包埋磷酸盐小球的合成及其对溶液中铅镉的吸附与固定
来源期刊:中国有色金属学报(英文版)2016年第8期
论文作者:王云燕 姚文斌 王庆伟 杨志辉 梁丽芬 柴立元
文章页码:2230 - 2237
关键词:吸附;固定;铅;镉;包埋磷酸盐海藻酸钙
Key words:adsorption; immobilization; lead; cadmium; phosphate-embedded calcium alginate beads
摘 要:采用海藻酸钠、磷酸二氢钙和碳酸氢钠成功合成了包埋磷酸盐的海藻酸钙小球。通过扫描电镜、傅里叶变换红外光谱、X射线衍射等分析表征了该小球的形貌与结构。研究pH值和初始金属离子浓度对铅镉去除率的影响,发现吸附铅镉的最佳pH值分别为4.0和5.5;铅镉的最适初始浓度分别为200 mg/L和25 mg/L,对应的去除率分别达94.2%和80%。XRD和FTIR的分析结果证实了该小球对铅镉的去除机理为:铅镉离子吸附到小球的表面,与小球的羧基发生反应,进而与磷酸根反应生成稳定的磷酸盐沉淀。铅镉的吸附符合Langmuir等温线方程,拟合系数R2分别为0.9957和0.988。根据Langmuir等温线方程计算得到铅镉的理论饱和吸附量分别为263.16 mg/g和82.64 mg/g。研究结果表明该小球对溶液中的铅镉离子有良好的处理效果,同时由于生成稳定的沉淀物,也能应用于处理被铅镉污染的水稻土。
Abstract: The phosphate-embedded calcium alginate beads were successfully synthesized based on sodium alginate, calcium dihydrogen phosphate and sodium hydrogen carbonate. Scanning electron microscopy, Fourier transformed infrared (FTIR) spectroscopy and X-ray diffraction (XRD) were conducted to characterize the morphology and structure of the phosphate-embedded calcium alginate beads. The effects of pH and the initial concentration of the metal ions on Pb(II) and Cd(II) sorption by the beads were investigated. The optimal pH values for Pb(II) and Cd(II) sorption are 4.0 and 5.5, respectively. The optimal initial concentrations of Pb(II) and Cd(II) are 200 mg/L and 25 mg/L, correspondingly, and the removal efficiencies are 94.2% and 80%, respectively. The sorption mechanism is that the heavy metal ions accessed the beads firstly due to the large surface area, combined with OH-, and then precipitated with phosphate radical, which was proven by FTIR and XRD. The sorption of Pb(II) and Cd(II) is fitted to Langmuir isotherm model with R2 values of 0.9957 and 0.988, respectively. The sorption capacities of Pb(II) and Cd(II) are 263.16 mg/g and 82.64 mg/g, respectively. The results indicate that the phosphate-embedded calcium alginate beads could be applied to treating Pb(II)/Cd(II)-containing wastewater and it could be implied that the synthesized beads also could be used as a kind of soil ameliorant for remediation of the heavy metal contaminated paddy soil.
Trans. Nonferrous Met. Soc. China 26(2016) 2230-2237
Yun-yan WANG1,2, Wen-bin YAO1,2, Qing-wei WANG1,2, Zhi-hui YANG1,2, Li-fen LIANG1,2, Li-yuan CHAI1,2
1. School of Metallurgy and Environment, Central South University, Changsha 410083, China;
2. Chinese National Engineering Research Center for Control & Treatment of Heavy Metal Pollution, Central South University, Changsha 410083, China
Received 10 June 2015; accepted 4 May 2016
Abstract: The phosphate-embedded calcium alginate beads were successfully synthesized based on sodium alginate, calcium dihydrogen phosphate and sodium hydrogen carbonate. Scanning electron microscopy, Fourier transformed infrared (FTIR) spectroscopy and X-ray diffraction (XRD) were conducted to characterize the morphology and structure of the phosphate-embedded calcium alginate beads. The effects of pH and the initial concentration of the metal ions on Pb(II) and Cd(II) sorption by the beads were investigated. The optimal pH values for Pb(II) and Cd(II) sorption are 4.0 and 5.5, respectively. The optimal initial concentrations of Pb(II) and Cd(II) are 200 mg/L and 25 mg/L, correspondingly, and the removal efficiencies are 94.2% and 80%, respectively. The sorption mechanism is that the heavy metal ions accessed the beads firstly due to the large surface area, combined with OH-, and then precipitated with phosphate radical, which was proven by FTIR and XRD. The sorption of Pb(II) and Cd(II) is fitted to Langmuir isotherm model with R2 values of 0.9957 and 0.988, respectively. The sorption capacities of Pb(II) and Cd(II) are 263.16 mg/g and 82.64 mg/g, respectively. The results indicate that the phosphate-embedded calcium alginate beads could be applied to treating Pb(II)/Cd(II)-containing wastewater and it could be implied that the synthesized beads also could be used as a kind of soil ameliorant for remediation of the heavy metal contaminated paddy soil.
