过氧化氢浓度对Ni-Cr合金表面性能的影响
来源期刊:中国有色金属学报(英文版)2016年第5期
论文作者:何美凤 王浩 江鸿 赵素 潘登
文章页码:1353 - 1358
关键词:过氧化氢(H2O2);齿科镍-铬合金;表面粗糙度;表面形貌;表面腐蚀产物
Key words:hydrogen peroxide (H2O2); dental Ni-Cr alloys; surface roughness; surface morphology; surface corrosion products
摘 要:使用表面轮廓仪和扫描电子显微镜对不同浓度的过氧化氢浸泡112 h之后的镍铬合金的表面粗糙度和表面形貌进行分析。采用能谱仪分析0%和30%过氧化氢浸泡的合金表面腐蚀产物。结果表明:不同过氧化氢浓度浸泡下的合金表面粗糙度从低到高排序依次为0<3.6%<10%<30%。随着过氧化氢浓度的增加,合金表面粗糙度随之增加,表面形貌也呈现不同程度的腐蚀。根据XPS测试结果,在研究样品表面形成的最外层的腐蚀产物为Ni(OH)2 和BeO。
Abstract: The effect of concentration of hydrogen peroxide (H2O2) on the surface properties of Ni-Cr alloys was studied. Surface roughness and surface morphology of Ni-Cr alloys were evaluated by surface profiler and scanning electron microscopy after being immersed in different concentrations of H2O2 for 112 h. Surface corrosion products of Ni-Cr alloys were analyzed by photoelectron spectrograph after being immersed in 0% and 30% H2O2. The order of increasing surface roughness of Ni-Cr alloys after being immersed in different concentrations of H2O2 was 0<3.6%<10%<30%. As the concentration of hydrogen peroxide increased, the surface roughness of Ni-Cr alloys increased and the surface morphology showed different degrees of corrosion. According to the XPS results, the corrosion products formed on the outmost surface layer of the studied samples are Ni(OH)2 and BeO.
Trans. Nonferrous Met. Soc. China 26(2016) 1353-1358
Mei-feng HE1, Hao WANG1, Hong JIANG1, Su ZHAO2, Deng PAN1
1. School of Materials Science and Engineering, University of Shanghai for Science and Technology,
Shanghai 200093, China;
2. School of Mechanical Engineering, Shanghai Dianji University, Shanghai 200001, China
Received 9 April 2015; accepted 3 March 2016
Abstract: The effect of concentration of hydrogen peroxide (H2O2) on the surface properties of Ni-Cr alloys was studied. Surface roughness and surface morphology of Ni-Cr alloys were evaluated by surface profiler and scanning electron microscopy after being immersed in different concentrations of H2O2 for 112 h. Surface corrosion products of Ni-Cr alloys were analyzed by photoelectron spectrograph after being immersed in 0% and 30% H2O2. The order of increasing surface roughness of Ni-Cr alloys after being immersed in different concentrations of H2O2 was 0<3.6%<10%<30%. As the concentration of hydrogen peroxide increased, the surface roughness of Ni-Cr alloys increased and the surface morphology showed different degrees of corrosion. According to the XPS results, the corrosion products formed on the outmost surface layer of the studied samples are Ni(OH)2 and BeO.
Key words: hydrogen peroxide (H2O2); dental Ni-Cr alloys; surface roughness; surface morphology; surface corrosion products
1 Introduction
With the development of oral repair techniques, porcelain fused-to-metal prosthesis is approved by patients because of its strength of metal and beautiful outlook of porcelain. For clinical dental applications, there are currently hundreds of alloys available for prosthodontic restorations. Among them, nickel-based (Ni-Cr) alloy prosthesis is widely used (especially in developing countries) in the inner crown of casting crown and bridge and porcelain-fused-to-metal crown and bridge owing to its simple fabrication process, low cost, and additionally, improvement of castability and oxidizability. However, the oxidizability increment is accompanied by an increment of the corrosion rate, which has the disadvantage to release nickel (which presents an allergen character) and chromium (able to be present as toxic chromate) in the human system. Nickel might activate monocytes and endothelial cells, suppressing or promoting the expression of intercellular adhesion molecule-1 (ICAM-1) by endothelial cells, depending on the ionic concentration [1].
