Pyrolysis of different sewage sludge feedstocks for biochar products: Characterization and application
来源期刊:中南大学学报(英文版)2020年第11期
论文作者:黄华军 刘平 王佳欣 赖发英
文章页码:3302 - 3319
Key words:sewage sludge; pyrolysis; biochar; heavy metals; adsorption
Abstract: Four sewage sludge (SS) feedstocks with distinct properties were converted into biochars by pyrolysis at 300-700 °C, in order to clarify the effects of the composition difference of SS feedstocks. The yields of biochars present a positive correlation with the contents of ash in SS. Notedly, the contents of organic matter (OM) in SS largely determine the quality of biochars. SS feedstocks with high content of OM are more likely to form stable biochars with higher aromaticity/carbonization degree, and the formed biochars possess higher calorific values. The contents of residual OM in biochars derived from SS feedstocks with low content of OM likely fail to meet the needs of soil improvement (10 wt.%). Most of heavy metals (HMs) existing in raw SS are remained in biochars after pyrolysis. The biochar produced from SS feedstocks with high content of HMs usually contains higher contents of HMs. Surprisingly, the leachability of HMs in biochars is all weakened to some extent compared to raw SS. In addition, the biochars show higher thermal stability and pH values, and P/K nutrients are enriched in biochars. The biochars prepared from four SS feedstocks exhibit different adsorption ability of methylene blue, especially at low dosage of biochar.
Cite this article as: WANG Jia-xin, LIU Ping, LAI Fa-ying, HUANG Hua-jun. Pyrolysis of different sewage sludge feedstocks for biochar products: Characterization and application [J]. Journal of Central South University, 2020, 27(11): 3302-3319. DOI: https://doi.org/10.1007/s11771-020-4548-y.

J. Cent. South Univ. (2020) 27: 3302-3319
DOI: https://doi.org/10.1007/s11771-020-4548-y

WANG Jia-xin(王佳欣)1, LIU Ping(刘平)2, LAI Fa-ying(赖发英)1, HUANG Hua-jun(黄华军) 1
1. School of Land Resources and Environment, Key Laboratory of Agricultural Resource and Ecology in the
Poyang Lake Basin of Jiangxi Province, Jiangxi Agricultural University, Nanchang 330045, China;
2. School of Animal Science and Technology, Jiangxi Agricultural University, Nanchang 330045, China
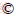 Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2020
Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2020
Abstract: Four sewage sludge (SS) feedstocks with distinct properties were converted into biochars by pyrolysis at 300-700 °C, in order to clarify the effects of the composition difference of SS feedstocks. The yields of biochars present a positive correlation with the contents of ash in SS. Notedly, the contents of organic matter (OM) in SS largely determine the quality of biochars. SS feedstocks with high content of OM are more likely to form stable biochars with higher aromaticity/carbonization degree, and the formed biochars possess higher calorific values. The contents of residual OM in biochars derived from SS feedstocks with low content of OM likely fail to meet the needs of soil improvement (10 wt.%). Most of heavy metals (HMs) existing in raw SS are remained in biochars after pyrolysis. The biochar produced from SS feedstocks with high content of HMs usually contains higher contents of HMs. Surprisingly, the leachability of HMs in biochars is all weakened to some extent compared to raw SS. In addition, the biochars show higher thermal stability and pH values, and P/K nutrients are enriched in biochars. The biochars prepared from four SS feedstocks exhibit different adsorption ability of methylene blue, especially at low dosage of biochar.
Key words: sewage sludge; pyrolysis; biochar; heavy metals; adsorption
Cite this article as: WANG Jia-xin, LIU Ping, LAI Fa-ying, HUANG Hua-jun. Pyrolysis of different sewage sludge feedstocks for biochar products: Characterization and application [J]. Journal of Central South University, 2020, 27(11): 3302-3319. DOI: https://doi.org/10.1007/s11771-020-4548-y.
1 Introduction
Biochar is a kind of solid product rich in carbon, which is prepared by thermal treatment of biomass, especially pyrolysis in anoxic atmosphere [1, 2]. The difference in the properties of biochar products is often very significant. The properties of biochar depend not only on the type of raw materials used to prepare biochar, but also on the pyrolysis conditions [3, 4]. In the thermal treatment process of biomass, the moisture and volatile substances are removed, so the resultant biochar usually has porous structure, maintained surface functional groups and mineral components [5]. These favorable properties make biochars widely used in various fields, such as agricultural production, climate change, environmental remediation and functional material development [6, 7].
Organic materials from biological organisms or compounds of organic and inorganic materials from the above sources are collectively referred to as biomass [4]. Therefore, various types of waste, such as livestock manure, sludge, waste paper and many industrial organic wastes, can also be regarded as biomass, which can be used to prepare biochar [7]. Sewage sludge (SS) is a kind of solid waste, which is mainly produced from the biochemical treatment process of wastewater. The research/development of sustainable SS treatment method is an important issue all over the world [8-10]. SS is a kind of heterogeneous material, which is composed of microorganism, undigested organic component and inorganic component, and contains a lot of water. SS is rich in nitrogen, phosphorus and potassium nutrients, and the content of micronutrients is also very high [11, 12]. There is no doubt that SS is a potential material for the preparation of biochar.
Thermochemical conversion technologies offer a new way of managing SS, not only by providing effective volume reduction, but also by enabling transformation of carbon-rich organic fraction into valuable products, including biooil, biochar and gases [13]. The prevailing thermochemical technologies applied to the treatment of SS include pyrolysis, combustion, incineration, gasification and hydrothermal carbonization/liquefaction, each of which has its own technological and socioeconomic merits and demerits [14]. In recent years, the preparation and application of biochar from SS by pyrolysis have attracted much attention. In particular, four aspects of work have been carried out: 1) the influences of process conditions, mainly including pyrolysis temperature, pyrolysis time, and activation treatment [15-18]; 2) co-pyrolysis of SS with lignocellulosic materials and microalgae, such as wooden waste, food shell or core, agricultural straw, and Chlorella [19-23]; 3) the occurrence characteristics (concentration and mobility) of heavy metals (HMs) in biochar [24-28]; 4) the utilization of SS-derived biochar as catalyst [29-31], adsorbent [32-34] and soil restoration/ improvement agent [35-38].
As mentioned before, the properties of raw biomass feedstocks are one of the important factors influencing the preparation of biochar. However, a few works have been carried out on the characterization/application of biochars obtained from different SS feedstocks [39, 40]. ZIELINSKA et al [39] compared the properties of biochars obtained from the pyrolysis of four anaerobic digested SS at 500-700 °C. LU et al [40] performed the characterization of biochars derived from the pyrolysis of three undigested SS at 300-600 °C. Notedly, above studies only analyzed the characteristics of biochars produced from a certain kind of SS (anaerobic digested SS or undigested SS) in a limited temperature range. Accurately speaking, the differences of the characteristics of SS feedstocks used in above each study are not obvious.
To further understand the effects of the initial properties of SS feedstocks, this study collected four kinds of SS feedstocks with distinct properties, including one anaerobic digested SS feedstock and three undigested SS feedstocks. These SS feedstocks were converted into biochar by pyrolysis at 300-700 °C, and the properties of corresponding biochars were analyzed and compared systematically. Finally, the SS-derived biochars were applied to the purification of dye wastewater by adsorption to clarify their adsorption potential.
2 Materials and methods
2.1 Sewage sludge
The raw SS materials used in this study are obtained from four sewage treatment plants located in the main urban areas of Nanchang City, Jiangxi Province, China. The four kinds of sludge are labeled with SS1 (Qingshanhu District), SS2 (Chaoyang District), SS3 (Honggutan District), and SS4 (Xianghu District), respectively. The source of sewage is mainly domestic sewage mixed with industrial/commercial wastewater of different proportions. The biological treatment process of sewage is mainly the activated sludge process, for example, A2/O process or oxidation ditch process. Except the SS1 feedstock with anaerobic digestion, the other three SS feedstocks are directly dewatered mechanically by the belt or rotary press filter after being concentrated. The dewatered sludge is collected, and it is first dried naturally in the room for several weeks, then crushed and screened through a 2 mm sieve. Finally, the sludge powder is dried in an oven at 105 °C for 12 h and put into a sealed bag, which is stored in a desiccator for use. As shown in Table 1, the properties of four SS feedstocks are obviously different.
The content of oxygen is determined by Eq. (1).
w(O)=100%-wAsh-w(H)-w(C)-w(N)-w(S) (1)
where w(Ash) is the content of ash in SS and biochar (wt.%); w(C), w(H), w(S), w(N) and w(O) are the mass percentages of carbon, hydrogen, sulfur, nitrogen and oxygen, respectively (wt.%).