Key words: adsorption; immobilization; lead; cadmium; phosphate-embedded calcium alginate beads
1 Introduction
Heavy metal pollutants, mainly derived from human activities, such as continuous exploitation of mineral resources, metallurgy, metal finishing, electroplating, printed circuit manufacture, have readily accumulated in the environment and have been frequently detected in sediments, rivers, lakes and other environment. Among various heavy metals, lead and cadmium are ranked as highly toxic and carcinogenic agents. The release of large quantities of heavy metals into the natural environment, e.g., irrigation of agricultural fields by using sewage, has resulted in a number of environmental problems. Heavy metals in the environment can accumulate in the food chain, and thus may pose a threat to human health due to their non-biodegradability and persistence [1-3].
Various methods have been suggested and applied for the removal of toxic metals from aqueous solution, such as chemical precipitation, evaporation, ion- exchange, adsorption, electrolysis and reverse osmosis. Due to the specific nature of industrial effluents, the effective removal of metal ions has proven to be a very difficult and costly process. Although adsorption is the most effective and widely used method, commercial chelating resin, one of the mostly utilized sorbents, is still an expensive material and mostly is non- biodegradable [4]. Hence, research is focused on the synthesis or preparation of novel, cheap and more effective sorbents [5-8].
A very promising material, that offers such advantages, is alginate, a natural anionic polymer. Alginate is a linear copolymer of α-L-guluronate and β-D-mannuronate, which constitutes 10%-40% of the dry mass of all species of brown algae [9]. The capability of this copolymer to form stable biodegradable gels in the presence of divalent cations has been known and studied extensively. These gelation properties can be attributed to the simultaneous binding of the divalent cations such as Ca2+ to different chains of α-L-guluronate blocks. Due to its ability to form stable structures, cross-linked alginate has been used for the immobilization and removal of heavy metal from wastewater. Gel beads of calcium alginate have been reported as a sorbent material for heavy metal removal from aqueous solutions [10].
It has been reported that phosphorus and phosphorus compounds could be used to immobilize Pb(II) and Cd(II) because of low solubility constants of Cd3(PO4)2 (3.6×10-32) and Pb(HPO4)2 (3.457×10-4) [11]. However, phosphorus and phosphorus compounds being applied to environment remediation extensively would cause eutrophication of water body. Phosphate would also decrease soil pH, which would activate heavy-metal. And microelements which were necessary for growing of plants would be decreased by phosphate too [12].
Sodium alginate is a kind of nature macromolecule organic matter, which possesses a large number of free carboxyl groups and whose sodium would be replaced by calcium when be cross-linked by calcium chloride. Sodium alginate has also been widely used to adsorb Pb(II) and Cd(II), for its amounts of carboxyl groups and its large specific surface area. Nanogel and superparamagnetic nanocomposite based on sodium alginate for sorption of heavy metal ions, biosorption of Pb(II) by Pleurotus ostreatus immobilized in calcium alginate gel and novel composite biopolymers of sodium alginate for adsorption of Pb(II) ions has been investigated [13-15]. However, sodium alginate was too fragile to be used alone.
Polyvinyl alcohol (PVA) is a water-soluble material containing large amounts of hydroxide groups which has been developed for biomedical applications since it is biocompatible [16]. As a kind of hydrophilic macromolecule organic matter, PVA is a polymer that is commonly used to remove heavy metals from waste water because it is non-toxic, safe and cheap. But PVA has tendency to agglomerate, therefore PVA is usually combined with alginate to remove Pb and Cd [17]. It has been reported that PVA gel has a higher mechanical strength and larger durability in high acid solutions than alginate gel, which could be used to overcome the frangibility of pure calcium alginate [18].
Combination of Ca(H2PO4)2 with sodium alginate would prevent phosphorous pollution in the environment. And  ,
, 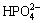 ,
,  would also increase the electronegativity of material, increasing the sorption capacity. In this research, sorption and immobilization of heavy metals by the phosphate-embedded calcium alginate beads synthesized by the dripping method was proposed to remove Pb(II) and Cd(II) ions from the aqueous solution. The effects of the initial pH of solution, initial ion concentration on Pb(II) or Cd(II) sorption were discussed. More importantly, the sorption isotherm was also studied by different models.
would also increase the electronegativity of material, increasing the sorption capacity. In this research, sorption and immobilization of heavy metals by the phosphate-embedded calcium alginate beads synthesized by the dripping method was proposed to remove Pb(II) and Cd(II) ions from the aqueous solution. The effects of the initial pH of solution, initial ion concentration on Pb(II) or Cd(II) sorption were discussed. More importantly, the sorption isotherm was also studied by different models.
2 Experimental
2.1 Materials
Calcium chloride dihydrate was used to prepare calcium alginate beads. PVA solution was prepared by dissolving PVA powder in deionized water at 80 °C. Pb(II) and Cd(II) solutions were prepared by dissolving required amount of cadmium chloride or lead nitrate in deionized water. Sodium hydroxide solution was used to adjust the pH of the solutions. All chemicals used are of analytical reagent (AR) grade.