Although Ni-Cr alloys are well-known for their good corrosion resistance in the body, the metal surface exposed to oral cavity rich in electrolytes would generate electrochemical corrosion after restoration [2,3]. The corrosion characteristics of metal alloys are dependent on the composition of the alloy, its potential values, the strain, the surface roughness, the degree of oxidation, the pH, the temperature of the media, the mixing velocity of the solution and the presence of inhibitors [4]. And it is all known that the oral cavity is a dynamic environment, subjecting to changes in pH and temperature, a continuous flow of saliva, microbiological activity, occlusal load, diet, unconscious regular contact with metal ions (contact with jewelry and cooking utensils), as well as many other factors [5]. Once the metal prosthesis is worn in mouth, the metal ions would release to oral cavity and then come to contact with cells and tissues in the immediate environment, or be distributed throughout the body [6,7]. And the metal ions released from dental alloys not only produced adverse effects on the morphology, viability and proliferation rate of gingival fibroblasts but also caused increased levels of Interleukin 2(IL-2) and Interleukin 6(IL-6) [ 8-12].
Tooth bleaching is an increasingly popular aesthetic procedure used in dentistry [13-17]. It is relatively simple and highly effective, and can often preclude the need for operative intervention [13,14]. There are various agents available for bleaching vital teeth, although they invariably involve the application or generation of hydrogen peroxide (HP), a strong oxidising agent. Despite increased popularity, controversy surrounds the use of peroxide-based bleaching systems to whiten teeth. The situation has not been helped by conflicting reports in the scientific literature and media, further compounded by a lack of standardisation in methodology or presentation of data. Some studies suggested the relatively high concentrations of peroxide used for topical bleaching altered the chemical structure of tooth tissues [15-18]. While vital bleaching does not appear to cause macroscopic changes to the dental hard tissues, microscopic changes have been reported, particularly where peroxide was applied at high concentrations [19,20].
Several bleaching methods exist, including in office bleaching with or without a light source [21,22], e.g., mouth guard bleaching under supervision of a dentist, and bleaching kits that were sold over the counter (where individuals apply the bleaching agent without the supervision of the dentist). Active HP concentrations may, however, vary enormously and can be as high as 35% [23] even though current UK law limits the peroxide content to 0.1%. Despite widespread debate, there is currently a trend towards employing greater concentrations of H2O2 as the active agent in tooth bleaching preparations [24,25]. Microstructural evaluation and corrosion properties of dental alloys subjected to bleaching have been investigated, and surface topographic alterations of Ni-Cr alloys occurred as a result of the application of 10% and 35% carbamide peroxide (CP) simulating at-home bleaching and in office bleaching during 14 d, respectively [26]. Ni-Cr alloys showed the highest surface roughness for both the control and home bleached subgroups [27,28].
The aim of the present study was therefore to investigate the effect of increasing H2O2 concentrations (0-30%) on surface properties from Ni-Cr alloys. This research will provide data in informing current discussion and scientific debate regarding the safety and efficacy of tooth bleaching agents.
2 Experimental
2.1 Sample preparation
The Ni-Cr dental casting alloy (77.36% Ni, 12.27% Cr, 4.84% Mo, 5.53% other elements) was used in the present study. The alloy samples were manufactured to a circle sheet of 10 mm in diameter and 1.5 mm in thickness, divided into 4 groups randomly, and then immersed in 0, 3.6%, 10%, and 30% H2O2 solution of pH 3 at (37±0.1) °C for 112 h. The immersion time of 112 h better reflects the actual clinical tooth bleach. The surface morphologies were observed by scanning electron microscopy (SEM), the surface roughness was detected by surface profile-meter (SPM), and the composition and structure of the passive film formed on the surface of nickel-chromium alloy were tested by X-ray photo-electron spectrometer (XPS). All measured data were statistically analyzed using one-way analysis of variance (ANOVA) and multiple comparisons test (LSD post hoc test) to examine the effect of the factor of hydrogen peroxide concentration.