Table 1 Elemental and proximate analysis of SS samples
2.2 Pyrolysis experiments
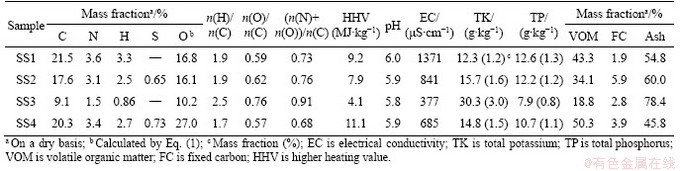
The pyrolysis of SS is carried out in a muffle furnace. First, a certain number of sludge samples (8.0 g) are placed in a round crucible with a cover, and then the crucible is placed in the heating area of the oven. In order to maintain the anoxic environment, nitrogen was introduced into the reaction chamber at a rate of 200 mL/min during pyrolysis. At the rate of 20 °C/min, the sludge sample is heated to the set reaction temperature (300, 400, 500, 600 and 700 °C). After reaching the reaction temperature, it stays for 1 h [21, 35]. After the reaction, stop heating, take out the crucible when the temperature drops to room temperature, and weigh the residue after sludge pyrolysis, i.e., biochar. The yield of biochar (YBiochar) is calculated according to the following equation:
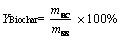 (2)
(2)
where mBC refers to the dry mass of biochar; mSS is the dry mass of SS subjected to pyrolysis.
2.3 Chemical analysis
2.3.1 Analysis of basic physicochemical properties
The compositions of elements (C, H, N and S) in raw SS feedstocks and biochar products are detected by an element analyzer (Flash EA1112, Thermo, America). In order to determine the proximate compositions of raw SS feedstocks and biochar products, a certain amount of SS and biochar samples are firstly burned in muffle furnace at 500 °C for 1 h, and then the mass loss rate of each sample is calculated, which is defined as the content of volatile organic matter (VOM). A certain number of samples are further burned in a muffle furnace at 850 °C for 1 h, and then the residue is collected and weighed. The percentage of residue in the test sample is defined as the content of ash. The content of organic matter (OM) is defined as the difference between 100% and ash content, while the content of fixed carbon (FC) is calculated by subtracting the content of OM and VOM [41, 42]. In the light of the solid-liquid ratio of 1:10, a certain amount of SS and biochar samples are fully mixed with deionized water. The pH and electrical conductivity (EC) values of each suspension are detected by a pH meter (PHS-3C, China) and a conductivity meter (DDS-11A, China), respectively. The calorific values (Q, higher heating value) of raw SS feedstocks and biochar products are calculated by the modified formula of Dulong on the basis of their element composition [43, 44].
 (3)
(3)
In order to determine the content of main nutrients, a certain amount of SS and biochar samples are first dissolved by sodium hydroxide, and then the concentrations of total phosphorus/ potassium (TP/TK) in the digestive solution are measured by a visible spectrophotometer (772, Yoke, China) and a flame photometer (FP6400, Yuefeng, China), respectively. The content of total nitrogen (TN) is estimated according to the results of elemental analysis.
2.3.2 FT-IR spectra and thermal stability analysis
The functional groups of raw SS and biochar samples are determined by a Fourier transform infrared spectrometer (FT-IR, Nicolet5700, USA) with the aid of potassium bromide pellet technology. It mainly collects spectra in the range of 4000 cm-1 to 400 cm-1. The thermal stability analysis of SS and biochar samples is conducted with the aid of a thermogravimetric analyzer (TGA-4000, PerkinElmer, USA). The test sample is heated from room temperature (30 °C) to 1000 °C at the rate of 20 °C/min, and nitrogen is injected continuously at the rate of 200 mL/min during the heating period.
2.3.3 Micromorphology and surface characteristics
The raw SS and biochar samples are analyzed by an environmental scanning electron microscope (SEM, JSM6701F, JEOL, Japan), and their microstructure is characterized. The specific surface area, pore size and pore volume of biochar products are measured by means of a specific surface area and pore size analyzer (Micromeritics TriStar II 3020, USA).
2.3.4 Heavy metal analysis
The mixed-acid digestion method (HNO3- HClO4-H2O2 (30%)) is adopted to determine the total contents of HMs in raw SS feedstocks and biochar products. The leaching concentration of HMs in raw SS feedstocks and biochar products is determined by a toxicity characteristic leaching procedure (TCLP). The specific steps of the above two methods can refer to the previous papers published by the author’s research group [21, 45-47]. The content of HMs in each digestion/ extraction solution is determined by an atomic absorption spectrophotometer (PinAAcle 900T, Perkin Elmer, USA). Six conventional HMs, such as copper, zinc, lead, cadmium, chromium and nickel, are mainly considered.
2.4 Adsorption experiments
A certain amount of biochar is weighed and mixed with 25 mL of methylene blue solution (pH=7.0, 200 mg/L), and then shaken at 200 r/min for 12 h at room temperature (30 °C). Then, the mixture is transferred to a centrifuge tube. After centrifugation (6000 r/min for 10 min), the concentration of methylene blue in the supernatant is determined according to the method of ultraviolet spectrophotometry (UV-6300, Mapada, China). Finally, according to Eqs. (4) and (5), the removal rate (q, %) of methylene blue and the adsorption capacity (Q, mg/g) of biochar are calculated, respectively, in order to characterize the adsorption potential of biochar.
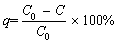 (4)
(4)
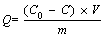 (5)
(5)
where C0 is the initial concentration of methylene blue (mg/L); C is the concentration of methylene blue after adsorption (mg/L); V is the volume of methylene blue solution (L); m is the mass of biochar (g).
3 Results and discussions
3.1 Biochar yield
Figure 1 depicts the yield of biochar obtained from the pyrolysis of different SS samples at various temperatures (300-700 °C). No matter what kind of SS, the yield of biochar decreases with the increase of pyrolysis temperature and the highest decline in yield is observed for SS4. During the pyrolysis of SS, organic matters contained in SS will be decomposed/transformed. The decrease of biochar yield with increasing pyrolysis temperature may be due to the more intense thermal degradation of SS at high pyrolysis temperature. Specifically, the primary decomposition of initial SS feedstock and the secondary reaction of stable residue will become more efficient [39]. As shown in Table 1, the SS4 feedstock contains the highest content of organic matters, which results in the largest decline in the yield of biochar (29.7% fall).
The yields of biochar obtained from the pyrolysis of SS are usually higher than those reported for lignocellulosic biomass [48, 49]. The reason may be that the SS feedstock usually contains higher content of inorganic matter (ash) compared to those traditionally used biomass [39]. In addition, as clearly shown in Figure 1, the yield of biochar produced from different SS essentially ranks in the following order: SS3 ((96.1-82.4) wt.%)>SS2 ((86.6-66.8) wt.%)>SS1 ((82.1- 63.1) wt.%)>SS4 ((84.4-54.7) wt.%), which is consistent with the sequence of ash content in SS (Table 1). In general, the yield of SS-derived pyrolysis biochar is not only influenced by the pyrolysis reaction conditions, for example, the reaction temperature discussed in this study, but also largely depends on the proximate compositions of raw SS feedstocks, such as the contents of ash and OM. In a research of LU et al [40], it is proposed that the presence of various metals in raw SS feedstocks, which may possess catalytical effects on the pyrolysis reaction, may be one of the reasons for the difference in the biochar yield among different SS feedstocks.
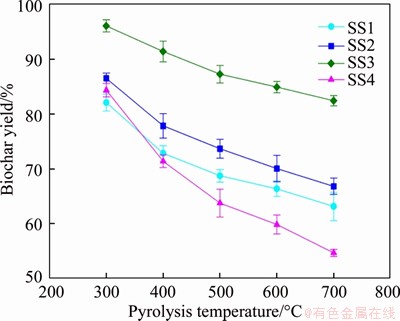
Figure 1 Yield of biochars obtained from different SS samples
3.2 Basic characteristics of biochars
3.2.1 Elemental compositions
The contents of C in different SS feedstocks range from 9.1 wt. % to 21.5 wt.% (Table 1). The contents of N and H are both at a low level, from 1.5 wt.% to 3.6 wt.% and from 0.86 wt.% to 3.3 wt.%, respectively. S is detected in SS2 (0.65 wt.%) and SS4 (0.73 wt.%), but not observed in SS1 and SS3. Significant variations are also observed in the contents of O in different SS feedstocks, ranging from 10.2 wt.% to 27.0 wt.%. The contents of C, N, and H in SS1 are the highest, whereas the lowest contents are found in SS3. The sample of SS4 contains the highest content of O, and the lowest content is found in SS3.
Generally, the pyrolysis of SS has a obvious influence on the change in the contents of C in biochars (Table 2). Compared with the original SS feedstock, the content of C in biochar decreases, and the higher the pyrolysis temperature, the greater the reduction. An exception is found for SS4 that the contents of carbon in biochars obtained at 300 and 400 °C slightly increase by 1.2% and 5.5%, respectively. The variations of the contents of N and H in biochars are similar to that of C. Except for some biochars produced at low pyrolysis temperature, the contents of N and H in all biochars will reduce, and the decrease will be more obvious with the increase of pyrolysis temperature. The decomposition of proteins in SS results in the decrease of N content in biochar; while the dehydration, decarboxylation and de-methanations reactions contribute to the removal of C and H during the pyrolysis of SS [50].