2.2 Synthesis of beads
Phosphate-embedded calcium alginate beads were synthesized by the following procedure: PVA solution was prepared by dissolving PVA powder in the deionized water at 80 °C. Calcium dihydrogen phosphate and sodium hydrogen carbonate were added into the PVA solution in sequence and fully dissolved with deionized water, and then sodium alginate powder was dispersed into the above solution and a homogeneous blend solution was formed. The whole process was performed under the conditions of continuous magnetic stirring, and the stirring was kept for an additional 10 min after the last addition of sodium alginate dispersion. The obtained homogeneous solution with the concentration of 60 g/L PVA, 10 g/L Ca(H2PO4)2, 25 g/L sodium alginate and 6 g/L NaHCO3 was slowly cooled to room temperature. 1% (w/v) calcium chloride powder was added into the saturated boric acid solution, and then pH of the solution was adjusted to 6.5-7.0 by dilute NaOH solution to prepare the crosslinking medium. The obtained aqueous dispersion was dripped into the crosslinking medium using a syringe. The spherical, smooth and homogenous beads were obtained, washed with distilled water two times and dried. The beads were stored at 4 °C before use.
2.3 Sorption experiments
50 mL of Pb(II) or Cd(II) solution and 0.05 g of phosphate-embedded calcium alginate beads were used in batch sorption experiments. The sorption experiments were performed at the temperature of 25 °C for 24 h by shaking the sorption mixture at 120 r/min in a thermostabilized warm bath. Samples were then filtered in quality paper to determine the final concentrations of metals. The initial concentration of the solutions prepared was in the range of 100-1000 mg/L Pb(II) and 20-200 mg/L Cd(II), respectively. The optimal concentration was determined according to the maximum Pb(II) or Cd(II) removal efficiency.
As pH is an important factor affecting the sorption process, the effects of pH on metal ions sorption by phosphate-embedded calcium alginate beads were conducted. The Pb(II) solutions were adjusted to pH values of 3.0, 3.5, 4.0, 4.5, 5.0 and 5.5 and Cd(II) solutions were adjusted to pH values of 3.0, 3.5, 4.0, 4.5, 5.0, 5.5 and 6.0 by adding required amount of diluted NaOH solution. In order to prevent Pb(II) and Cd(II) from forming the sedimentation, the maximum pH value was controlled at 6.0. The pH was measured using a pH meter. 50 mL of pH-adjusted solution with the Pb(II) concentration of 500 mg/L, Cd(II) concentration of 200 mg/L and 0.05 g phosphate-embedded calcium alginate beads were used in batch experiments. The pH value at the maximum Pb(II) or Cd(II) removal efficiency was determined.
The removal efficiency of metal ions was calculated by
 (1)
(1)
where R is the removal efficiency of heavy metal ion by the beads, CI and CE (mg/L) are the initial and final metal concentrations in the solution.
All experiments were performed in duplicate and mean values were used in the data analysis.
2.4 Adsorption isotherm
The adsorption isotherm of Pb(II) or Cd(II) solution by phosphate-embedded calcium alginate beads at the optimal conditions was conducted to evaluate the saturated sorption capacity. In the pre-experiment, Pb(II) or Cd(II) sorption onto phosphate-embedded calcium alginate beads reached an equilibrium within 24 h. Therefore, 0.05 g beads were fully dispersed in 50 mL of Pb(II) or Cd(II) solution with different concentrations, and then vigorously stirring for 24 h at 25 °C followed by filtration. After filtration, the concentrations of Pb(II) or Cd(II) in filtrate were determined and used to calculate the maximal sorption capacity according to
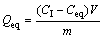 (2)
(2)
where sorption capacity Qeq is the amount of ions per unit of bead in equilibrium, Ceq is the equilibrium metal concentration in the solution, V is the volume of the solution used, and m is the mass of beads used.
Several isotherm equations have been used for the equilibrium modeling of the sorption processes. The most commonly used equations are Langmiur and Freundlich equations, which are widely used to analyze the sorption process in wastewater treatment. The Langmuir equation assumed a monolayer sorption of a solution from a liquid solution. The linearized mathematical description of the model is given by
 (3)
(3)
The main characteristics of the Langmuir equation, contants Kd and Qm can be determined from a linearized form of the Langmuir equation, as follows:
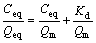 (4)
(4)
Therefore, a plot of Ceq/Qeq versus Ceq, gives a straight line of slope 1/Qm and intercept 1/(KdQm). Ceqand Qeq show the residue metal concentration and the amount of metal adsorbed on the beads at equilibrium. While Qm and Kd are the Langmuir constants, which relate to the maximum sorption capacity and sorption energy, respectively.
