthesized using glucose as template by a polyethylene glycol (PEG)-assisted one-pot hydrothermal method, and the photocatalytic activity of the samples has been investigated in detail. The results showed that the formation of heterojunction between ZnO and ZnGa2O4 enhanced photocatalytic performance for the photocatalytic degradation of MO aqueous solution compared with pure ZnO and ZnGa2O4 alone. Meanwhile, by adjusting the molar ratio of Zn to Ga in the final composites and calcination temperature led to the high photocatalytic activity, and the highest degradation rate of MO reached 97.1% within 60 min under the simulated light irradiation. The synthesis strategy may provide new design and controlled fabrication of composite materials for environmental purification.
References
[1] FUJISHIMA K, HONDA K. Electrochemical photolysis of water at a semiconductor electrode [J]. Nature, 1972, 238(7): 37-38.
[2] CHEN Chiiing-chang. Degradation pathways of ethyl violet by photocatalytic reaction with ZnO dispersions [J]. Journal of Molecular Catalysis A, 2007, 264: 82-89.
[3] LI Cheng-yong, JIA Yan-rong, ZHANG Xiang-chao, ZHANG Shi-ying, TANG Ai-dong. Photocatalytic degradation of formaldehyde using mesoporous TiO2 prepared by evaporation- induced self-assembly [J]. Journal of Central South University, 2014, 21: 4066-4070.
[4] LIU Yan, ZHANG Yong-cai, XU Xiao-fei. Hydrothermal synthesis and photocatalytic activity of CdO2 nanocrystals [J]. Journal of Hazardous Materials, 2009, 163(2/3): 1310-1314.
[5] SARAVANAN R, KHAN M M, GUPTA V K, MOSQUERA E, GRACIA F, NARAYANAN V, STEPHEN A. ZnO/Ag/CdO nanocomposite for visible light-induced photocatalytic degradation of industrial textile effluents [J]. Journal of Colloid and Interface Science, 2015, 452: 126-133.
[6] SHARMA A, FRANKLIN J B, SINGH B, ANDERSSON G G, LEWIS D A. Electronic and chemical properties of ZnO in inverted organic photovoltaic devices [J]. Organic Electronics, 2015, 24: 131-136.
[7] WANG Ze-yan, HUANG Bai-biao, DAI Ying, QIN Xiao-yan, ZHANG Xiao-yang, WANG Peng, LIU Hai-xia, YU Jiao-xian. Highly photocatalytic ZnO/In2O3 heteronanostructures synthesized by a coprecipitation method [J]. Journal of Physical Chemistry C, 2009, 113: 4612-4617.
[8] ZHENG Li-rong, ZHENG Yuan-hui, CHEN Chong-qi, ZHAN Ying-ying, LIN Xing-yi, ZHENG Qi, WEI Ke-mei, ZHU Jie-fang. Network structured SnO2/ZnO heterojunction nanocatalyst with high photocatalytic activity [J]. Inorganic Chemistry, 2009, 48: 1819-1825.
[9] HAMEED A, MONTINI T, GOMBAC V, FORNASIERO P. Photocatalytic decolourization of dyes on NiO-ZnO nanocomposites [J]. Photochemical and Photobiological Sciences, 2009, 8: 677-682.
[10] ZHANG Li, YAN Jian-hui, ZHOU Min-jie, YANG Hai-hua, LIU You-nian. Fabrication and photocatalytic properties of spheres ZnO/ZnAl2O4 composites hollow microspheres [J]. Applied Surface Science, 2013, 268: 237-245.
[11] LIU Liang-liang, HUANG Jian-feng, CAO Li-jun, WU Jian-peng, FEI Jie, OUYANG Hai-bo, YAN Chun-yao. Influence of temperature on the morphology and photocatalytic activity of ZnGa2O4 crystallites prepared by hydrothermal method [J]. Ceramics International, 2013, 39: 3165-3171.