2.2 Surface roughness
Following immersion test, the surface roughness of the samples was measured again using a surface profile-meter (SPM) that was calibrated by setting the appropriate zero setting prior to roughness measurement of the samples. The roughness of the uppermost surface was then measured by moving the stylus across its diameter. This procedure was repeated eight times for each sample, altering the orientation each time, and the results were averaged.
2.3 Surface analysis
X-ray photoelectron spectroscopy measurement was performed to analyze the composition and structure of the passive film formed on the surface of nickel-chromium alloy by means of a PHI 5000 photoelectron spectrometer (VersaProbe, Japan) with monochromatized Al Kα radiation (1486.6 eV). The take-off angle for photoelectron detection was set at 45° for the sample surface. The vacuum level of the analyzing chamber during measurement was of the order of 10-8 Pa. The XPS data were converted into VAMAS file format and imported into XPSPEAK software package for manipulation and curve-fitting.
2.4 Statistical analysis
A two-way ANOVA (element by concentration) revealed a significant interaction between concentration and elements (p<0.001) indicating that difference between solutions was different across elements. The two-way ANOVA was followed by a one-way ANOVA and Dunnett Post Hoc test for multiple comparisons between solutions for each element. The roughness measurements were analyzed using a paired t-test.
3 Results and discussion
3.1 Surface morphology
Figure 1 shows the surface morphology of the dental Ni-Cr casting alloy after being immersed in different concentrations of H2O2 (0, 3.6%, 10%, and 30%) for 112 h. The more serious corrosion happened on the surface of nickel-chromium alloy after being soaked in different concentrations of hydrogen peroxide for 112 h. The pore sizes of point erosion with the uneven distribution on the surface were observed. The corrosion of the alloys was the most conspicuous after being immersed in 30% hydrogen peroxide, while the surface of the alloys did not change significantly after being immersed in 0% H2O2 as the control group (deionized water). The corrosion of the alloys happened more and more conspicuously with the increase of hydrogen peroxide concentration.

Fig. 1 SEM images on surface of nickel-chromium alloys after being immersed in different concentrations of H2O2 for 112 h: (a) 0% H2O2; (b) 3.6% H2O2; (c) 10% H2O2; (d) 30% H2O2
3.2 Surface roughness
The average roughness values for each group before and after treatment are listed in Table 1. The surface roughness of nickel-chromium alloy changed after being soaked in different concentrations of hydrogen peroxide. In addition, the surface of the alloys became more and more rough with the increase of hydrogen peroxide concentration.
3.3 X-ray photoelectron spectrometry (XPS)
Figure 2 shows the full XPS survey spectra of the Ni-Cr dental alloy surface after being immersed in 30% and in 0% H2O2 for 112 h. It can be found that, in 30% and 0% H2O2, the dominant elements are O, Be and Ni.