In addition, a molecule attached to a surface may affect another molecule attaching to neighbouring sites. The Freundlich model can describe ion sorption on a heterogeneous surface with an empirical equation:
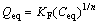 (5)
(5)
and can be expressed in the linearized form as Eq. (6), which is also used to confirm the applicability of the model:
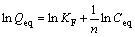 (6)
(6)
where KF and n are the Freundlich constant and indicator of sorption capacity and sorption intensity, respectively.
Selection of the best fitting model among the candidate models was done by using the Akaike’s information criterion corrected for small sample sizes (AICc) [19]: AICc=Nlg(RSS/N)+2k+2k(k+1)/(N-k-1), where N is the sample size, RSS is the residual sum of squares, k is the number of estimated parameters. The model with the smallest AICc was considered to be the best fitting model among the candidate models.
2.5 Analysis and characterization
The concentration of Pb(II) or Cd(II) in the filtrates obtained from both kinetic and isotherm experiments was determined by ICP-OES (iCAP6300). The synthesized phosphate-embedded alginate bead was ground into fine powders for the measurements of their physical and mechanical properties. The crystal structure of beads was characterized by an X-ray diffraction (XRD, D/max 2550 VB+X). The morphology of the beads before and after sorption of Cd(II) ion or Pb(II) was examined using a scanning electron microscope (SEM, Nova NanoSEM 230). Fourier transform infrared (FTIR NICOLET IS10) spectral measurements were carried out to identify the potential molecules of the beads. The sample was scanned under 400 to 4000 cm-1 at 4 cm-1 resolution.
3 Results and discussion
3.1 Characterization of phosphate-embedded calcium alginate beads
Generally, the wet beads are spherical (about 5 mm in diameter) and have a smooth surface. After drying, the diameter of the beads shrinks to about 2 mm and keeps a satisfactory spherical shape. SEM images directly demonstrate the microstructure of the beads surface. Figure 1(a) clearly shows that a lot of pleats exist on the bead surface, which enlarges the surface area. There are many grains on the surface (Fig. 1(b)) with the estimated diameter of 0.2 μm (Fig. 1(c)). The micro-porous structure of the beads is shown in Fig. 1(d), in which the bead surface consists of small and uniform spheres which favors the adsorption of metal ions in the aqueous solution and this property is different from other alginate and PVA beads [20,21].
FTIR could be used to detect the molecular structure of chemical compounds and it is useful for the characterization of biopolymers. Figure 2 shows FTIR spectra in the interval 500-4000 cm-1 of the beads before and after sorption the metal ions Pb(II), Cd(II) was adsorbed by phosphate-embedded calcium alginate beads.
Figure 2(a) reveals that anion functional groups (hydroxyl, amine) exist on the beads’ surface. It had been reported that 3000-3400 cm-1 is band of —OH or —NH. Bands at 2946, 1635, 1495, 1112 cm-1 refer to the vibration stretching of —CH, O—H stretch of—COOH, symmetric —CO2 stretch and C—O in the six-membered ring of sodium alginate [16], the band at 1028 cm-1 attributes to  , and the band at 822 cm-1may be due to
, and the band at 822 cm-1may be due to 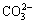 [17]. Therefore, a wide strong band about 3400 cm-1 may be due to the vibration stretching of the O—H bond and suggests that the presence of the hydroxyl (OH-) groups or amine (NH2) groups in the beads. But there is no N element in the beads. The band at 3000-3400 cm-1 represents —OH. And the bands at 2925, 1630, 1440 and 1100 cm-1 in the FTIR spectra refer to the bands of —CH, —COOH, C—O and P—O, respectively.
[17]. Therefore, a wide strong band about 3400 cm-1 may be due to the vibration stretching of the O—H bond and suggests that the presence of the hydroxyl (OH-) groups or amine (NH2) groups in the beads. But there is no N element in the beads. The band at 3000-3400 cm-1 represents —OH. And the bands at 2925, 1630, 1440 and 1100 cm-1 in the FTIR spectra refer to the bands of —CH, —COOH, C—O and P—O, respectively.

Fig. 1 SEM images of beads

Fig. 2 FTIR spectra before (a) and after sorption of Pb(II) (b) and Cd(II) (c) by beads
Figures 2(b) and (c) indicate no modification in FTIR spectra after sorption of metal ions Pb(II) and Cd(II). There is only a decrease in the intensity of the vibration due to the sorption process. The wider and weaker bands at 3000-3400 cm-1 after the bead sorption of Pb(II) or Cd(II) illustrate that the metal ions combining with groups of those beds affect the band length of —OH. Carboxyl may also be present in the beads because 800 cm-1 band attributes to the combination of heavy metal ions and 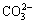 or
or 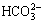 . The fact suggests that metal ions are combined with the functional hydroxyl, phenolic and carboxyl groups in the phosphate-embedded calcium alginate beads.
. The fact suggests that metal ions are combined with the functional hydroxyl, phenolic and carboxyl groups in the phosphate-embedded calcium alginate beads.
