
Analysis of electron structure of γ/a2 phase boundaries in ternary TiAl intermetallics
KONG Fan-tao(孔凡涛), CHEN Yu-yong(陈玉勇)
School of Materials Science and Engineering, Harbin Institute of Technology, Harbin 150001, China
Received 28 July 2006; accepted 15 September 2006
Abstract: The electron structure of γ/α2 phase boundaries in lamellar colonies in Ti-47Al-2M(M=Nb, Cr, V) (mole fraction, %) alloys was theoretically investigated by Empirical Electron Theory of Solid and Molecules (EET) and the bond-length-difference (BLD) method. Average-Atom-Model was employed to calculate valence electron structure of TiAl intermetallics containing site substitution elements. On this basis, the boundary condition of electron movement was employed in the improved Thomas-Fermi-Dirac (TFD) theory to decide the continuity of the electron density of the lamellar colonies interface and it is found that of γ/a2 interface is continuous(?ρ<10%). Furthermore, it is found that adding alloying elements (including Nb, Cr, and V) can improve the electron density (ρ) of γ/a2 interfaces, and decrease the electron density difference(?ρ) of γ/α2 interfaces. Adding V element decreasing ?ρ is more remarkable than other site substitution elements. According to electron structure study of γ/α2 interfaces in Ti-47Al-2M alloys, the added elements improve mechanical properties of the alloy in the following order: V>Cr>Nb.
Key words: TiAl alloy; alpha phase; gamma phase; electron structure; phase boundaries; electron density
1 Introduction
γ-TiAl based alloys have found wide applications in the aerospace, power generation and automotive industry due to their high strength, low density and good oxidation resistance. At present, the fundamental behavior of the gamma alloys is well understood. However, the disadvantages of TiAl based alloys are low ductility and toughness at room temperature, and they have poor workability. Now the majority of research is focused on alloy modifications through compositional controls and alloying additions[1-4].
In general, the electron structure of atoms is very important for properties of materials. Through the electron structure analysis, the understanding of relationships between materials composition and properties can be improved in essence. However, research on electron structures of γ/a2 phase boundaries in γ-TiAl is little. In this article, the electron structures of γ/a2 phase boundaries in Ti-47Al-2M(M=Nb, Cr, V) (at%) alloys were investigated by Empirical Electron Theory of Solid and Molecules (EET) and improved Thomas-Fermi-Dirac (TFD) theory [5,6].
2 Experimental
Four alloy ingots with compositions of Ti-47Al and Ti-47Al-2M(M=Nb, Cr, V) (mole fraction, %) were prepared by induction skull melting(ISM). The ingots were homogenized by hot isostatic pressing (HIP) at
1 250 ℃ for 4 h under an argon pressure of 170 MPa. Composition analysis of phases (g/a2) in Ti-47Al-2M (M=Nb, Cr, V) alloys were performed by transmission electron microscopy (TEM/EDAX9100). The lattice parameter of g and a2 phases were studied by X-ray diffractometry (XRD).
3 Electron structure of γ/a2 phase boundaries in TiAl based alloys
3.1 Site substitution behaviour of alloying elements and Average-Atom-Model
Ti-47Al based alloys are double phases (a2/γ) alloys. The crystal structures of γ phase and a2 phase are L10 and D019, respectively. Phase stabilization and equilibrium of ternary dual-phase TiAl alloys can be related to the site substitution behaviour of alloying elements in the individual phases. In general, those of Nb, W, Mo and Y atoms in g phase strongly favor the Ti sites for Ti-47Al alloys. In a2 phase, Nb, V, Mn, Cr, W, Mo and Y atoms only occupy the Ti sites[7-9]. Because alloying elements occupying Ti sites or Al sites are disordered, Average-Atom-Model in EET must be employed to calculate the characteristic parameters of valence electron structure of g phase and a2 phase[6].
3.2 Lattice parameter and adding element contents of γ phase and a2 phase
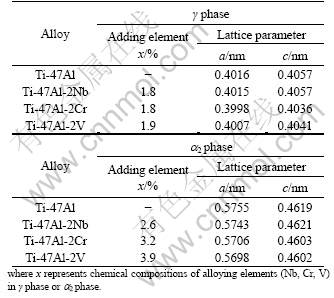
Table 1 shows alloying element M composition and lattice parameter of γ or a2 phases in Ti-47Al-2M (M=Nb, Cr, V) alloys, which were analyzed using EDAX and XRD.
