Recovery of antimony from antimony-bearing dusts through reduction roasting process under CO–CO2 mixture gas atmosphere after firstly oxidation roasted
来源期刊:中南大学学报(英文版)2018年第8期
论文作者:李磊 钟大鹏 谈诚
文章页码:1904 - 1913
Key words:antimony-bearing dust; separation of arsenic and antimony; antimony recovery; reduction roasting; waste utilization
Abstract: This paper mainly investigated the antimony recovery from antimony-bearing dusts through reduction roasting process after the dust firstly oxidation roasted. CO–CO2 mixture gas was used as reducing agent, and the antimony-containing phase was reduced into Sb4O6, volatilized into smoke, and finally recovered through the cooling cylinder. The antimony recovery rate increased from 66.00 wt% to 73.81 wt% in temperature range of 650 to 800 °C, and decreased with temperature increased further to 900 °C due to the reduction of Sb4O6 to the nonvolatile Sb. Similarly, the CO partial pressure also played a double role in this test. Under optimized conditions of roasting temperature of 800 °C, CO partial pressure of 7.5 vol% and roasting time of 120 min, 98.40 wt% of arsenic removal rate and 80.40 wt% antimony recovery rate could be obtained. In addition, the “As2O3” product could be used for preparing ferric arsenate which realized the harmless treatment of it.
Cite this article as: ZHONG Da-peng, LI Lei, TAN Cheng. Recovery of antimony from antimony-bearing dusts through reduction roasting process under CO–CO2 mixture gas atmosphere after firstly oxidation roasted [J]. Journal of Central South University, 2018, 25(8): 1904–1913. DOI: https://doi.org/10.1007/s11771-018-3880-y.

J. Cent. South Univ. (2018) 25: 1904-1913
DOI: https://doi.org/10.1007/s11771-018-3880-y

ZHONG Da-peng(钟大鹏)1, 2, LI Lei(李磊)1, 2, 3, TAN Cheng(谈诚)1, 2
1. State Key Laboratory of Complex Non-ferrous Metal Resources Clean Utilization,
Kunming University of Science and Technology, Kunming 650093, China;
2. Engineering Research Center of Metallurgical Energy Conservation and Emission Reduction of
Ministry of Education, Kunming University of Science and Technology, Kunming 650093, China;
3. Faculty of Metallurgical and Energy Engineering, Kunming University of Science and Technology, Kunming 650093, China
 Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2018
Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2018
Abstract: This paper mainly investigated the antimony recovery from antimony-bearing dusts through reduction roasting process after the dust firstly oxidation roasted. CO–CO2 mixture gas was used as reducing agent, and the antimony-containing phase was reduced into Sb4O6, volatilized into smoke, and finally recovered through the cooling cylinder. The antimony recovery rate increased from 66.00 wt% to 73.81 wt% in temperature range of 650 to 800 °C, and decreased with temperature increased further to 900 °C due to the reduction of Sb4O6 to the nonvolatile Sb. Similarly, the CO partial pressure also played a double role in this test. Under optimized conditions of roasting temperature of 800 °C, CO partial pressure of 7.5 vol% and roasting time of 120 min, 98.40 wt% of arsenic removal rate and 80.40 wt% antimony recovery rate could be obtained. In addition, the “As2O3” product could be used for preparing ferric arsenate which realized the harmless treatment of it.
Key words: antimony-bearing dust; separation of arsenic and antimony; antimony recovery; reduction roasting; waste utilization
Cite this article as: ZHONG Da-peng, LI Lei, TAN Cheng. Recovery of antimony from antimony-bearing dusts through reduction roasting process under CO–CO2 mixture gas atmosphere after firstly oxidation roasted [J]. Journal of Central South University, 2018, 25(8): 1904–1913. DOI: https://doi.org/10.1007/s11771-018-3880-y.