The phosphate-embedded calcium alginate beads were burned at 800 °C to remove the organic matter, increasing the concentration of phosphate. The residues were determined by XRD. Figures 3(a)-(c) show XRD patterns of the residues of the original beads, the beads sorbed Pb(II) and Cd(II), respectively. Compounds existed in the residues are listed in Table 1.
Figure 3 and Table 1 demonstrate that compounds of lead and phosphorus (Pb4P2O9 and Pb2O3) exist in the residue of the beads after Pb(II) sorption. Cadmium phosphate occurs in the residue of the beads after Cd(II) sorption. Cadmium phosphate and compounds of lead and phosphorus might be produced via two ways: the sorption process was followed by precipitation or formation in the beads burning process. Therefore, the sorption mechanism of Pb(II) and Cd(II) is that heavy metal ions are sorbed on the surface of beads, combined with the functional hydroxyl, phenolic and carboxyl groups, and finally precipitated with phosphate based on the SEM image and spectra of FTIR and XRD.
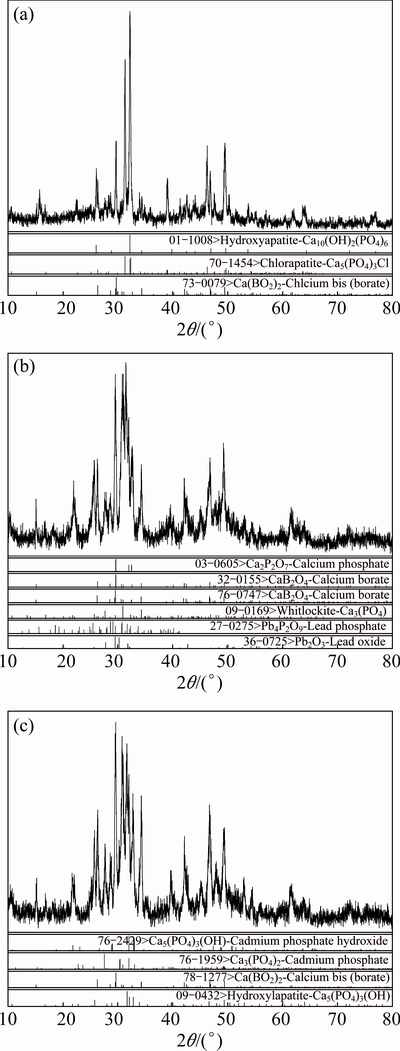
Fig. 3 XRD patterns of residuals of original beads (a), beads adsorbed Pb(II) (b) and Cd(II) (c)
Table 1 Compounds existed in different residuals

3.2 Effect of pH
Sorption process of heavy metal cations onto beads can be affected by pH because there is a competitive mechanism with the negative carboxylate and phosphate groups between H+ and metal ions. On the other hand, the pH value of the solution affects the degree of ionization, the surface charge and the speciation of metals, and the ionization state of the functional groups can affect the sorption mechanism and the uptake capacity [22,23].
Effects of pH on removal efficiency of Pb(II) and Cd(II) are shown in Fig. 4. When the pH value is lower than 3.0, there is no heavy metal removal in the presence of plenty of H+ ion because of reversal of the sorption process. It is obvious that the removal efficiency increases abruptly with the increasing initial pH from 3.0 to 4.0 and the maximum sorption capacity is at pH 4.0. For the competition between Cd(II) ions and H+ for the protonated active sites, the sorption capacity is significantly low when the pH is below 4.0. When the pH exceeds 4.0, the removal efficiency decreases slightly, while slumps after the pH high than 5.0. This phenomenon could be explained by those two reasons: firstly, the species of Pb(II) in solution would affect the sorption process. As pH increases, metal ions could transform into MOH+ through hydrolysis [24-26]. For Pb(II) cation, when pH increases to 4.0, it exists in the form of PbOH+. However, this form is not easier to transfer into beads comparing to Pb2+ [18]. Secondly, H+ would promote dissolution of Ca(H2PO4)2, and  would precipitate Pb2+ in liquor. The concentration of
would precipitate Pb2+ in liquor. The concentration of  and sorption rate of Pb(II) decreases with H+ decreasing.
and sorption rate of Pb(II) decreases with H+ decreasing.