Table 1 Surface roughness of nickel-chromium alloys after being immersed in different concentrations of H2O2 for 112 h (mean±SD, n=4)
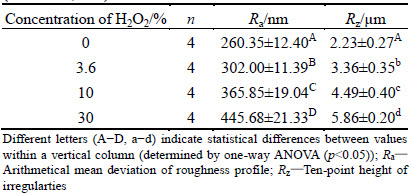
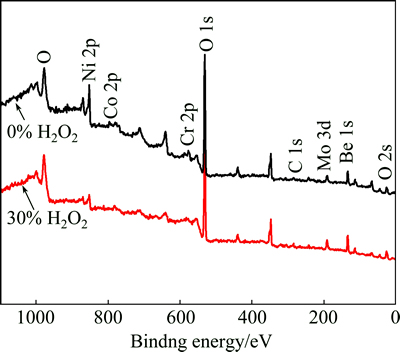
Fig. 2 XPS survey spectra of nickel-chromium alloys after being immersed in 0% and 30% H2O2 for 112 h
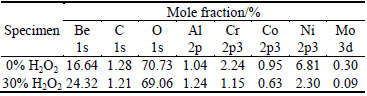
A small amount of Be and Al are often added to Ni-Cr alloys to improve their cast ability [29]. Table 2 lists the composition of the surface oxide films on the Ni-Cr alloys after being immersed in 30% and 0% H2O2 for 112 h determined by XPS. In 30% H2O2, the amounts of three dominant elements are 69.06%, 24.32%, and 2.30% for O, Be, and Ni, respectively (Table 2). It is obvious that in 0% H2O2, the Ni, Cr, C and O concentrations increase, while the Al and Be concentrations decrease. The main component of O 1s peak at 532 eV is related to H—O or O—O bonding, and its presence is attributed to the H2O2.
Table 2 Concentration of elements of outermost surface layer on nickel-chromium alloys after being immersed in 0% and 30% H2O2 for 112 h
Because the contents of Cr, Co and Mo are too low, they are not found in typical high resolution XPS spectra. High resolution XPS spectra of Be after being immersed in different concentrations of H2O2 (0% and 30%) for 112 h are shown in Fig. 3. The Be0 and Be2+ (BeO) peaks in the spectra indicate a metallic and oxide states. The Be0 peaks in the spectra at 111.3 eV correspond to the Ni-Cr alloy. The Be2+ peaks in the spectra at 113.64 eV correspond to BeO. The peak area ratio of BeO to Be is 2204.915:743.4307=2.97 in Fig. 3(a). The peak area ratio of BeO to Be is 1794.638:253.7229=7.07 in Fig. 3(b). Compared with 0% H2O2, the composition of Be2+ of Ni-Cr alloy after being immersed in 30% H2O2 for 112 h is significantly high.

Fig. 3 High-resolution XPS spectra of Be after being immersed in 0% (a) and 30% (b) H2O2 for 112 h
High resolution XPS spectra of Ni after being immersed in 30% and 0% H2O2 for 112 h are shown in Fig. 4. The spectra exhibit two dominant peaks before argon ion sputtering, namely Ni2+ 2p3/2 at 855.6 eV and Ni2+ 2p1/2 at 871.8 eV, respectively. The dominant Ni 2p3/2 and Ni 2p1/2 peaks are decomposed into two peaks, respectively. The Ni0 and Ni2+ peaks in the Ni 2p3/2 and Ni 2p1/2 spectra indicate a metallic and oxide states. The Ni0 peaks in the Ni 2p3/2 and Ni 2p1/2 spectra at 852.8 eV and 869.9 eV also correspond to the Ni-Cr alloy. The peak area ratio of Ni to Ni2+ is (5722.08+5504.477): (1961.26+6877.058)=1.27:1 in Fig. 4(a). The peak area ratio of Ni to Ni2+ is (657.8834+1593.158):(901.7117+ 3372.353)=1.28:1 in Fig. 4(b). Compared with 0% H2O2, the composition of Ni2+ of Ni-Cr alloy after being immersed in 30% H2O2 for 112 h is lower.