Table 2 Elemental and proximate analysis of biochars

After pyrolysis, the contents of O in all biochars decrease significantly compared to the initial SS feedstocks. For example, the contents of O in biochar obtained at 700 °C reduce by 90.2%-96.3%. On the contrary, the contents of S in biochars slightly increase after pyrolysis. In special, the contents of S in SS2-derived biochars range from 1.3 wt.% to 1.6 wt.% versus 0.65 wt.% in raw SS2, while the contents of S in SS4-derived biochars range from 1.4 wt.% to 1.6 wt.% versus 0.73 wt.% in raw SS4. The caloric values of biochars are lower than those of raw SS samples and decline with the elevation of pyrolysis temperature, which is mainly because of the high content of ash in SS feedstocks ((45.8-78.4) wt.%), now that the energy enrichment of pyrolysis process only occurs in organic components of raw materials [51].
For the raw SS feedstocks, the molar ratios of H/C, O/C, and (N+O)/C lay within the ranges of 1.7-2.5, 0.57-0.76 and 0.73-0.91, respectively (Table 1). After pyrolysis, all the above molar ratios of biochars significantly decline. In general, the higher the reaction temperature is, the lower these molar ratios are (Table 2). In a certain extent, the aromaticity, carbonization degree, and polarity of biochar can be indicated by the molar ratios of H/C, O/C, and (N+O)/C, respectively [52, 53]. The lower the molar ratios of H/C, the higher content of the aromatic carbon compounds, which makes it not easy to be decomposed by microorganisms. In addition, the lower the molar ratio of O/C, the higher the carbonization degree of biochar. Thus, compared with raw SS feedstocks, biochars have a longer half-life in the soil and will perform better in long-term soil carbon sequestration [39]. It has been suggested that the H/C molar ratio of a stable biochar should be lower than 0.7 [51]. Biochars derived from SS1, SS2, and SS4 at higher pyrolysis temperature present desired H/C molar ratios, but all SS3-derived biochars fail to meet the requirements, which indicates that the SS feedstock with lower content of OM is not conducive to the formation of stable biochar. The biochar with higher (N+O)/C molar ratio usually possesses higher polarity and a better adsorption capacity for polar substances [17]. On the whole, compared to raw SS feedstocks, the biochar products have higher stability and lower polarity. And this trend becomes more obvious with the increase of reaction temperature.
3.2.2 Contents of ash, VOM and FC
The proximate compositions of raw SS feedstocks and biochars are also listed in Tables 1 and 2. The SS4 feedstock contains the highest content of VOM (50.3 wt.%) and the lowest content of ash (45.8 wt.%). The situation is just the opposite for the SS3 feedstock, which possesses the highest content of ash (78.4 wt.%) and the lowest content of VOM (18.8 wt.%). The content of FC in all SS feedstocks is relatively low, ranging from 1.9 wt.% to 5.9 wt.%. Although the proximate compositions of raw SS feedstocks are different, the changing trends of the proximate compositions in biochars are similar after SS pyrolysis. The contents of VOM in biochars are all lower than those in raw SS feedstocks and further decrease with the elevation of reaction temperature. The changing trend of ash content in biochar is just the opposite. These results are associated with the removal of volatile organic decomposition products and the concentration of non-volatile mineral constituents forming ash, especially at higher pyrolysis temperatures [39]. In addition, the pyrolysis of SS has no definite effect on the content of FC in biochar. The content of VOM in biochar is also seen as an indicator of carbonization extent. In special, the biochar with lower VOM content possesses higher carbonization degree [54]. Obviously, the biochar obtained at higher pyrolysis temperature usually has a higher carbonization degree.
3.2.3 pH and EC
The pH value is mainly depending on the compositions of inorganic components and organic functional groups in raw SS feedstocks and biochar products [50]. The raw SS feedstocks present weak acid characteristics with the pH values of 5.8-6.0. After pyrolysis, the pH values of biochars are higher than that of SS feedstock and increase gradually with the increase of pyrolysis temperature. This phenomenon can be ascribed to the following three reasons: 1) the polymerization/condensation reactions of aliphatic compounds; 2) the dehydration of SS feedstock resulting in the decrease of the amount of acidic surface groups; 3) the removal of alkali metal salts from the organic matrix with elevating temperature [39]. The EC of raw SS feedstocks is ranging from 377-1371 μS/cm. It is consistent that the EC values of biochars are lower than that of raw SS feedstock, which is due to the decomposition of dissolved salts during the pyrolysis process [15].
3.2.4 Nutrient components
The essential nutrients for soil improvement and fertility maintenance mainly include nitrogen, phosphorus and potassium. The contents of N/P/K in raw SS feedstocks and biochar products can refer to Tables 1 and 2. The content of TN in SS3 feedstock is the lowest with the value of 15.0 g/kg. The contents of TN in other three SS feedstocks are close, ranging from 31.0 to 36.0 g/kg. For biochars obtained from SS1-SS3, the contents of TN are all lower than those in raw SS feedstocks and decrease with the rise of pyrolysis temperature. As regards biochars produced from SS4 at lower pyrolysis temperature (300-400 °C), the contents of TN are higher than that in raw SS4 feedstock. However, when the reaction temperature further increases, the content of TN presents a downward trend. YUAN et al [15] reported similar results and concluded that low reaction temperature sometimes would facilitate the enrichment of N in biochar. Nitrogen is mainly removed in three forms: the loss of NH4-N, the loss of NO3-N fraction, and the loss of volatile matter containing N groups. The residual nitrogen in biochar mainly organically bonded in recalcitrant forms [55].
The contents of TP and TK in SS feedstocks are ranging from 7.9 to 12.6 g/kg and from 12.3 to 30.3 g/kg, respectively. After pyrolysis, the contents of TP/TK in biochars are generally higher than those in raw SS feedstocks and increase with elevating reaction temperature from 300 to 700 °C. In other words, the pyrolysis treatment facilitates the accumulation of P/K in biochars. Phosphorus in raw SS feedstocks mainly exists in the form of phosphate mineral, which is difficult to degrade and its crystallinity will become higher with the increase of reaction temperature. This is just the reason why phosphorus is enriched in biochar [15, 17]. Potassium is also mainly associated with the inorganic fractions in raw SS feedstock, resulting in the increase of K content in biochars [55].
In the light of a sludge disposal standard carried out in China (GB/T 24600-2009), the pH value of treated sludge used for soil improvement should be between 5.5 and 10, while the contents of total nutrients (TN+TP+TK) and OM need to be higher than 1 wt.% and 10 wt.%, respectively [56]. The biochars prepared from SS1, SS2, and SS4 show the required pH value and the contents of total nutrients and OM. The contents of OM in biochars produced from SS3 at higher pyrolysis temperatures are not up to the mark. The main reason is that the content of OM in raw SS3 feedstock is as low as 21.6 wt.%.
3.3 FT-IR analysis of biochars
The FT-IR analysis results of raw SS feedstocks and biochars are presented in Figure 2. The vibrational peaks of —OH in mineral components are usually present around 3600 and 3700 cm-1 [55]. These two peaks are observed in the FT-IR curves of SS2, SS3 and SS4 feedstocks, but not found for the corresponding biochars. Interestingly, the characteristic peak around 3700 cm-1 appears on the infrared spectra of SS1-derived biochar but the SS1 feedstock has no related peak. Obvious characteristic peak around 3400 cm-1, representing the vibrations of —OH in the organic matter, appears in all four SS feedstocks. And the intensity of this peak weakens in the corresponding biochars, indicating that a large lot of organic matter containing hydroxyl groups have been degraded/transformed in the process of SS pyrolysis [40].
The stretching vibration peaks of asymmetric/symmetric aliphatic CH3 are present around 2950 and 2840 cm-1, respectively [55]. These two peaks disappear in the FT-IR curves of biochars obtained from SS2, SS3 and SS4 feedstocks and weaken for the SS1-derived biochar, indicating the decomposition of organic fatty hydrocarbons.The bending vibration peak of C—N—C appears around 1650 cm-1. This characteristic peak is present in raw SS feedstocks but obviously weakens in biochar products, indicating the decomposition of nitrogenous organic matter, for example amine and amides [40]. The peaks below 600 cm-1 belong to the stretching vibrations of metal-halogen, originating from the organic/inorganic halogen compounds. The wagging vibrations of C—H present in the range of 600-800 cm-1 represent the aromatic structure of aromatic/heteroaromatic compounds [55].