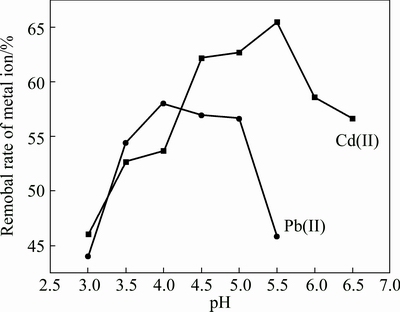
Fig. 4 Effect of pH on removal efficiency of metal cations
A similar tendency is observed for the effect of pH on Cd(II) adsorption. The efficiency was increased with the pH ascending to 5.5. This results may attribute to that amounts of protons(H+) would be available to the carboxyl groups at the lower pH, in which the available binding sites in the alginate molecule would be reduced. The high level hydrogen ions in acidic media occupy the negative binding sites, which is not favorable for metal sorption [21]. However, a striking decrease of the uptake capacity was observed at pH values higher than 5.5. The maximun sorption capacity at pH 4.0 and 5.5 for Pb(II) and Cd(II) were 58.05% (230.1 mg/g) and 62.17% (61.92 mg/g), respectively. When the pH was 3.0, the removal efficiencies of Pb(II) and Cd(II) were 43.5% (176.3 mg/g) and 45.03% (42.6 mg/g), respectively. However, in the previous literature, Cd reached the maximum sorption (40 mg/g) onto Ca-alginate beads at pH 4.0, while no Cd was adsorbed at pH 3.0 [19]. The result in this study implies that the phosphate-embedded calcium alginate beads can be used under the acidic condition.
3.3 Effects of initial concentration
Figure 5 shows the effect of the initial concentration on sorption capacity and removal efficiency of Pb(II) and Cd(II) from the solution. The results illustrate that the increase of the sorption capacity of metal ions on the beads is due to the initial concentration increase of cation.
The behavior of Pb(II) removal was affected by the initial concentration of Pb(II) in the solution. The removal efficiency increases with the initial concentration increasing from 100 mg/L to 200 mg/L with the maximum removal efficiency of 94.2%, and then it decreases as the initial concentration increases further. Sorption capacity of Pb(II) becomes almost constant at 250 mg/g when initial Pb(II) concentration exceeds 800 mg/L; however, the removal efficiency of 62.5% is too low. Consequently, the optimal initial concentration of Pb(II) is 200 mg/L. Sorption capacity of Cd(II) increases gradually with increasing initial concentration. The removal efficiency of Cd(II) decreases with the increases in ion dipole force. So, the optimal initial Cd(II) concentration is 25 mg/L, meanwhile the removal efficiency of 80% is maximum.

Fig. 5 Effect of initial concentration on sorption capacity and removal efficiency of Pb(II)(a) and Cd(II)(b)
Table 2 Parameters of Langmuir and Freundlich mathematic models related to sorption process of Pb(II) and Cd(II) on beads

3.4 Sorption isotherms
Sorption isotherms of Pb(II) and Cd(II) ions on the beads are shown in Table 2. Parameters of Langmuir and Freundlich mathematic models are linearized by data obtained from the equilibrium of the sorption isotherms of the Pb(II) and Cd(II) ions onto the beads. Langmuir model employed to describe the sorption of Cd(II) by the beads is more satisfactory than Freundlich model because Langmuir model shows a lower AICc value and high R2 than Freundlich model, which suggests that the adsorption of Pb(II) and Cd(II) is mono-layer sorption occurred in its sorption process [19].
According to the Langmuir isotherm model, the sorption capacities of Pb(II) (263.16 mg/g) and Cd(II) (82.64 mg/g) by phosphate-embedded calcium alginate beads are higher than those by double network gel (100 mg/g for Pb) [13], PVA/gelatin hydrogel beads (203 mg/g for Pb) [27] and Alginate-Moringa oleifera beads (60 mg/g for Cd) [28].
The maximum sorption capacity (Qm) within Langmuir’s model is supposed to coincide with the saturation of a fixed number of active sites on the beads’ surface, which is affected by several factors, such as the number of sites in the beads, accessibility of sites, chemical state of the sites and affinity between the site and the metal ions. It may also be associated with the functional groups on the surface. Langmuir’s parameter (Kd) is a constant that represents the interaction force between the sorbent and the sorbed and is highly relevant in sorption studies. According to Table 2, high Kd shows high bonding energy between metals and sorbents. Among Freundlich’s parameters, n indicates the reactivity of the sorbent’s active sites, which is entirely related to the solid’s heterogeneity. Table 2 shows that n rates were higher than 1, indicating that strong evidence exists on the presence of highly energetic sites. The higher the difference between n and 1, the greater the distribution of the bonding energy on the beads’ surface. The results may also imply the occurrence of cooperative sorption in which strong interaction between molecules of the sorbate is involved.
4 Conclusions
Phosphate-embedded calcium alginate beads were successfully synthesized based on sodium alginate Ca(H2PO4)2 and NaHCO3. This phosphate-embedded calcium alginate beads can be used as absorbent to remove Pb(II) and Cd(II), and the sorption capacities of Pb(II) and Cd(II) are 263.16 mg/g and 82.64 mg/g, respectively. The sorption mechanism is that the heavy metal ions access to the beads firstly due to the large surface area, combined with OH-, and then precipitated with phosphate radical. The optimal pH values for Pb(II) and Cd(II) sorption are 4.0 and 5.5, respectively. Langmuir mathematical model employed to describe the sorption of Cd(II) by the beads is satisfactory, which suggests that mono-layer sorption is predominant in the sorption process. A further study will focus on the metal species competition in multi-metal solutions, and issues of the performance in remediation of the heavy metal contaminated soil will also be addressed in later work.