[12] LIU Liang-liang, HUANG Jian-feng, CAO Li-jun, WU Jian-peng, FEI Jie, OUYANG Hai-bo, MA Feng-lan, ZHOU Chang-jiang. Synthesis of ZnGa2O4 octahedral crystallite by hydrothermal method with the aid of CTAB and its photocatalytic activity [J]. Materials Letters, 2013, 95: 160-163.
[13] MENG Sun, LI Dan-zhen, ZHANG Wen-juan, CHEN Zhi-xin, HUANG Han-jie, LI Wen-juan, HE Yun-hui, FU Xian-zhi. Rapid microwave hydrothermal synthesis of ZnGa2O4 with high photocatalytic activity toward aromatic compounds in air and dyes in liquid water [J]. Journal of Solid State Chemistry, 2012, 190: 135-142.
[14] LIU Qi, WU Di, ZHOU Yong, SU Hai-bin, WANG R, ZHANG Chun-feng, YAN Shi-cheng, XIAO Min, ZOU Zhi-gang. Single- Crystalline, ultrathin ZnGa2O4 nanosheet scaffolds to promote photocatalytic activity in CO2 reduction into methane [J]. ACS Applied Materials & Interfaces, 2014, 6(4): 2356-2361.
[15] HIRANO M. Hydrothermal synthesis and characterization of ZnGa2O4 spinel fine particles [J]. Journal of Materials Chemistry, 2000, 10: 469-472.
[16] YUAN Yu-feng, HUANG Jun-jian, TU Wei-xia, HUANG Si-min. Synthesis of uniform ZnGa2O4 nanoparticles with high photocatalytic activity [J]. Journal of Alloy and Compounds, 2014, 616: 461-467.
[17] YU Jia-guo, YU Jian-qiang, HO Wing-kei, LEUNG M K P, CHENG Bei, ZHANG Cao-ke. Effects of alcohol content and calcination temperature on the textural properties of bimodally mesoporous titania [J]. Applied Catalysis A, 2003, 255: 309-320.
[18] LEE M, YONG K. Highly efficient visible light photocatalysis of novel CuS/ZnO heterostructure nanowire arrays [J]. Nanotechnology, 2012, 23(19): 194014.
[19] KANG B K, LIM H D, MANG S R, SONG K M, JUNG M K, YOON D H. Synthesis and characteristics of ZnGa2O4 hollow nanostructures via carbon  Ga(OH)
Ga(OH) Zn(OH)2 by a hydrothermal method [J]. Crystengcomm, 2012, 17: 2267-2272.
Zn(OH)2 by a hydrothermal method [J]. Crystengcomm, 2012, 17: 2267-2272.
[20] ZHAO Xiao-fei, WANG Lei, XU Xin, LEI Xiao-dong, XU Sai-long, ZHANG Fa-zhi. Fabrication and photocatalytic properties of novel ZnO/ZnAl2O4 nanocomposite with ZnAl2O4 dispersed inside ZnO network [J]. AIChE Journal, 2012, 58(2): 573-582.
[21] WANG Gang, HUANG Bai-biao, LOU Zai-zhu, WANG Ze-yan, QIN Xiao-yan, ZHANG Xiao-yang, DAI Ying. Valence state heterojunction Mn3O4/MnCO3: Photo and thermal synergistic catalyst [J]. Applied Catalysis B: Environmental, 2015, 180: 6-12.
[22] ZHANG Ying, GU Jie, MURUGANANTHAN M, ZHANG Yan-rong. Development of novel α–Fe2O3/NiTiO3 heterojunction nanofibers material with enhanced visible-light photocatalytic performance [J]. Journal of Alloys and Compounds, 2015, 630: 110-116.
[23] MIN Shi-xiong, WANG Fang, JIN Zhi-liang, XU Jing. Cu2O nanoparticles decorated BiVO4 as an effective visible-light-driven p-n heterojunction photocatalyst for methylene blue degradation [J]. Superlattices and Microstructures, 2014, 74: 294-307.
[24] LEI X F, XUE X X, YANG H, CHEN C, LI X, MIU M C, GAO X Y, YANG Y T. Effect of calcination temperature on the structure and visible-light photocatalytic activities of (N, S and C) co-doped TiO2 nano-materials [J]. Applied Surface Science, 2015, 332: 172-180.