Figure 2 FT-IR curves of raw SS feedstocks and biochars produced at 500 °C
3.4 Thermal stability of biochars
Figure 3 presents the TG/DTG curves of raw SS feedstocks and biochar products. Analyzing the thermal analysis results for SS feedstocks, three main stages could be observed: 1) the first stage (30-200 °C) belongs to the removal of physically combined water particles; 2) the second stage (200-800 °C) is assigned to the loss of volatile substances, i.e., the removal/degradation of the main components in raw SS feedstock; 3) the third stage (up to 1000 °C) is associated with the removal of inorganic matter (for example, the decomposition of CaCO3) [39, 44]. Compared with the original SS feedstock, the weight loss of biochars is greatly reduced in the same temperature range. For example, the weight loss of SS4-derived biochars in the temperature range of 200-1000 °C is 13.3 wt.% versus 47.6 wt.% for raw SS4 feedstock. In addition, all biochars are stable up to about 400 °C. After this temperature, the decomposition of carbon containing substances and the migration of volatile degradation substances begins to take place gradually. In other words, the SS-derived biochars produced in this study possess higher thermal stability compared to the raw SS feedstocks. Analyzing the DTG curves of raw SS feedstocks, three obvious degradation peaks are found at 30-200, 200-400, 800-1000 °C, respectively, corresponding to the aforementioned three stages. However, no significant peak is present in the DTG curves of biochars.
3.5 Surface properties of biochars
Figure 4 presents the N2 adsorption/desorption isotherms for four different biochars. In the light of the classification of IUPAC, the isotherms listed in Figure 4 could be ascribed to Type IV, representing physical adsorption [57]. Well-developed hysteresis loops are observed for all biochars in the medium- high pressure range, indicating the development of the mesopores structure. In addition, these hysteresis loops can be assigned to the capillary condensation and classified as the H3 type, suggesting the slit-like pore shape [57].
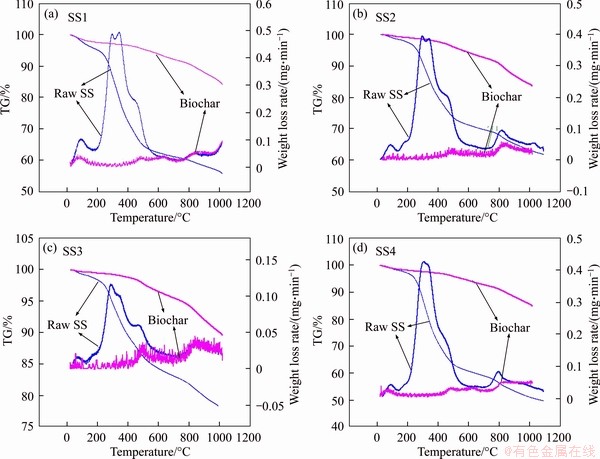
Figure 3 TG-DTG curves of SS and biochars produced at 500 °C
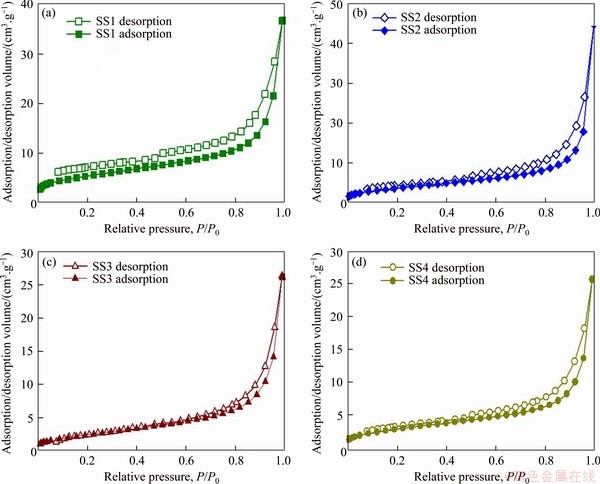
Figure 4 N2 adsorption/desorption of biochars produced at 500 °C
Interestingly, for the biochar derived from SS1 feedstock, there is also an isotherm hysteresis in the low relative pressure range of P/P0<0.4, which is lower than the beginning of common capillary condensation. This may be due to the expansion of biochar particles in the adsorption process. The expansion of biochar particles might loosen the structure, for example, by breaking the weak bond of primary particle, allowing the adsorbate to enter an area previously inaccessible. Therefore, part of adsorbate molecules might be trapped, and their desorption is very slow and even has no desorption phenomenon, as the deformation of structure is completely reversible [39].
In accordance with the pore size, the pores of biochar can be divided into three categories, including micropores (less than 2 nm), mesopores (2-50 nm) and macropores (more than 50 nm) [41, 58]. As listed in Table 3, the average pore diameters of biochars range from 16.0 to 20.5 nm, indicating that the biochars obtained in this research are mesopores materials. The specific surface area of SS1-derived biochar is the highest, up to 18.6 m2/g, followed by SS2-derived biochar (12.9 m2/g), SS4-derived biochar (9.4 m2/g), and SS3-derived biochar (7.9 m2/g). In the research of LU et al [40], the specific surface area of SS-derived biochar is ranging from 4 to 26.5 m2/g. The specific surface area of biochar depends on two factors: one is the ash content and the other is the degradation of VOM. Inorganic compounds can fill or even block pores, so higher ash content often leads to lower specific surface area. However, the degradation or removal of VOM is beneficial to the formation of pore structure, thus increasing the specific surface area [39]. The pore volumes of all biochars are relatively close, distributed in the range of 0.04-0.07 cm3/g. Figure 5 shows the particle size distribution curve of four different biochars. According to this figure, different biochars present similar particle size distribution patterns, with the peak horizontal position close to 2 nm.
3.6 SEM analysis of biochars
The micromorphology of biochar is exhibited in Figure 6. In order to highlight the morphological changes, the SEM image of the raw SS feedstock is also shown in Figure 6. In Figure 6(a), the surface of raw SS feedstock is smooth and compact. However, the surface of biochar is rough and has irregular structure, which contains more developed pore structure (Figure 6(b)). These pores are mainly caused by the escape of volatiles during pyrolysis. Three types of pores are usually observed in biochar: pores among biochar particles, i.e., interstitial pores; macropores remained after pyrolysis of raw materials, i.e., residual pores and turbostratic pores on carbon, i.e., pyrogenic nanopores [59].
Table 3 Specific surface area, pore size and pore volume of different biochars (500 °C for 60 min)
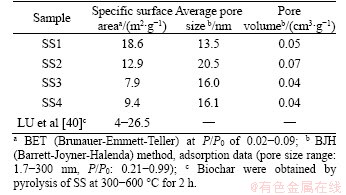
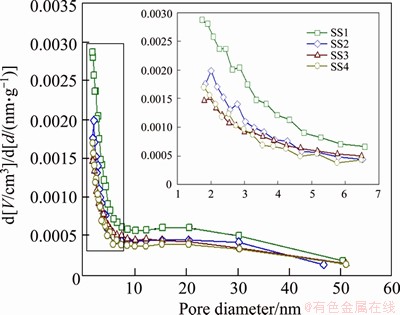
Figure 5 Pore size distribution of biochars produced at 500 °C
3.7 HMs in biochars
3.7.1 Total content of HMs
The comparison results of the total concentrations of HMs in raw SS feedstocks and biochars are shown in Figure 7. The allowable values of HMs are also presented in Figure 7, cited from a quality standard of SS used in land improvement [56]. The contents of HMs in raw SS feedstocks collected from different urban sewage treatment plants have some differences. The content of HMs in SS2 feedstock is relatively high, while that in SS3 feedstock is relatively low. This is mainly due to the difference in the composition of wastewater accepted by sewage treatment plants, such as the proportions of domestic wastewater, commercial wastewater, and industrial wastewater. In addition, among the six HMs investigated in this study, the contents of Zn/Cu in each SS feedstock are generally higher, which could be ascribed to the large-scale application of galvanized pipes and the mixing of industrial wastewater [40]. Except for Cd in the SS2 sample, the content of HMs in all SS feedstocks is below the safety value required by acid soil (pH<6.5).