1 Introduction
As a valuable metal, antimony and its compounds have been widely used in the production of ceramics, glass, paint, firework materials, and fire prevention materials etc. [1–5]. However, antimony reserves which occur chiefly as the gray sulfide mineral stibnite (Sb2S3) [5, 6] are very scarce. The extractable global resources of antimony will be exhausted before 2050 if the antimony extraction rate continues to increase by the current pace [5, 7]. It is necessary to find other resources to extract antimony, in addition from the ore. There is an antimony-bearing dust generated from the reduction-smelting of non-ferrous metals, and most of which also contains other valuable metals, such as zinc, lead, copper, bismuth, and even some rare metals [8–10]. The antimony content in the dust exceeds 30 wt% in general,which provides the value of reutilization. Nevertheless, arsenic, a toxic element companioning with antimony [11, 12], should be firstly removed for the antimony recovery due to the similarity of their physical and chemical properties.
Main phases of arsenic and antimony existing in antimony-bearing dusts are As2O3 and Sb2O3, respectively. The As2O3 is easy to volatilize and its volatilization rate outstripped 93.0 wt% in 120 min at 460 °C [13], and the Sb2O3 volatilization rate also exceeded 95.0 wt% in 140 min at 600 °C under nitrogen atmosphere [14]. However, when oxygen concentration exceeded 10%, PADILLA et al [15] found that the antimony volatilization was inhibited effectively by the formation of a non-volatile compound, SbO2. So, the arsenic could be separated effectively if the Sb2O3 was selectively oxidized from As2O3 and Sb2O3 to SbO2. Based on this, a selective oxidation roasting process for treating high As–Sb dusts was used in the studies of LI et al [16] and TANG et al [17], but their studies showed that only about 60 wt% of arsenic was removed and the antimony lost was higher than 10 wt%. The reason might be that the As2O3 and Sb2O3 were combined and transformed into a heteronuclear compound AsxSbyO6 (where x=1, 2 or 3, and x+y=4) during roasting process, which inhibited the arsenic volatilization and simultaneously promoted antimony volatilization at 200 to 800 °C [18–20]. A better separation rate of arsenic and antimony could be obtained from the hydrometallurgy process, in which the arsenic was selectively leached into solution and separated from the antimony using acid [21, 22], alkali [23–25], chlorination [26] or alkaline [27, 28]. The antimony was transformed into insoluble matter during the leaching process and separated from the solution by filtration [24, 29–31]. The arsenic removal rate could be up to 92 wt% using the hydrometallurgy process [23–28], but it consumes a lot of reagents and the arsenic containing wastewater poses a potential threat to the environment. Thus, a more reasonable process is urgently proposed to treat the antimony-bearing dust.
Using CuO as a weak oxidant for treating the antimony-bearing dust, ZHONG et al [32] found that about 91.50 wt% of arsenic was removed with only 8.63 wt% of antimony lost under the conditions of roasting temperature of 400 °C, roasting time of 100 min and N2 flow rate of 30 mL/min. A higher separation rate of arsenic and antimony was obtained. After being treated, the antimony existed in the roasted residues in the form of Sb2O4, but the corresponding recovery method was not proposed in the research above. In addition, the treatment method of copper existing in the roasted residue was not also put forward. Based on the volatility of Sb2O3, two stage roasting processes including oxidation and reduction for treating the antimony-bearing dust are put forward in this paper. For the first oxidation roasting stage, the process was carried out under the optimum process parameters proposed by ZHONG et al [32]. The second stage followed the first stage finished. During the second stage of reduction roasting, the CO acted as a reductant and effects of processing parameters on recovery of antimony had been investigated systematically, including roasting temperature, CO partial pressure and roasting time.
2 Experimental
2.1 Materials
The antimony-bearing dust used in this study was provided by a plant for treating tin anode slime using a pyrometallurgical process, which locates in Yunnan province, China. The chemical composition of it is presented in Table 1. Table 1 shows that the major elements presented are “As” (36.28 wt%), “Sb” (28.72 wt%) and “O” (22.35 wt%). “Others” in Table 1 is mainly composed of “Ca”, “K” and “S”, etc. Mineral constituent of the raw material was analyzed using XRD and EMPA reported in Figures 1 and 2. Figure 1 shows that the main phases are As2O3, Sb2O3, As4O6 and (Sb,As)2O3. Figure 2 shows that part of arsenic and antimony exists independently in the form of white arsenic ore and valentinite respectively. Simultaneously, the combined phases of arsenic and antimony are observed, including variant white arsenic ore and altered red sulfur arsenic antimony sodium ore.