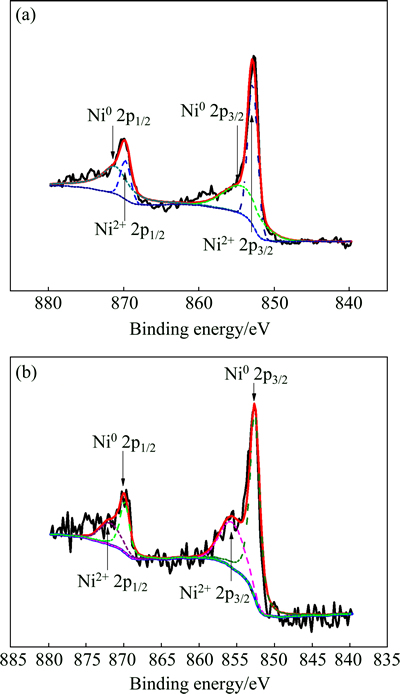
Fig. 4 High-resolution XPS spectra of Be after being immersed in 0% (a) and 30% (b) H2O2 for 112 h
Figure 5 reveals the O 1s core level after being immersed in 30% and 0% H2O2 for 112 h. As shown in Fig. 5(a), the O 1s core level spectrum is curve-fitted with one peak component indicating Ni(OH)2. As shown in Fig. 5(b), the O 1s core level spectrum is curve-fitted with two peaks components indicating Ni(OH)2 and BeO. In Fig. 5(a), peak 1 at 531.5 eV is attributed to Ni(OH)2. In Fig. 5(b), peak 2 at 532.50 eV is Be—O bonding.
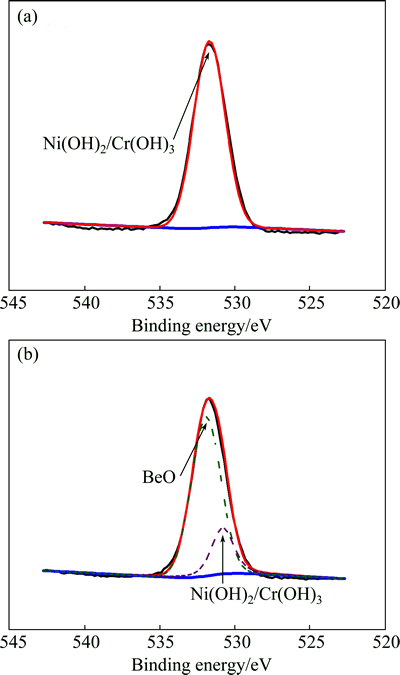
Fig. 5 High-resolution XPS spectra of O after being immersed in 0% (a) and 30% (b) H2O2 for 112 h
According to the XPS results, the corrosion resistances of Ni-Cr alloys in different concentrations of H2O2 were studied and the corrosion products formed on the outmost surface layer of the studied samples are Ni(OH) 2 and BeO.
4 Conclusions
1) The more serious corrosion happened on the surface of nickel-chromium alloy, after being soaked in different concentrations of hydrogen peroxide for 112 h. The pore sizes of point erosion with the uneven distribution on the surface were observed. The corrosion of the alloys was the most conspicuous after being immersed in 30% H2O2, while the surface of the alloys did not change significantly after being immersed in 0% H2O2 as the control group (deionized water). The corrosion of the Ni-Cr alloys happened more and more conspicuously with the increase of the concentration of hydrogen peroxide.
2) The surface roughness of nickel-chromium alloy after being soaked in different concentrations of H2O2 was observed. The surface of the nickel-chromium alloys became rougher and rougher with the increase of the concentration of H2O2.
3) The oxide content on the surface of nickel-chromium alloy after being soaked in 30% H2O2 is lower than that in 0% H2O2, and the corrosion resistance of the alloys becomes poor. The corrosion products formed on the outmost surface layer of the studied samples are Ni(OH)2 and BeO.
4) Too much bleach will inevitably come into contact with nickel-chromium alloy and other materials, which will promote the corrosion of the alloys, and then more metal ions are released and to further damage body tissues in the clinical bleaching treatment.
References
[1] WATAHA J C, SUN Z L, HANKS C T, FANG D N. Effect of Ni ions on expression of intercellular adhesion molecule 1 by endothelial cells [J]. J Biomed Mater Res, 1997, 36: 145-151.