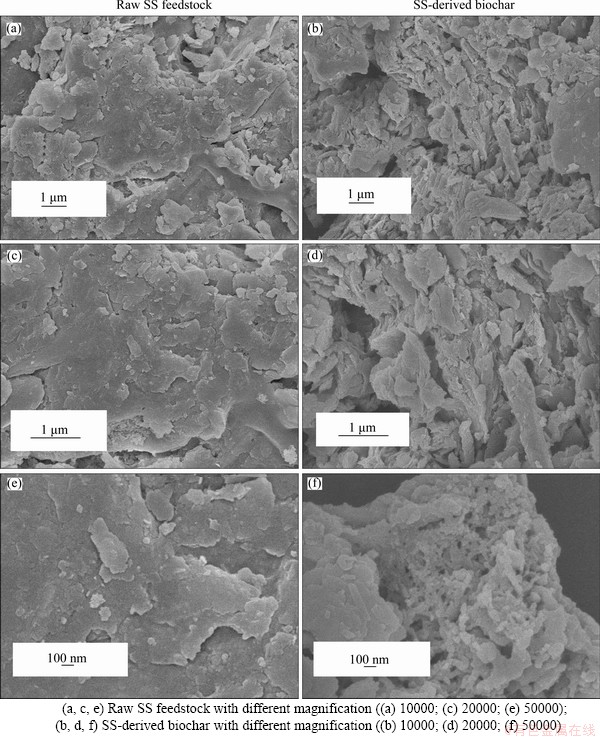
Figure 6 SEM images of raw SS (taking SS2 for example) and its derived biochar at 500 °C:
Obviously, the total contents of HMs in biochars are all higher than those in raw SS feedstocks, suggesting that the HMs are concentrated in biochars. And, the enrichment is intensified as the pyrolysis temperature increases. In general, the HMs have better thermo-stability than other compositions of SS feedstock. The binding forms of HMs in raw SS feedstocks mainly include: mineral salts, sulfides, hydroxides, oxides and chelates. During pyrolysis, the HMs bound to mineral salts and hydroxides would usually be transformed into oxides or sulfides, which has higher thermal stability. Thus, the bulk of HMs will still exist in biochar. It is also found that different HMs present distinct enrichment degrees, which might be associated with the various chemical speciation/decomposition temperatures (boiling point) of the aforementioned HMs [15, 17].
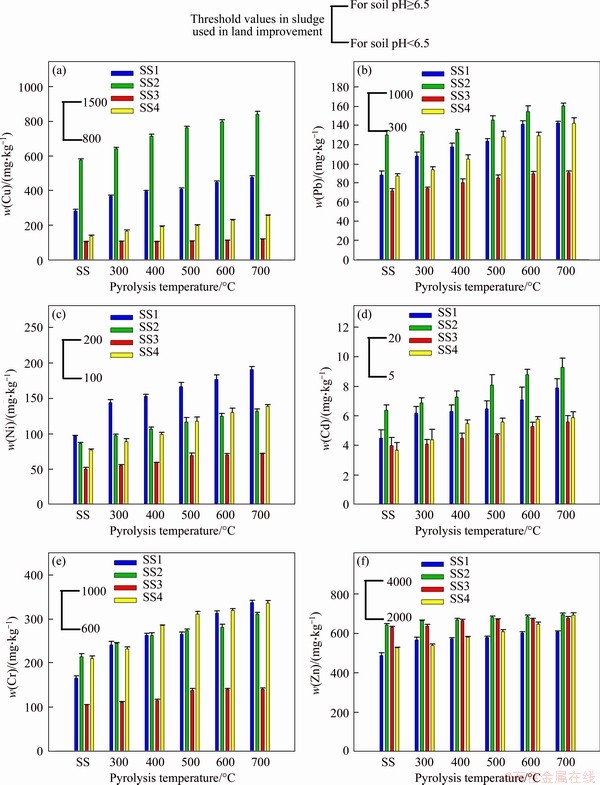
Figure 7 Total content of heavy metals in raw SS and biochars
The total contents of Pb, Cr, and Zn in all biochars are below the safe level for acid soil (pH<6.5). The total contents of Cu, Ni, and Cd in some biochars exceed the standard value of acid soil but are still below the standard value of neutral- alkaline soil (pH≥6.5). Hence, the ecological risks caused by HMs in biochars for neutral/alkaline soil improvement may not be considered, but when it is used in acid soil, the influence of Cu, Ni, and Cd must be considered for some biochars. Meanwhile, it is found that the total contents of HMs in biochars mainly depended on those in the corresponding raw SS feedstocks. In general, if the total content of HMs in raw SS feedstocks is high, the content of HMs in biochar is also high. Thus, in the selection of raw SS materials for the preparation of biochar, raw SS feedstocks with low content of HMs should be selected under the same conditions.
3.7.2 Leachable content of HMs
TCLP is usually used to evaluate the leaching toxicity of HMs in various materials. Table 4 summarizes the leaching content of HMs in raw SS feedstocks and biochars. As shown in this table, it can be found that the concentrations of leachable HMs in biochars are lowered compared to raw SS feedstocks. Leachable Pb and Cr are not detected in both SS feedstocks and biochars. The leachable contents of Cu in SS1-SS4 are 9.9, 75.0, 3.4 and 3.6 mg/kg, respectively. For the biochars produced from SS1, SS3 and SS4, leachable Cu is not detected. In the case of SS2-derived biochar, the content of leachable Cu is reduced to 1.5-9.1 mg/kg. The change situation of leachable Ni is similar to Cu. Leachable Ni is detected in three SS samples, i.e., SS1 (26.7 mg/kg), SS2 (16.9 mg/kg), and SS4 (19.5 mg/kg), higher than the threshold value (5.0 mg/kg). Except for individual biochar (SS1-BC-700), the contents of leachable Ni in other biochars are all below the corresponding standard value.
As the content of leachable Cd in raw SS feedstocks is lower than the standard value, the content of leachable Cd in biochar is even lower, sometimes it is not detected. The leachable contents of Zn in SS1-SS4 are 80.2, 103.7, 61.6 and 97.9 mg/kg, respectively, much higher than the threshold value (5.0 mg/kg). It’s a little pity that the content of leachable Zn in biochar has not decreased greatly, and its content is still above the standard. It is undeniable that compared with the original sludge, the leaching toxicity of HMs in biochar is reduced to a certain extent. The reduction in the bioavailability of HMs can be explained from two aspects: one is the formation of organometallic chelates; the other is that compared with the raw SS feedstock, biochar has developed pore structure and higher specific surface area, which is conducive to the adsorption/fixation of HMs [15]. Meanwhile, it is found that the increase of reaction temperature imparts no definite effect on the leachable content of HMs in biochar. A reason may be that the pyrolysis temperature used in this study is generally relatively low, and the rise of reaction temperature is not enough to significantly change the chemical speciation of HMs in biochar [15, 21].
Table 4 Leachable content of heavy metals in biochar

3.8 Adsorption potential of biochars
Figure 8 presents the effect of adsorbent dosage on the adsorption of methylene blue by different biochars. According to this figure, the removal rate of methylene blue gradually increases with the elevation of biochar dosage. When the dosage of biochar is up to 400 mg, the removal rate of methylene blue is all higher than 94%. After that, further increasing biochar dosage, the removal rate of methylene blue increases slowly and tends to be stable. Generally speaking, the increase of the amount of biochar will increase the surface area and active sites for methylene blue adsorption [60-62]. When the amount of biochar is low, methylene blue will quickly adsorb on the surface of biochar, so that the removal rate of methylene blue increases rapidly. However, when the amount of biochar exceeds a certain limit, the adsorption of methylene blue will be limited because of the splitting effect of flux (concentration gradient) between biochar and methylene blue [63]. Quite the opposite, the adsorption capacity of biochar generally decreases with the increase of the dosage of biochar. For a certain volume of methylene blue solution, the number of methylene blue molecules in it is certain. Therefore, when increasing the amount of biochar (meaning more adsorption sites), the driving force between methylene blue molecule and adsorption site reduces, which results in the decrease of the adsorption capacity of biochar [63].
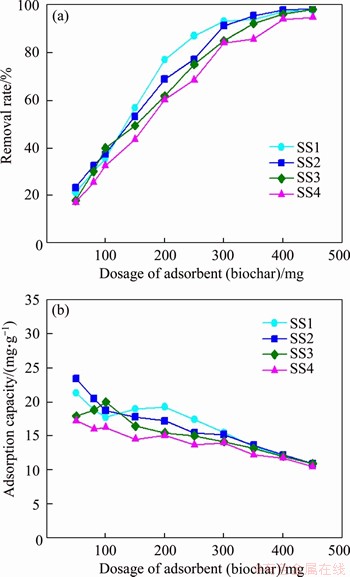
Figure 8 Adsorption performance of different biochars produced at 500 °C
Meanwhile, it is also found that there are some differences in the adsorption performance of four kinds of biochars, especially at low doses of biochar, which may be caused by their different characteristics. However, according to the current analysis of biochar characteristics in this study, it is difficult to explain these differences. For example, as shown in Figure 8, the adsorption performance of biochars does not fully match the change trend of the specific surface area of biochars. This is mainly due to the complex adsorption mechanism of methylene blue by biochar, including ion exchange, functional group complexation, π-π adsorption, hydrogen bonding, and so on [64]. In the future, it is necessary to further study the relevant characteristics that affect the adsorption potential of biochar, such as the Boehm titration, the adsorption kinetic/thermodynamic parameters, and the release of cations. In practice, these SS-derived biochars can also be further activated by physical or chemical methods, so as to further improve their application value [7].