References
[1] ISLAM M S, AHMED M K, RAKNUZZAMAN M, HABIBULLAH-AI-MAMUN M, ISLAM M K. Heavy metal pollution in surface water and sediment: A preliminary assessment of an urban river in a developing country [J]. Ecological Indicators, 2015, 48(2): 282-291.
[2] GUO Wei, HUO Shou-liang, XI Bei-dou, ZHANG Jing-tian, WU Feng-chang. Heavy metal contamination in sediments from typical lakes in the five geographic regions of China: Distribution, bioavailability, and risk [J]. Ecological Engineering, 2015, 81(8): 243-255.
[3] CHEN Hai-yang, TENG Yan-guo, LU Si-jin, WANG Ye-yao, WANG Jin-sheng. Contamination features and health risk of soil heavy metals in China [J]. Science of the Total Environment, 2015, 512-513(4): 143-153.
[4] MISHRA P C, PATEL R K. Removal of lead and zinc from water by low cost adsorbents [J]. Journal of Hazardous Materials, 2009, 168(1): 319-325.
[5] WANG Hui-min, MIN Xiao-bo, CHAI Li-yuan, SHU Yu-de. Biological preparation and application of poly-ferric sulfate flocculant [J]. Transactions of Nonferrous Metals Society of China, 2011, 21(11): 2542-2547.
[6] SU Yan rong, CHAI Li yuan, YANG Zhi hui, YOU Xiang yu, ZHU Yong hua. Biosorption characteristics of Pb2+ by Pannonibacter phragmitetus T1 [J]. The Chinese Journal of Nonferrous Metals, 2011, 21(12): 3211-3217. (in Chinese).
[7] LI Qing-Zhu, QIN Wen-qing, CHAI Li-yuan, WANG Qing-wei. Characteristics of Pb(II) adsorption on esterified spent grain [J]. The Chinese Journal of Nonferrous Metals, 2013, 23(4): 1152-1159. (in Chinese).
[8] MIAO Yu, MIN Xiao-bo, CHAI Li-yuan, YIN Yi-nan. Removal of fluoride and lead in wastewater with iron-bioflocculant [J]. The Chinese Journal of Nonferrous Metals, 2012, 22(8): 2366-2373. (in Chinese).
[9] PAPAGEORGIOU S K, KATSAROS F K, KOUVELOS E P, NOLAN J W, LE DELT H, KANELLOPOULOS N K. Heavy metal sorption by calcium alginate beads from Laminaria digitata [J]. Journal of Hazardous Materials, 2006, 137(3): 1765-1772.
[10] CATALDO S E, GIANGUZZA A, PETTIGNANO A, VILLAESCUSA I. Mercury(II) removal from aqueous solution by sorption onto alginate, pestate and polygalacturonate calcium gel beads: A kinetic and speciation based equilibrium study [J]. Reactive and Functional Polymers, 2013, 73(1): 207-217.
[11] MIGNARDI S, CORAMI A, FERRINI V. Evaluation of the effectiveness of phosphate treatment for the remediation of mine waste soils contaminated with Cd, Cu, Pb, and Zn [J]. Chemosphere, 2012, 86(4): 354-360.
[12] LAKOURAT M M, MOJERLOU F, ZARE E N. Nanogel and superparamagnetic nanocomposite based on sodium alginate for sorption of heavy metal ions [J]. Carbohydrate Polymers, 2014, 106(6): 34-41.
[13] BEE A, TAIBOT D, ABRAMSON S, DUPUIS V. Magnetic alginate beads for Pb(II) ions removal from wastewater [J]. Journal of Colloid and Interface Science, 2011, 362(2): 486-492.
[14] RIYAJAN S A, TANGBORIBOONRAT P. Novel composite biopolymers of sodium alginate/natural rubber/coconut waste for adsorption of Pb(II) ions [J]. Polymer Composites, 2014, 35(5): 1013-1021.
[15] SILA C C, PINHEIRO A G, MIRANDA M A R, GOES J C, SOMBRA A S B. Structural properties of hydroxyapatite obtained by mechanosynthesis [J]. Solid State Sciences, 2003, 5(4): 553-558.
[16] ZHANG Yun, LI Yan-feng, LI Xiao-li, YANG Liu-qing, BAI Xue, YE Zheng-fang, ZHOU Lin-cheng, WANG Li-yuan. Selective removal for Pb2+ in aqueous environment by using novel macroreticular PVA beads [J]. Journal of Hazardous Materials, 2010, 181(1-3): 898-907.
[17] GOBURLAOUEN C, PIQUEMAL J P, PARISEL O. [Pb(H2O)]2+ and [Pb(OH)]+: four-component density functional theory calculations, correlated scalar relativistic constrained-space orbital variation energy decompositions, and topological analysis [J]. Journal of Chemical Physics, 2006, 124(17): 174311.