The oxidation treatment for this dust had been investigated in the previous study of ZHONG et al [32], and chemical composition of the roasted residue for this roasting process is presented in Table 2. It shows that the major elements are “Sb” (36.54 wt%), “O” (26.54 wt%) and “Cu”(23.67 wt%). “Others” in Table 2 is mainly composed of “Ca”,“K” and “S”, etc. Mineral constituent of the roasted residue was characterized with XRD and EMPA. Figure 3 shows that main phases of the roasted residue are CuO, Sb2O4, Cu2O and Cu3(AsO4)2. Figure 4 shows that most antimony phase (Sb2O4) coexists with copper phase (Cu2O), and other copper exists independently in the form of Cu2O and CuO respectively.
Table 1 Chemical composition of antimony-bearing dust (mass fraction, %)
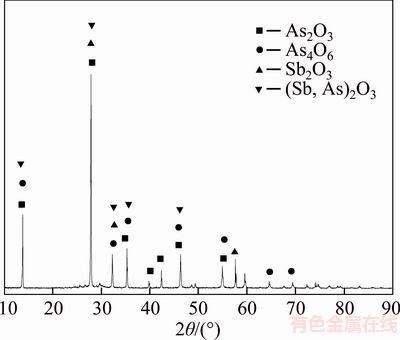
Figure 1 XRD pattern of antimony-bearing dust
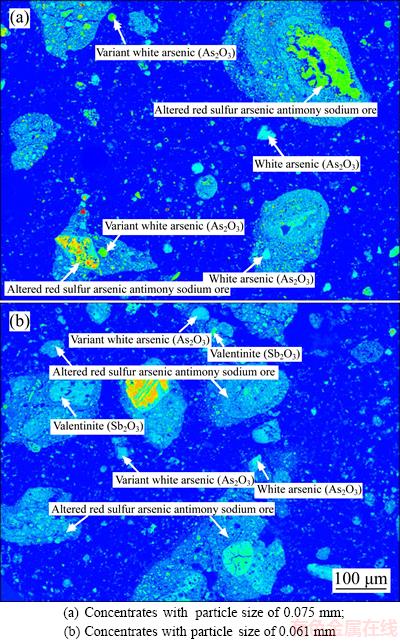
Figure 2 Mineral phase of distribution of antimony- bearing dust obtained by EPMA:
Table 2 Chemical composition of roasted residue for oxidation roasting process (mass fraction, %)

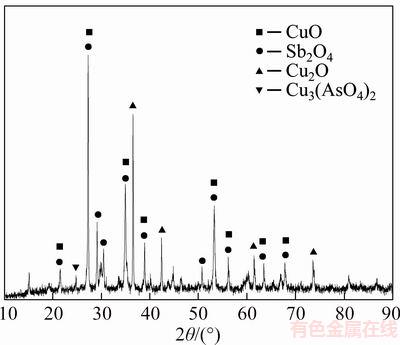
Figure 3 XRD pattern of roasted residue for oxidation roasting process

Figure 4 EPMA analysis of roasted residue for oxidation roasting process obtained:
The CuO reagent was used as oxidant in the oxidation roasting stage, and the purity of it is over 99.00 wt%. N2 (purity of 99.3%), CO (purity of 99.9%) and CO2 (purity of 99.9%) were procured from local suppliers.
2.2 Roasting experiments
In this work, two stage roasting processes including oxidation and reduction were used to deal with the antimony-bearing dust. For the purpose of separating arsenic from the dusts drastically,30.000 g of the dust mixed with 34.54 wt% CuO was firstly roasted in the tube furnace (Figure 5) at 400 °C for 100 min with N2 flow rate of 30 mL/min based on the previous study [32]. During this oxidation roasting process, keep the valve “14” off and “15” on. The off-gas from the reaction tube continuously passed through a water-cooled condenser (Device “10”), which collected the volatile matter, and then was directed to the solution of 1 mol/L NaOH to remove harmful components. The reduction roasting followed the oxidation finished. With the temperature being raised further to a set value, the roasted residue for the oxidation roasting was further roasted in the tube furnace for 20–140 min under CO–CO2 gas mixture atmosphere. The total inlet gas flow rate was fixed at 500 mL/min. After being cooled down to the room temperature under N2 atmosphere, the roasted residue was pulled out for analysis. During the reduction roasting stage, maintain the valve “15” off and “14” on. The off-gas from the reaction tube continuously passed through a cooling cylinder (Device“12”), which collected the volatile matter, and then was directed to the solution of 1 mol/L Na2S to remove harmful components. At last, the volatiles in cooling cylinder (Device “12”) and water-cooled condenser (Device “10”) were put out for analysis respectively.