Table1 Lattice parameter and adding element contents of γ phase and a2 phase
3.3 Valence electron structure of g phase and a2 phase
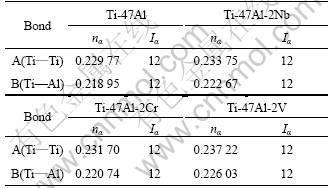
In general, there are lots of lamellar colonies in Ti-47Al-2M alloys. The microstructure consists of lamellar colonies that are composed of alternating layers of α2 and γ platelets aligned according to the (0001)α2 //(111)γ and <1120>α2//<110>γ crystallographic relations (for example, SAED pattern of α2/γ crystallographic relations of lamellar colonies in Ti-47Al alloy is shown in Fig.1). (0001) crystal plane of α2 phase and (111) crystal plane of γ phase are shown in Fig.2. According to the bond-length-difference (BLD) method of EET method[5,6], average atom model[10] and Table 1, the valence electron structure of g phase and a2 phase were calculated. The calculation results are shown in Tables 2, 3 and 4, where σ refers to hybridization level, nc refers to number of covalent electrons, n1 refers to number of crystal lattice electrons, R(l) refers to single bond radius, Iα refers to bond number, and nα refers to pair number of a bond covalent electron.
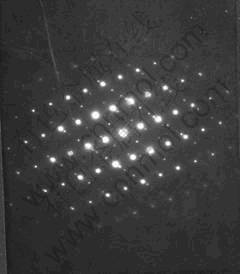
Fig.1 SAED pattern of α2/γ crystallographic relations
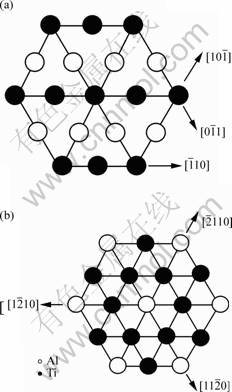
Fig.2 Crystal planes of g phase and a2 phase: (a) (111) plane of g phase; (b) (0001) plane of a2 phase
Table 2 Valence electron structures of atoms in Ti-47Al-2M alloys

Table 3 nα and Iα on (111) crystal plane of γ phases
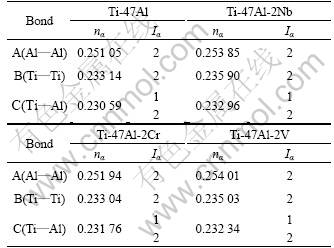
Table 4 nα and Iα on (0001) crystal plane of a2 phases
3.4 Electron structure of γ/a2 phase boundaries
Together with the valence electron structures of (111) crystal plane of γ phases and (0001) crystal plane of a2 phases in Ti-47Al based alloys, the boundary condition of electron movement in the improved Thomas-Fermi-Dirac (TFD) theory was employed to decide the continuity of the electron density between γ phases and a2 phases interfaces in the lamellar colonies[6].
According to Fig.1, Table 3 and Table 4, the electron density of (0001) crystal plane of α2 phase and (111) crystal plane of γ phase and the electron density difference of γ/a2 phase boundaries were calculated. For the electron state group in Table 3 and Table 4, the total covalent electron number on (111) crystal planes of γ phase and (0001) crystal planes of α2 phase could be obtained by equations (1) and (2). Comparing the
Calculated 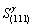 with
with 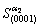 in equations (3) and (4),
in equations (3) and (4),
electron density of (111) crystal planes and (0001) crystal planes could be calculated (see equations (5) and (6).
Substituting 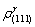 and
and 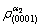 into equation (7), the electron density differences (Δρ) in g/a2 phase boundaries were obtained. The calculation results of the electron structure parameters in γ/a2 phase boundaries are shown in Table 5.
into equation (7), the electron density differences (Δρ) in g/a2 phase boundaries were obtained. The calculation results of the electron structure parameters in γ/a2 phase boundaries are shown in Table 5.