[25] INAGAKI M. Pores in carbon materials-importance of their control[J]. New Carbon Materials, 2009, 24: 193-232.
[26] HUANG Jian-hui, DING Kai-ning, WANG Xin-chen, FU Xian-zhi. Nanostructuring cadmium germanate catalysts for photocatalytic oxidation of benzene at ambient conditions [J]. Langmuir, 2009, 25(14): 8313-8319.
[27] YU Jia-guo, WANG Guo-hong, CHENG Bei, ZHOU Ming-hua. Effects of hydrothermal temperature and time on the photocatalytic activity and microstructures of bimodal mesoporous TiO2 powders [J]. Applied Catalysis B, 2007, 69(3/4): 171-180.
[28] YU Jia-guo, Liu Sheng-wei, YU Huo-gen. Microstructures and photoactivity of mesoporous anatase hollow microspheres fabricated by fluoride-mediated self-transformation [J]. Journal of Catalysis, 2007, 249: 59-66.
[29] SONG Gan, DING Yu-dong, ZHU Xun, LIAO Qiang. Carbon dioxide adsorption characteristics of synthesized MgO with various porous structures achieved by varying calcination temperature [J]. Colloids and Surface A: Physicochemical and Engineering Aspects, 2015, 470: 39-45.
[30] LIU Bin, KHARE A, AYDIL E S. TiO2-B/anatase core-shell heterojunction nanowires for photocatalysis [J]. ACS Applied Materials & Interfaces, 2011, 3(11): 4444-4450.
[31] LI Fang-bai, LI Xiang-zhong, HOU Mei-fang, CHEAH K W, CHOY W C H. Enhanced photo-catalyticactivity of Ce3+ TiO2 for 2-mercaptobenzothiazole degradation in aqueous suspension for odour control [J]. Applied Catalysis A: General, 2005, 285: 181-189.
[32] ZHANG Xia, ZHAO Yan, ZHANG Cai-pei. Preparation and photocatalytic performance of TiO2 nanomaterials under the control of organic molecular [J]. Journal of Functional Materials, 2003, 34: 436-438. (in Chinese)
[33] LI Di, HANEDA H. Morphologies of zinc oxide particles and their effects on photocatalysis [J]. Chemosphere, 2003, 51: 129-137.
[34] YAN Shi-cheng, OUYANG-Shuxin, GAO Jun, YANG Ming, FENG Jian-yong, FAN Xiao-xing, WANG Li-juan, LI Zhao-sheng, YE Jin-hua, ZHOU Yong, ZOU Zhi-gang. A room-temperature reactive- template route to mesoporous ZnGa2O4 with improved photocatalytic activity in reduction of CO2 [J]. Angewandte Chemie International Edition, 2010, 49: 6400-6404.
[35] BARD A J, PARSONS R, JORDAN J. Standard Potentials in Aqueous Solution [M]. New York: Marcel Dekker, 1985: 237-239.
[36] ZHANG Wei-wei, ZHANG Jun-ying, LAN Xiang, CHEN Zi-yu, WANG Tian-min. Photocatalytic performance of ZnGa2O4 for degradation of methylene blue and its improvement by doping with Cd [J]. Catalysis Communacations, 2010, 11: 1104-1108.
[37] QU Ping, ZHAO Jin-cai, SHEN Tao, HIDAKA Hisao. TiO2-assisted photodegradation of dyes: A study of two competitive primary processes in the degradation of RB in an aqueous TiO2 colloidal solution [J]. Journal of Molecular Catalysis A: Chemical, 1998, 129: 257-268.
(Edited by YANG Bing)
Foundation item: Projects(21306041, 21271071) supported by the National Natural Science Foundation of China; Project(15A076) supported by the Scientific Research Foundation of Hunan Provincial Education Department of China
Received date: 2015-11-27; Accepted date: 2016-03-17
Corresponding author: YAN Jian-hui; Professor; PhD; Tel: +86-730-8640436; E-mail: yanjh58@163.com