[2] ELIASSON A, ARNELUND C F, JOHANSSON A. A clinical evaluation of cobalt-chromium metal-ceramic fixed partial dentures and crowns: A three- to seven-year retrospective study [J]. J Prosthet Dent, 2007, 98(1): 6-16.
[3] BERZINS D W, KAWASHIMA I, GRAVES R. Electrochemical characteristics of high-Pd alloys in relation to Pd-allergy [J]. Dent Mater, 2000, 16(4): 266-273.
[4] WYLIE C M, SHELTON R M, FLEMING G J. Corrosion of nickel-based dental casting alloys [J]. Dent Mater, 2007, 23(6): 714-723.
[5] GEIS-GERSTORFER J. In vitro corrosion measurements of dental alloys [J]. J Dent, 1994, 22(4): 247-251.
[6] LU Y, CHEN W, KE W. Nickel-based (Ni-Cr and Ni-Cr-Be) alloys used in dental restorations may be a potential cause for immune-mediated hypersensitivity [J]. Med Hypotheses, 2009, 73(5): 716-717.
[7] FACCIONI F, FRANCESCHETTI P, CERPELLONI M. In vivo study on metal release from fixed orthodontic appliances and DNA damage in oral mucosa cells [J]. Am J Orthod Dent of acial Orthofacil Orthop, 2003, 124(6): 687-693.
[8] AGAOGLU G, ARUN T, IZGI B. Nickel and chromium levels in the saliva and serum of patients with fixed orthodontic appliances [J]. Angle Orthod, 2001, 71(5): 375-379.
[9] LOPEZ-ALIAS J F, MARTINEZ-GOMIS J, ANGLADA J M. Ion release from dental casting alloys as assessed by a continuous flow system: Nutritional and toxicological implications [J]. Dent Mater, 2006, 22(9): 832-837.
[10] ISSA Y, BRUNTON P, WATERS C M. Cytotoxicity of metal ions to human oligodendroglial cells and human gingival fibroblasts assessed by mitochondrial dehydrogenase activity [J]. Dent Mater, 2008, 24(2): 281-287.
[11] WATAHA J C. Alloys for prosthodontic restoration [J]. J Prosthet Dent, 2002, 87(4): 351-363.
[12] SHETTLEMORE M G, BUNDY K J. Assessment of dental material degradation product toxicity using a bioluminescent bacterial assay [J]. Dent Mater, 2002, 18(6): 445-453.
[13] SCHMALZ G, GARHAMMER P. Biological interactions of dental casting alloys with oral tissues [J]. Dent Mater, 2002, 18(5): 396-406.
[14] SULIEMAN M, ADDY M, MACDONALD E, REES J S. The bleaching depth of a 35% hydrogen peroxide based in-office product: A study in vitro [J]. Journal of Dentistry, 2005, 33(1): 33-40.
[15] TURKER S B, BISKIN T. The effect of bleaching agents on the microhardness of dental aesthetic restorative materials [J]. Journal of Oral Rehabilitation, 2002, 29(7): 657-661.
[16] FRIEDMAN S, ROTSTEIN I, LIBFELD H, STABHOLZ A, HELING I. Incidence of external root resorption and esthetic results in 58 bleached pulpless teeth [J]. Endodontics & Dental Traumatology, 1988, 4(1): 23-26.
[17] MADISON S, WALTON R. Cervical root resorption following bleaching of endodontically treated teeth [J]. Journal of Endodontics, 1990, 16(12): 570-574.
[18] ROTSTEIN I, LEHR Z, GEDALIA I. Effect of bleaching agents on inorganic components of human dentin and cementum [J]. Journal of Endodontics, 1992, 18(6): 290-293.
[19] DAHL J E, PALLESEN U. Tooth bleaching—A critical review of the biological aspects [J]. Critical Reviews in Oral Biology & Medicine, 2003, 14(4): 292-304.