2.3 Characterization
Chemical composition and mineralogy of the samples were characterized by chemical analysis and electron probe microanalysis (EPMA) respectively. Especially, the “O” contents in the samples were detected by XRF analysis (MINIPAL4, PANalytical, Netherlands). Phase compositions of all the samples were detected by an XRD with a Cu-Kα radiation (the scanning rate was 10°/min and 2θ was 10°–90°). FactSage 7.0 software was used to calculate the standard Gibbs free energy changes and equilibrium compositions of the reaction system. Mathematical expressions of volatilization rate of arsenic (RA) and recovery rate of antimony (RS) were defined as follows:
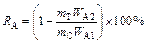 (1)
(1)
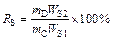 (2)
(2)
where mC stands for the mass of antimony-bearing dusts used; wA1 and wS1 stand for arsenic and antimony contents in the origin antimony-bearing dust, respectively; mT and mD stand for the mass of roasted residue and collected matter in device “12” (Figure 5) respectively; WA2 stands for the arsenic content in the roasted residue; WS2 stands for the antimony content in the collected matter in device “12” (Figure 5).
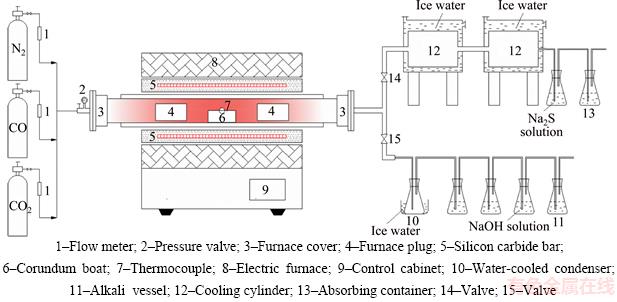
Figure 5 Experimental setup:
3 Thermodynamic analysis
Based on the mineral constituent of the roasted residue for the oxidation roasting process, the main reactions which may occur during the followed reduction roasting are as follows:
Sb2O4(s)+CO(g)=Sb4O6(g)+CO2(g) (3)
1/3Sb4O6(g)+CO(g)=2/3Sb(l)+CO2(g) (4)
1/6Sb4O6(g)+CO(g)=1/3Sb2(g)+CO2(g) (5)
1/6Sb4O6(g)+CO(g)=1/6Sb4(g)+CO2(g) (6)
2/13Cu3(AsO4)2(s)+CO(g)=2/13Cu3As(s)+1/26As4O6(g)+CO2(g) (7)
2CuO(s)+CO(g)=Cu2O(s)+CO2(g) (8)
Cu2O(s)+CO(g)=2Cu(s)+CO2(g) (9)
1/6As4O6(g)+CO(g)=1/3As(l)+CO2(g) (10)
Figure 6 shows that the Sb2O4 can be reduced to Sb4O6, and further reduced to lower valence states of antimony compounds (Sb, Sb2, Sb4, etc.). In order to increase the antimony volatilization and recovery rate, the reduction roasting process should be carried out by accurately controlling the temperature and CO partial pressure so as to increase the formation of Sb4O6 while avoid its further reduction.

Figure 6 Standard Gibbs free energy changes of Eqs. (3)–(10) with temperature
4 Results and discussion
All of the original antimony-bearing dusts were firstly roasted with CuO, which was carried out under the process parameters of roasting temperature of 400 °C, roasting time of 100 min, CuO amount of 34.54 wt% and N2 flow rate of 30 mL/min [32]. Then the roasted residue for the oxidation roasting stage was further roasted under CO–CO2 gas mixture atmosphere to recover antimony.