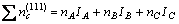 (1)
(1)
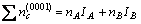 (2)
(2)
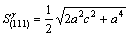 (3)
(3)
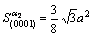 (4)
(4)
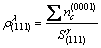 (5)
(5)
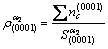 (6)
(6)
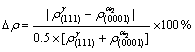 [(7)
[(7)
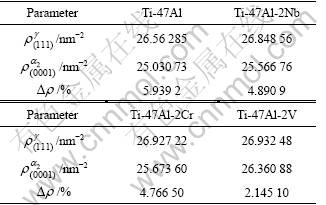
Table 5 Electron structure parameters in γ/a2 phase boundaries
4 Discussion
From Table 5, it is found that g/a2 interfaces in TiAl based alloys are continuous (?ρ<10%). Furthermore, it is found that adding alloying elements (including Nb, Cr, and V) can improve the electron density (ρ) of (0001)α2 crystal plane and (111)γ crystal plane, and decrease the electron density difference (?ρ) of γ/a2 phase boundaries. LIU et al[6] have pointed out that bond strength of phase boundaries increases gradually with increasing electron density. In addition, with the electron density difference in phase boundaries decreasing, interface stress has the tendency of decreasing, and continuous electron density in phase boundaries can be improved. In general, TiAl based alloys are mostly transcrystalline fracture along lamellar phase boundaries at room temperature, so improving bond strength of a2/γ interfaces and decreasing a2/γ interfaces stress will increase RT ductility and strength of TiAl based alloys. With the electron structure analysis together with properties analysis, the effect mechanism of adding elements (Nb, Cr, V) to improve mechanical properties of TiAl based alloys can be explained through the electron structure in γ/a2 phase boundaries. The order of adding elements improving RT mechanical properties in theory is as follows: V>Cr>Nb.
5 Conclusions
EET and the Bond-Length-Difference(BLD) method were employed to calculate the valence electron structures of γ/a2 phase boundaries in lamellar colonies in Ti-47Al based alloys. On this basis, the boundary condition of electron movement in the improved Thomas-Fermi-Dirac (TFD) theory was used to decide the continuity of the electron density of the lamellar colonies interfaces and it is found that the γ/a2 interfaces are continuous. Furthermore, it is found that adding alloying elements (including Nb, Cr, and V) can improve the electron density (ρ) of γ/a2 interface, and decrease ?ρ of γ/a2 interface. With the electron structure analysis together with properties analysis, the order of adding elements improving RT mechanical properties in theory is as follows: V > Cr > Nb.
References
[1] WU Xin-hua.  Review of alloy and process development of TiAl alloys [J]. Intermetallics, 2006, 14(10-11): 1114-1122.
Review of alloy and process development of TiAl alloys [J]. Intermetallics, 2006, 14(10-11): 1114-1122.
[2] CHEN Yu-yong, KONG Fan-tao, TIAN Jing, CHEN Zi-yong, XIAO Shu-long. Recent developments in engineering γ-TiAl intermetallics research [J]. Trans Nonferrous Met Soc China, 2002, 12(4): 605-609.
[3] CHEN Yu-yong, KONG Fan-tao, HAN Jie-cai, CHEN Zi-yong, TIAN Jing. Influence of yttrium on microstructure, mechanical properties and deformability of Ti-43Al-9V alloy [J]. Intermetallics, 2005, 13(3-4): 263-266.
[4] KIM Young-won. Advances in the fundamental understanding for designing engineering gamma TiAl alloys [J]. Journal of the Chinese Institute of Engineers, 1999, 22(1): 13-25.
[5] ZHANG Rui-lin. Empirical Electron Theory of Solid and Molecule [M]. Changchun: Jilin Science and Technology Press, 1993.(in Chinese)
[6] LIU Zhi-lin, LIU Wei-dong. Electron Structure and Properties of Boundaries [M]. Beijing: Science Press, 2002.(in Chinese)
[7] SONG Y, YANG R, LI D, HU Z Q, GUO Z X. First principles study of the influence of alloying elements on TiAl: Site preference [J]. Intermetallics, 2000, 8(5-6): 563-568.
[8] HAO Y L, YANG R, CUI Y Y. Effect of Ti/Al ratio on the site occupancies of alloying elements in γ-TiAl [J]. Intermetallics, 2000, 8(5-6): 633-636.
[9] HAO Y L, XU D S, CUI Y Y. Site occupancies of alloying elements in TiAl and Ti3Al alloys [J]. Acta Mater, 1999, 47(4): 1129-1139.
(Edited by PENG Chao-qun)
Foundation item: Project(50674037) supported by the National Natural Science Foundation of China
Corresponding author: KONG Fan-tao; Tel: +86-451-86418802; E-mail: kft@hit.edu.cn