[20] ATTIN T, HANNIG C, WIEGAND A, ATTIN R. Effect of bleaching on restorative materials and restorations—A systematic review [J]. Dental Materials, 2004, 20: 852-861.
[21] SULIEMAN M, ADDY M, REES J S. Surface and intra-pulpal temperature rises during tooth bleaching: An in vitro study [J]. British Dental Journal, 2005, 199(1): 37-40.
[22] LEONARD R H, SHARMA A, HAYWOOD V B. Use of different concentrations of carbamide peroxide for bleaching teeth: An in vitro study [J]. Quintessence International, 1998, 29(8): 503-507.
[23] LEE J H, KIM H I, KIM K H, KWON Y H. Effect of bleaching agents on the fluoride release and microhardness of dental materials [J]. Journal of Biomedical Materials Research, 2002, 63(5): 535-541.
[24] MORRIS C D N. Tooth whiteners—The legal position [J]. British Dental Journal, 2003, 194(7): 375-376.
[25] KELLEHER M G D, ROE F J C. The safety-in-use of 10% carbamide peroxide (Opalescence) for bleaching teeth under the supervision of a dentist [J]. British Dental Journal, 1999, 187(4): 190-194.
[26] YU Hao, ZHANG Chang-yuan, CHENG Shao-long, CHENG Hui. Effects of bleaching agents on dental restorative materials: A review of the literature and recommendation to dental practitioners and researchers [J]. Journal of Dental Sciences, 2015, 89(4): 425-429.
[27] MOHSEN C A. The effect of bleaching agents on the surface topography of ceramometal dental alloys [J]. Journal of Prosthodontics, 2010, 19(1): 33-41.
[28] WU Zhan-wen, CHEN Ji, PIAO Nan, SUN Cheng, HASSAN W, ZHANG Xin-hang, XIE Yu-jun. Electrochemical corrosion behavior of bulk ultra-fine grained Fe-Ni-Cr alloy [J]. Transactions of Nonferrous Metals Society of China, 2014, 24(6): 1989-1994.
[29] SAJI Viswanathan S, CHOE Han-Cheol. Electrochemical behavior of Co-Cr and Ni-Cr dental cast alloys [J]. Transactions of Nonferrous Metals Society of China, 2009, 19(4): 785-790.
何美凤1,王 浩1,江 鸿1,赵 素2,潘 登1
1. 上海理工大学 材料科学与工程学院,上海 200093;
2. 上海电机学院 机械学院,上海 200001
摘 要:使用表面轮廓仪和扫描电子显微镜对不同浓度的过氧化氢浸泡112 h之后的镍铬合金的表面粗糙度和表面形貌进行分析。采用能谱仪分析0%和30%过氧化氢浸泡的合金表面腐蚀产物。结果表明:不同过氧化氢浓度浸泡下的合金表面粗糙度从低到高排序依次为0<3.6%<10%<30%。随着过氧化氢浓度的增加,合金表面粗糙度随之增加,表面形貌也呈现不同程度的腐蚀。根据XPS测试结果,在研究样品表面形成的最外层的腐蚀产物为Ni(OH)2 和BeO。
关键词:过氧化氢(H2O2);齿科镍-铬合金;表面粗糙度;表面形貌;表面腐蚀产物
(Edited by Xiang-qun LI)
Foundation item: Projects (13ZR1427700, 13ZR1427900) supported by the Natural Science Foundation of Shanghai, China; Project (51304136) supported by the National Natural Science Foundation of China; Projects (Slgl4049, Slgl4050) supported by the Shanghai Education Development Foundation “Selection and Training the Excellent Young College Teacher” Project, China
Corresponding author: Hong JIANG; Tel/Fax: +86-21-55271708; E-mail: hongjiangsh@usst.edu.cn
DOI: 10.1016/S1003-6326(16)64238-3