4.1 Effects of roasting temperature
Under roasting time of 120 min and CO partial pressure of 5 vol%, six roasting temperatures of 650, 700, 750, 800, 850 and 900 °C were chosen for studying the effects on volatilization rates of arsenic and antimony.
Figure 7 shows that arsenic volatilization rate almost remains constant with temperature from 650 to 900 °C, which is due to most of the arsenic having been volatilized in the oxidation roasting stage [32]. Moreover, a high temperature provides a favorable kinetic condition for the selective reduction of Sb2O4 to Sb4O6 and further the Sb4O6 volatilization, which results in the antimony recovery rate increase with temperature from 650 to 800°C. However, the generated Sb4O6 could be further reduced into nonvolatile Sb and its amount increases with temperature from 800 to 900 °C (Figure 8), resulting in the gradual decrease of antimony recovery rate. Hence, the reduction roasting temperature is determined as 800 °C.
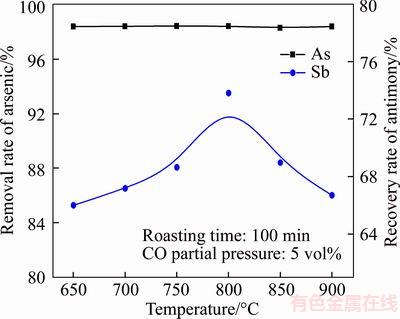
Figure 7 Effects of roasting temperature on removal rate of arsenic and recovery rate of antimony
4.2 Effects of CO partial pressure
The FactSage 7.0 was used to calculate the equilibrium of roasted products for the reduction roasting process in Gibbs free energy minimization under isothermal, isobaric and fixed mole conditions. Required data for computation were provided by FactPS database of the program.

Figure 8 XRD patterns of roasted residues at 800 °C, 850 °C and 900 °C
In Equilib program of FactSage, Cu3(AsO4)2(s)(0.0012 mol), Sb2O4(s)(0.0321 mol), CuO(s)(0.0602 mol) and Cu2O(s)(0.0321 mol) were selected as reactant precursors, and CO–CO2 gas mixtures was an reactant gas to strictly control the retained O2 content in the atmosphere. Total quantity of CO and CO2 was fixed at 2.679 mol due to the total inlet gas flow rate of 500 mL/min and roasting time of 120 min. The temperature and atmosphere pressure were fixed at 800 °C and 1.01325×105 Pa, respectively. This calculation was performed at antimony-bearing dusts of 30 g and CO partial pressure range of 0 to 15 vol%. The phases of Cu3(AsO4)2(s), Cu3As(s), CuO(s), Cu2O(s), Cu(s), Sb(l) and Sb2O4(s) were assumed to be present in the roasted products, and the phases of Sb4O6(g), Sb2(g), Sb4(g), As4O6(g) were deemed to be volatilized and went into the smoke. The results of their equilibrium are present in Figures 9(a) and (b). Amounts of Sb2(g) and Sb4(g) are too little in Figure 9(a) to consider their effects during the reduction roasting. With CO partial pressure from 0 to 5 vol%, Figure 9(a) shows that Sb2O4(s) amount decreases and Sb4O6(g) amount increases attributed to the occurrence of Eq.(3). When CO partial pressure increases above 5 vol%, the Sb(l) phase appears going with a rapid decrease of Sb4O6(g) amount, which is related to the further reduction of Sb4O6(g) to Sb(l) (Eq.(4)). Improving the Sb4O6(g) formation amount and antimony recovery rate, the reduction roasting should be performed at CO partial pressure being in the range of 3 vol% to 5 vol% seen from Figure 9(a).
With CO partial pressure being from 0 to 2 vol%, the CuO(s) is reduced to Cu2O(s), resulting in a rapid decrease of CuO(s) and gradual increase of Cu2O(s) in Figure 9(b). When CO partial pressure is above 2 vol%, the Cu(s) phase appears accompanying with a rapid decrease of Cu2O(s) amount attributed to the further reduction of Cu2O(s) to Cu(s).