4 Conclusions
The properties of raw SS feedstock have a definite influence on the formation/characteristics of its derived pyrolysis biochars. The yields of biochars decline with the increase of reaction temperature and present a positive correlation with the content of ash in raw SS feedstock. In relation to the raw SS feedstock, the biochars possesses higher thermal stability, aromaticity, carbonization degree, and pH values. Meanwhile, the biochars have rich pore structure and P/K nutrients. The HMs contained in raw SS feedstock are enriched in biochars and the content of HMs in biochars largely depends on that in raw SS feedstock. Importantly, the leachability of HMs in biochars is all alleviated. All biochars show a good adsorption potential for methylene blue. It is suggested that SS with low content of HMs should be selected to prepare biochar. And the content of OM in raw SS feedstock should not be too low, now that the low content of OM is not conducive to the formation of stable biochar and will make the content of OM in biochar not meet the requirements of land use.
Contributors
HUANG Hua-jun provided the idea of the study, developed the overarching research goal, and led the research activity planning and execution. LIU Ping made great contribution to the improvement of manuscript after the initial draft finished. WANG Jia-xin conducted the experiments, analyzed the test data, and wrote the initial draft of the manuscript. LAI Fa-ying offered some valuable suggestions for the contents of the manuscript and polished the language of the manuscript. All authors replied to reviewers’ comments and revised the final version.
Conflict of interest
HUANG Hua-jun, LIU Ping, LAI Fa-ying and WANG Jia-xin declare that they have no conflict of interest.
References
[1] XIAO Xin, CHEN Bao-liang, CHEN Zai-ming, ZHU Li-zhong, SCHNOOR J L. Insight into multiple and multilevel structures of biochars and their potential environmental applications: A critical review [J]. Environmental Science & Technology, 2018, 52: 5027-5047. DOI: https://doi.org/10.1021/acs.est.7b06487.
[2] YUAN Shen-nan, TAN Zhong-xin, HUANG Qiao-yun. Migration and transformation mechanism of nitrogen in the biomass–biochar–plant transport process [J]. Renewable and Sustainable Energy Reviews, 2018, 85: 1-13. DOI: https://doi.org/10.1016/j.rser.2018.01.008.
[3] LENG Li-jian, HUANG Hua-jun. An overview of the effect of pyrolysis process parameters on biochar stability [J]. Bioresource Technology, 2018, 270: 627-642. DOI: https://doi.org/10.1016/j.biortech.2018.09.030.
[4] TRIPATHI M, SAHU J N, GANESAN P. Effect of process parameters on production of biochar from biomass waste through pyrolysis: A review [J]. Renewable and Sustainable Energy Reviews, 2016, 55: 467-481. DOI: https://doi.org/ 10.1016/j.rser.2015.10.122.
[5] TAN Xiao-fei, LIU Shao-bo, LIU Yun-guo, GU Yan-ling, ZENG Guang-ming, HU Xin-jiang, WANG Xin, LIU Shao-heng, JIANG Lu-hua. Biochar as potential sustainable precursors for activated carbon production: Multiple applications in environmental protection and energy storage [J]. Bioresource Technology, 2017, 227: 359-372. DOI: https://doi.org/10.1016/j.biortech.2016.12.083.
[6] QIAN Ke-zhen, KUMAR A, ZHANG Hai-lin, BELLMER D, HUHUKN R. Recent advances in utilization of biochar [J]. Renewable and Sustainable Energy Reviews, 2015, 42: 1055-1064. DOI: https://doi.org/10.1016/j.rser.2014.10.074.
[7] CHA Jin-sun, PARK S H, JUNG S C, RYU C, JEON J K, SHIN M C, PARK Y K. Production and utilization of biochar: A review [J]. Journal of Industrial and Engineering Chemistry, 2016, 40: 1-15. DOI: https://doi.org/10.1016/ j.jiec.2016.06.002.
[8] RAHEEM A, SIKARWAR V S, HE Jun, DASTYAR W, DIONYSIOU D D, WANG Wei, ZHAO Ming. Opportunities and challenges in sustainable treatment and resource reuse of sewage sludge: A review [J]. Chemical Engineering Journal, 2018, 337: 616-641. DOI: https://doi.org/10.1016/j.cej. 2017.12.149.
[9] HUANG Hua-jun, YUAN Xing-zhong. Recent progress in the direct liquefaction of typical biomass [J]. Progress in Energy and Combustion Science, 2015, 49: 59-80. DOI: https://doi.org/10.1016/j.pecs.2015.01.003.
[10] PAN Zi-qian, HUANG Hua-jun, ZHOU Chun-fei, LAI Fa-ying, HE Xiao-wu, XIONG Jiang-bo, XIAO Xiao-feng. Distribution and transformation behaviors of heavy metals during liquefaction process of sewage sludge in ethanol- water mixed solvents [J]. Journal of Central South University, 2019, 26(10): 2771-2784. DOI: https://doi.org/10.1007/ s11771-019-4212-6.
[11] UDAYANGA W D C, VEKSHA A, GIANNIS A, LISAK G, CHANG V W, LIM T T. Fate and distribution of heavy metals during thermal processing of sewage sludge [J]. Fuel, 2018, 226: 721-744. DOI: https://doi.org/10.1016/j.fuel. 2018.04.045.
[12] HUANG Hua-jun, YUAN Xing-zhong. The migration and transformation behaviors of heavy metals during the hydrothermal treatment of sewage sludge [J]. Bioresource Technology, 2016, 200: 991-998. DOI: https://doi.org/10. 1016/j.biortech.2015.10.099.
[13] GAO Ning-bo, KAMRAM K, QUAN Cui, WILLIAMS P T. Thermochemical conversion of sewage sludge: A critical review [J]. Progress in Energy and Combustion Science, 2020, 79: 100843. DOI: https://doi.org/10.1016/j.pecs.2020. 100843.
[14] SYED H S S A, WANG Yi, HU Song, SU Sheng, XIANG Jun. Thermochemical processing of sewage sludge to energy and fuel: Fundamentals, challenges and considerations [J]. Renewable and Sustainable Energy Reviews, 2017, 80: 888-913. DOI: https://doi.org/10.1016/j.rser.2017.05.262.
[15] YUAN Hao-ran, LU Tao, HUANG Hong-yu, ZHAO Dan-dan, NORIYUKI K, CHEN Yong. Influence of pyrolysis temperature on physical and chemical properties of biochar made from sewage sludge [J]. Journal of Analytical and Applied Pyrolysis, 2015, 112: 284-289. DOI: https://doi. org/10.1016/j.jaap.2015.01.010.
[16] CHEN Hui, CHEN De-zhen, HONG Liu. Influences of activation agent impregnated sewage sludge pyrolysis on emission characteristics of volatile combustion and De-NOx performance of activated char [J]. Applied Energy, 2015, 156: 767-775. DOI: https://doi.org/10.1016/j.apenergy.2015. 05.098.
[17] YUAN Hao-ran, LU Tao, ZHAO Dan-dan, HUANG Hong-yu, NORIYUKI K, CHEN Yong. Influence of temperature on product distribution and biochar properties by municipal sludge pyrolysis [J]. Material Cycles and Waste Manage, 2013, 15(3): 357-361. DOI: https://doi.org/10. 1007/s10163-013-0126-9.
[18] MENDEZ A, TERRADILLOS M, GASCO G. Physicochemical and agronomic properties of biochar from sewage sludge pyrolysed at different temperatures [J]. Journal of Analytical and Applied Pyrolysis, 2013, 102: 124-130. DOI: https://doi.org/10.1016/j.jaap.2013.03.006.
[19] CHEN Qin-dong, LIU Hu, KO J, WU Hua-nan, XU Qi-yong. Structure characteristics of bio-char generated from co-pyrolysis of wooden waste and wet municipal sewage sludge [J]. Fuel Processing Technology, 2019, 183: 48-54. DOI: https://doi.org/10.1016/j.fuproc.2018.11.005.
[20] ZHAO Bing, Xu Xin-yang, Xu Shu-cong, CHEN Xi, Li Hai-bo, ZENG Fan-qiang. Surface characteristics and potential ecological risk evaluation of heavy metals in the bio-char produced by co-pyrolysis from municipal sewage sludge and hazelnut shell with zinc chloride [J]. Bioresource Technology, 2017, 243: 375-383. DOI: https://doi.org/10. 1016/j.biortech.2017.06.032.
[21] HUANG Hua-jun, YANG Ting, LAI Fa-ying, WU Guo-qiang. Co-pyrolysis of sewage sludge and sawdust/rice straw for the production of biochar [J]. Journal of Analytical and Applied Pyrolysis, 2017, 125: 61-68. DOI: https://doi. org/10.1016/j.jaap.2017.04.018.
[22] ZHOU Yuan, LIU Yong-ze, JIANG Wen-bo, SHAO Lin-lin, ZHANG Li-qiu, FENG Li. Effects of pyrolysis temperature and addition proportions of corncob on the distribution of products and potential energy recovery during the preparation of sludge activated carbon [J]. Chemosphere, 2019, 221: 175-183. DOI: https://doi.org/10.1016/ j.chemosphere.2019.01.026.