[18] NURUDDIN M F, KHAN S U, SHAFIG N, AYUB T. Strength development of high-strength ductile concrete incorporating metakaolin and PVA fibres [J]. The Scientific World Journal, 2014, 567(10): 505-510.
[19] KATSANEVAKIS S. Density surface modelling with line transect sampling as a tool for abundance estimation of marine benthic species: The Pinna nobilis example in a marine lake [J]. Marine Biology, 2007, 152(1): 77-85.
[20] CHU Lin, LIU Cheng-bin, ZHOU Gui-yin, XU Rui, TANG Yan-hong, ZENG Ze-bing, LUO Sheng-lian. A double network gel as low cost and easy recycle adsorbent: Highly efficient removal of Cd(II) and Pb(II) pollutants from wastewater [J]. Journal of Hazardous Materials, 2015, 300: 153-160.
[21] HUI Bing, ZHANG Yi, YE Lin. Structure of PVA/gelatin hydrogel beads and adsorption mechanism for advanced Pb(II) removal [J]. Journal of Industrial and Engineering Chemistry, 2015, 21(4): 868-876.
[22] ARICA M Y, ARPA C, ERGENE A, BAYRAMOGLU G, GENC O. Ca-alginate as a support for Pb(II) and Zn(II) biosorption with immobilized Phanerochaete chrysosporium [J]. Carbohydrate Polymers, 2003, 52(2): 167-174.
[23] LI Ying, YUE Qing-yan, GAO Bao-yu. Adsorption kinetics and desorption of Cu(II) and Zn(II) from aqueous solution onto humic acid [J]. Journal of Hazardous Materials. 2010, 178(1-3): 455-461.
[24] BABICH H, STOTZKY G. Toxicity of zinc to fungi, bacteria, and coliphages: Influence of chloride ions [J]. Applied and Environmental Microbiology, 1978, 36(6): 906-914.
[25] SUN Xiao-xia, UYAMA H. Poly(vinyl alcohol)/sodium alginate blend monolith with nanoscale porous structure [J]. Nanoscale Research Letters, 2013, 8(1): 411-415.
[26] ZHANG Dan, ZHANG Yu, SHEN Fei, WANG Ji-peng, LI Wei, LI En-xia, FALANDYSZ J. Removal of cadmium and lead from heavy metals loaded PVA–SA immobilized Lentinus edodes [J]. Desalination and Water Treatment, 2014, 52(25-27): 4792-4801.
[27] IDRIS A, ISMAIL N M, HASSAN N, MISRAN E, NGOMSIK A F. Synthesis of magnetic alginate beads based on maghemite nanoparticles for Pb(II) removal in aqueous solution [J]. Journal of Industrial and Engineering Chemistry, 2012, 18(5): 1582-1589.
[28] MONALISA F, JESUS B H, JOSE E S P. Use of Alginate-Moringa oleifera beads on Cu(II) and Cd(II) adsorption from aquatic systems [J]. International Journal of Chemical Engineering and Application, 2013, 4(6): 373-376.
王云燕1,2,姚文斌1,2,王庆伟1,2,杨志辉1,2,梁丽芬1,2,柴立元1,2
1. 中南大学 冶金与环境学院,长沙 410083;2. 中南大学 国家重金属污染防治工程技术研究中心,长沙 410083
摘 要:采用海藻酸钠、磷酸二氢钙和碳酸氢钠成功合成了包埋磷酸盐的海藻酸钙小球。通过扫描电镜、傅里叶变换红外光谱、X射线衍射等分析表征了该小球的形貌与结构。研究pH值和初始金属离子浓度对铅镉去除率的影响,发现吸附铅镉的最佳pH值分别为4.0和5.5;铅镉的最适初始浓度分别为200 mg/L和25 mg/L,对应的去除率分别达94.2%和80%。XRD和FTIR的分析结果证实了该小球对铅镉的去除机理为:铅镉离子吸附到小球的表面,与小球的羧基发生反应,进而与磷酸根反应生成稳定的磷酸盐沉淀。铅镉的吸附符合Langmuir等温线方程,拟合系数R2分别为0.9957和0.988。根据Langmuir等温线方程计算得到铅镉的理论饱和吸附量分别为263.16 mg/g和82.64 mg/g。研究结果表明该小球对溶液中的铅镉离子有良好的处理效果,同时由于生成稳定的沉淀物,也能应用于处理被铅镉污染的水稻土。
关键词:吸附;固定;铅;镉;包埋磷酸盐海藻酸钙
(Edited by Xiang-qun LI)
Foundation item: Project (51504299) supported by the National Science Found for Young Scientists of China; Project (2012GS430101) supported by the National Science and Technology Program for Public Wellbeing, China
Corresponding author: Qing-wei WANG; Tel: +86-731-88830875; Fax: +86-731-88710171; E-mail: qw_wang@csu.edu.cn
DOI: 10.1016/S1003-6326(16)64340-6