[23] BOLOGNESI S, BERNARDI G, CALLEGARI A, DONDI D, CAPODAGLIO A G. Biochar production from sewage sludge and microalgae mixtures: Properties, sustainability and possible role in circular economy [J]. Biomass Conversion and Biorefinery, 2019. DOI: https://doi.org/ 10.1007/s13399-019-00572-5.
[24] LIU Ting-ting, LIU Zhen-gang, ZHENG Qing-fu, LANG Qian-qian, XIA Yu, PENG Nana, GAI Chao. Effect of hydrothermal carbonization on migration and environmental risk of heavy metals in sewage sludge during pyrolysis [J]. Bioresource Technology, 2018, 247: 282-290. DOI: https://doi.org/10.1016/j.biortech.2017.09.090.
[25] XU Xue-bin, HU Xin, DING Zhu-hong, CHEN Yi-jun. Effects of copyrolysis of sludge with calcium carbonate and calcium hydrogen phosphate on chemical stability of carbon and release of toxic elements in the resultant biochars [J]. Chemosphere, 2017, 189: 76-85. DOI: https://doi.org/ 10.1016/j.chemosphere.2017.09.021.
[26] CHEN Fang-fang, HU Yu-yan, DOU Xiao-min, CHEN De-zhen, DAI Xiao-hu. Chemical forms of heavy metals in pyrolytic char of heavy metal-implanted sewage sludge and their impacts on leaching behaviors [J]. Journal of Analytical and Applied Pyrolysis, 2015, 116: 152-160. DOI: https:// doi.org/10.1016/j.jaap.2015.09.015.
[27] ZIELINSKA A, OLESZCZUK P. The conversion of sewage sludge into biochar reduces polycyclic aromatic hydrocarbon content and ecotoxicity but increases trace metal content [J]. Biomass and Bioenergy, 2015, 75: 235-244. DOI: https://doi.org/10.1016/j.biombioe.2015.02.019.
[28] JIN Hong-mei, ARAZO R O, GAO Jun, CAPAREDA S, CHANG Zhi-zhou. Leaching of heavy metals from fast pyrolysis residues produced from different particle sizes of sewage sludge [J]. Journal of Analytical and Applied Pyrolysis, 2014, 109: 168-175. DOI: https://doi.org/10.1016/ j.jaap.2014.06.016.
[29] ZHAI Yun-bo, CHEN Hong-mei, XU Bi-bo, XIANG Bo-bin, CHEN Zhong, LI Cai-ting, ZENG Guang-ming. Influence of sewage sludge-based activated carbon and temperature on the liquefaction of sewage sludge: Yield and composition of bio-oil, immobilization and risk assessment of heavy metals [J]. Bioresource Technology, 2014, 159: 72-79. DOI: https://doi.org/10.1016/j.biortech.2014.02.049.
[30] YUAN Yong, YUAN Tian, WANG Ding-mei, TANG Jia-huan, ZHOU Shun-gui. Sewage sludge biochar as an efficient catalyst for oxygen reduction reaction in an microbial fuel cell [J]. Bioresource Technology, 2013, 144: 115-120. DOI: https://doi.org/10.1016/j.biortech.2013.06. 075.
[31] BANDOSZ T J, BLOCK K. Effect of pyrolysis temperature and time on catalytic performance of sewage sludge/ industrial sludge-based composite adsorbents [J]. Applied Catalysis B, Environmental, 2006, 67(1): 77-85. DOI: https://doi.org/10.1016/j.apcatb.2006.04.006.
[32] TANG Yao, ALAM M S, KONHAUSER K O, ALESSI D S, XU Sheng-nan, TIAN Wei-Jun, LIU Yang. Influence of pyrolysis temperature on production of digested sludge biochar and its application for ammonium removal from municipal wastewater [J]. Journal of Cleaner Production, 2019, 209: 927-936. DOI: https://doi.org/10.1016/ j.jclepro.2018.10.268.
[33] ANTUNES E, SCHUMANN J, BRODIE G, JACOB M V, SCHNEIDER P A. Biochar produced from biosolids using a single-mode microwave: Characterisation and its potential for phosphorus removal [J]. Journal of Environmental Management, 2017, 196: 119-126. DOI: https://doi.org/ 10.1016/j.jenvman.2017.02.080.
[34] MIAN M M, LIU Gui-jian. Sewage sludge-derived TiO2/Fe/ Fe3C-biochar composite as an efficient heterogeneous catalyst for degradation of methylene blue [J]. Chemosphere, 2019, 215: 101-114. DOI: https://doi.org/10.1016/ j.chemosphere.2018.10.027.
[35] MENDEZ A, PAZ J, ARAUJO F, GASCO G. Biochar from pyrolysis of deinking paper sludge and its use in the treatment of a nickel polluted soil [J]. Journal of Analytical and Applied Pyrolysis, 2014, 107: 46-52. DOI: https://doi.org/10.1016/j.jaap.2014.02.001.
[36] GWENZI W, MUZAVA M, MAPANDA F, TAURO T P. Comparative short-term effects of sewage sludge and its biochar on soil properties, maize growth and uptake of nutrients on a tropical clay soil in Zimbabwe [J]. Journal of Integrative Agriculture, 2016, 15(6): 1395-1406. DOI: https://doi.org/10.1016/S2095-3119(15)61154-6.
[37] BREULMANN M, van AFFERDEN M, MULLER R A, SCHULZ E, FUHNER C. Process conditions of pyrolysis and hydrothermal carbonization affect the potential of sewage sludge for soil carbon sequestration and amelioration [J]. Journal of Analytical and Applied Pyrolysis, 2017, 124: 256-265. DOI: https://doi.org/10.1016/j.jaap.2017.01.026.
[38] YUAN Hao-ran, LU Tao, WANG Ya-zhuo, CHEN Yong, LEI Ting-zhou. Sewage sludge biochar: Nutrient composition and its effect on the leaching of soil nutrients [J]. Geoderma, 2016, 26: 17-23. DOI: https://doi.org/10.1016/j.geoderma. 2015.12.020.
[39] ZIELINSKA A, OLESZCZUK P, CHARMAS B, SKUBIZEWSKA J, PASIECZNA S. Effect of sewage sludge properties on the biochar characteristic [J]. Journal of Analytical and Applied Pyrolysis, 2015, 112: 201-213. DOI: https://doi.org/10.1016/j.jaap.2015.01.025.
[40] LU Huan-liang, ZHANG Wei-hua, WANG Shi-zhong, ZHUANG Lu-wen, YANG Yu-xi, QIU Rong-liang. Characterization of sewage sludge-derived biochars from different feedstocks and pyrolysis temperatures [J]. Journal of Analytical and Applied Pyrolysis, 2013, 102: 137-143. DOI: https://doi.org/10.1016/j.jaap.2013.03.004.
[41] PAN Zi-qian, HUANG Hua-jun, ZHOU Chun-fei, XIAO Xiao-feng, HE Xiao-wu, LAI Fa-ying, XIONG Jiang-bo. Highly efficient conversion of camphor tree sawdust into bio-oil and biochar products by liquefaction in ethanol-water cosolvent [J]. Journal of Analytical and Applied Pyrolysis, 2018, 136: 186-198. DOI: https://doi.org/10.1016/j.jaap. 2018.10.006.
[42] AGRAFIOTI E, BOURAS G, KALDERIS D, DIAMADOPOULOS E. Biochar production by sewage sludge pyrolysis [J]. Journal of Analytical and Applied Pyrolysis, 2013, 101: 72-78. DOI: https://doi.org/10.1016/ j.jaap.2013.02.010.
[43] HOSOKAI S, MATSUOKA K, KURAMOTO K, SUZUKI Y. Modification of Dulong's formula to estimate heating value of gas, liquid and solid fuels [J]. Fuel Processing Technology, 2016, 152. DOI: https://doi.org/10.1016/j.fuproc.2016.06. 040.
[44] HUANG Hua-jun, CHANG Yan-chao, LAI Fa-ying, ZHOU Chun-fei, PAN Zi-qian, XIAO Xiao-feng, WANG Jia-xin, ZHOU Chun-huo. Co-liquefaction of sewage sludge and rice straw/wood sawdust: The effect of process parameters on the yields/properties of bio-oil and biochar products [J]. Energy, 2019, 173: 140-150. DOI: https://doi.org/10.1016/j.energy. 2019.02.071.
[45] YANG Ting, HUANG Hua-jun, LAI Fa-ying. Pollution hazards of heavy metals in sewage sludge from four wastewater treatment plants in Nanchang, China [J]. Transactions of Nonferrous Metals Society of China, 2017, 27(10): 2249-2259. DOI: https://doi.org/10.1016/S1003- 6326(17)60251-6.
[46] YUAN Xing-zhong, HUANG Hua-jun, ZENG Guang-ming, LI Hui, WANG Jing-yu, ZHOU Chun-fei, ZHU Hui-na, PEI Xiao-kai, LIU Zhi-feng, LIU Zhan-tao. Total concentrations and chemical speciation of heavy metals in liquefaction residues of sewage sludge [J]. Bioresource Technology, 2011, 102(5): 4104-4110. DOI: https://doi.org/10.1016/j.biortech. 2010.12.055.
[47] HUANG Hua-jun, YUAN Xing-zhong, ZENG Guang-ming, ZHU Hui-na, LI Hui, LIU Zhi-feng, JIANG Hong-wei, LENG Li-jian, BI Wen-kai. Quantitative evaluation of heavy metals' pollution hazards in liquefaction residues of sewage sludge [J]. Bioresource Technology, 2011, 102(22): 10346-10351. DOI: https://doi.org/10.1016/j.biortech.2011. 08.117.
[48] ZHANG Xiao-xiao, ZHANG Pei-zhen, YUAN Xiang-ru, LI Yan-fei, HAN Lu-jia. Effect of pyrolysis temperature and correlation analysis on the yield and physicochemical properties of crop residue biochar [J]. Bioresource Technology, 2020, 296: 122318. DOI: https://doi.org/ 10.1016/j.biortech.2019.122318.
[49] HE Xin-yan, LIU Zhao-xia, NIU Wen-juan, YANG Li, ZHOU Tan, QIN Di, NIU Zhi-you, YUAN Qiao-xia. Effects of pyrolysis temperature on the physicochemical properties of gas and biochar obtained from pyrolysis of crop residues [J]. Energy, 2018, 143: 746-756. DOI: https://doi.org/10. 1016/j.energy.2017.11.062.
[50] UDAYANGA W D C, VEKSHA A, GIANNIS A, LIM T. Pyrolysis derived char from municipal and industrial sludge: Impact of organic decomposition and inorganic accumulation on the fuel characteristics of char [J]. Waste Management, 2019, 83: 131-141. DOI: https://doi.org/10.1016/j.wasman. 2018.11.008.
[51] NANSUBUGA I, BANADDA N, RONSSE F, VERSTRAETE W, RABAEY K. Digestion of high rate activated sludge coupled to biochar formation for soil improvement in the tropics [J]. Water Research, 2015, 81: 216-222. DOI: https://doi.org/10.1016/j.watres.2015.05.047.
[52] XIONG Jiang-bo, PAN Zi-qian, XIAO Xiao-feng, HUANG Hua-jun, LAI Fa-ying, WANG Jia-xin, CHEN Shuai-wei. Study on the hydrothermal carbonization of swine manure: The effect of process parameters on the yield/properties of hydrochar and process water [J]. Journal of Analytical and Applied Pyrolysis, 2019, 144: 104692. DOI: https://doi.org/ 10.1016/j.jaap.2019.104692.
[53] LENG Li-jian, HUANG Hua-jun, LI Hui, LI Jun, ZHOU Wen-guang. Biochar stability assessment methods: A review [J]. Science of the Total Environment, 2019, 647: 210-222. DOI: https://doi.org/10.1016/j.scitotenv.2018.07.402.
[54] CONTI R, FABBRI D, VASSURA I, FERRONI L. Comparison of chemical and physical indices of thermal stability of biochars from different biomass by analytical pyrolysis and thermogravimetry [J]. Journal of Analytical and Applied Pyrolysis, 2016: 160-168. DOI: https://doi.org/ 10.1016/j.jaap.2016.10.003.
[55] HOSSAIN M K, STREZOV V, CHAN K Y, ZIOLKOWSKI A, NELSON P F. Influence of pyrolysis temperature on production and nutrient properties of wastewater sludge biochar [J]. Journal of Environmental Management, 2011, 92(1): 223-228. DOI: https://doi.org/10.1016/j.jenvman. 2010.09.008.
[56] GB/T 24600—2009. Disposal of sludge from municipal wastewater treatment plant-Quality of sludge used in land improvement [S]. (in Chinese)
[57] SING K S W, EVERETT D H, HAUL R A W, MOSCOU L, PIEROTTI R A, ROUQUEROL J, SIEMIENIEWSKA T. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity [J]. Pure and Applied Chemistry, 1985, 57: 603-619. DOI: https://doi.org/10.1351/pac198557040603.
[58] LAI Fa-ying, CHANG Yan-chao, HUANG Hua-jun, WU Guo-qiang, XIONG Jiang-bo, PAN Zi-qian, ZHOU Chun-fei. Liquefaction of sewage sludge in ethanol-water mixed solvents for bio-oil and biochar products [J]. Energy, 2018, 148: 629-641. DOI: https://doi.org/10.1016/j.energy.2018. 01.186.
[59] SRINIVASAN P, SARMAH A K, SMERNIK R, DAS O, FATID M, GAO Wei. A feasibility study of agricultural and sewage biomass as biochar, bioenergy and biocomposite feedstock: Production, characterization and potential applications [J]. Science of the Total Environment, 2015, 512-513: 495-505. DOI: https://doi.org/10.1016/j.scitotenv. 2015.01.068.
[60] STREIT A F M, CORTES L N, DRUZIAN S P, GODINHO M, COLLAZZO G C, PERONDI D, DOTTO G L. Development of high quality activated carbon from biological sludge and its application for dyes removal from aqueous solutions [J]. Science of the Total Environment, 2019, 660: 277-287. DOI: https://doi.org/10.1016/j.scitotenv. 2019.01.027.
[61] ZHOU Yan-bo, LU Jian, ZHOU Yi, LIU Yong-di. Recent advances for dyes removal using novel adsorbents: A review [J]. Environmental Pollution (Barking, Essex: 1987), 2019, 252(Pt A): 352-365. DOI: https://doi.org/10.1016/j.envpol. 2019.05.072.
[62] SALLEH M A M, MAHMOUD D K, KARIM W A W A, IDRIS A. Cationic and anionic dye adsorption by agricultural solid wastes: A comprehensive review [J]. Desalination, 2011, 280(1): 1-13. DOI: https://doi.org/10.1016/j.desal.2011. 07.019.
[63] WANG Hou, YUAN Xing-zhong, ZENG Guang-ming, LENG Li-jian, PENG Xin, LIAO Kai-ling-li, PENG Li-juan, XIAO Zhi-hua. Removal of malachite green dye from wastewater by different organic acid-modified natural adsorbent: kinetics, equilibriums, mechanisms, practical application, and disposal of dye-loaded adsorbent [J]. Environmental Science and Pollution Research International, 2014, 21(19): 11552-11564. DOI: https://doi.org/10.1007/ s11356-014-3025-2.
[64] LENG Li-jian, YUAN Xing-zhong, HUANG Hua-jun, SHAO Jian-guang, WANG Hou, CHEN Xiao-hong, ZENG Guang-ming. Bio-char derived from sewage sludge by liquefaction: Characterization and application for dye adsorption [J]. Applied Surface Science, 2015, 346: 223-231. DOI: https://doi.org/10.1016/j.apsusc.2015.04.014.
(Edited by YANG Hua)
中文导读
不同污水厂污泥热解生物炭的特性和应用研究
摘要:为了明确污泥原料本身构成差异对其热解制备生物炭的影响,本研究对四种具有不同性质的污泥原料在300~700 °C条件下进行了热解。生物炭的产率与污泥中灰分的含量呈现正相关。更重要的是,污泥中有机质含量在很大程度上决定了生物炭的品质。有机质含量高的污泥原料更容易形成稳定的生物炭(更高的芳香度/碳化度),且生物炭具有更高的热值。有机质含量低的污泥原料热解生成的生物炭中有机质残留量很大概率不能满足土壤改良的需要(10 wt.%)。污泥中的重金属大部分在热解后仍存在于生物炭中污泥中。重金属含量高的污泥原料制备的生物炭所含重金属含量也高。有趣的是,与污泥原料相比,生物炭中重金属的浸出能力都有所减弱。此外,生物炭具有较高的热稳定性和pH值,且富含K/P营养元素。四种污泥原料制备的生物炭对亚甲基蓝的吸附能力不同,在生物炭用量低的情况下尤为明显。
关键词:污泥;热解;生物炭;重金属;吸附
Foundation item: Project(21707056) supported by the National Natural Science Foundation of China; Project(20192BAB203019) supported by the Natural Science Foundation of Jiangxi Province, China
Received date: 2020-02-27; Accepted date: 2020-07-15
Corresponding author: HUANG Hua-jun, PhD, Associate Professor; Tel: +86-791-83828028; E-mail: huanghuajun2004@126.com; ORCID: https://orcid.org/0000-0002-4104-9471; LIU Ping, PhD, Associate Professor; E-mail: pingliujx@ 163.com