Figure 9 Equilibrium amounts of species in roasted products as function of CO partial pressure:
The CO partial pressure ranging from 1.25 vol% to 15.0 vol% was selected to investigate its effects on volatilization rates of arsenic and antimony, and the results are shown in Figure 10. Obviously, the CO partial pressure hardly affects the arsenic removal rate due to its little content in the roasted residue for the oxidation roasting. The recovery rate of antimony increases from 48.10 wt% to 80.41 wt% with CO partial pressure from 1.25 vol% to 7.50 vol%. However, the Sb(l) is formed at CO partial pressure of 7.5 vol% and its amount increases with CO partial pressure (Figure 11), resulting in the recovery rate of antimony decreasing gradually. Hence, the CO partial pressure is fixed at 7.5%. During this roasting stage, the copper phases are mainly transformed into Cu and Cu2O (Figure 10), and they can be recycled into this two stage roasting processes after it reoxidized to CuO.
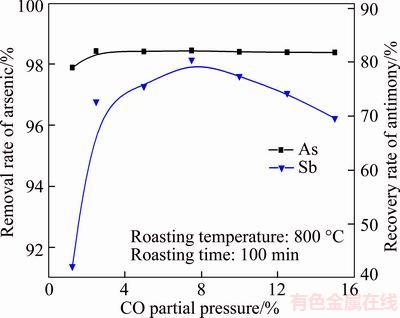
Figure 10 Effects of CO partial pressure on removal rate of arsenic and recovery rate of antimony
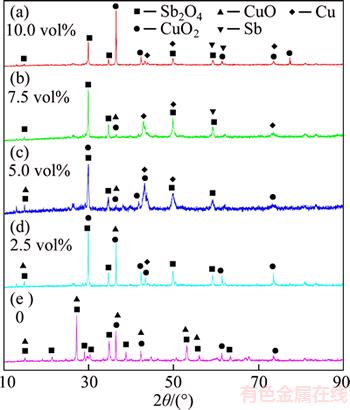
Figure 11 XRD patterns of roasted residues under different CO partial pressures
4.3 Effects of roasting time
The effect of roasting time was studied under the following conditions: 800 °C for roasting temperature and 7.5 vol% for CO partial pressure. Figure 12 reveals that the removal rate of arsenic is almost unchanged during this text. Simultaneously, the recovery rate of antimony increases gradually in the primary 120 min, from 61.0 wt% to 80.4 wt%, and then remains nearly constant. To decrease the process energy consumption and improve antimony recovery rate, the roasting time is fixed at 120 min.

Figure 12 Effects of roasting time on removal rate of arsenic and recovery rate of antimony
Based on the discussion above, it can be concluded that recovering antimony from the antimony-bearing dust through reduction roasting under CO–CO2 mixture gas atmosphere after oxidation roasted is viable. Under conditions of roasting temperature of 800 °C, CO partial pressure of 7.5 vol% and roasting time of 120 min,98.40 wt% of arsenic removal rate and 80.40 wt% antimony recovery rate could be obtained. Meanwhile, the products of As2O3 and Sb2O3 could be collected from the water-cooled condenser (device “10” in Figure 5) and cooling cylinder (device “12” in Figure 5) respectively, and XRD patterns of them are shown in Figures13 and 14 respectively. In addition, the “As2O3” product can be used for preparing ferric arsenate which realizes the harmless treatment of it [33, 34]. Meanwhile, this process can obtain a high antimony recovery rate.

Figure 13 XRD pattern of product in water-cooled condenser (device “10” in Figure 5)
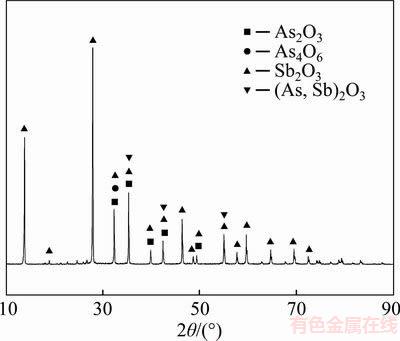
Figure 14 XRD pattern of product in cooling cylinder (device “12” in Figure 5)
5 Conclusions
The effective recovery of antimony from the antimony-bearing dust through reduction roasting under CO–CO2 mixture gas atmosphere after oxidation roasted is feasible. Several parameters of reduction roasting stage were studied, i.e., the roasting temperature, CO partial pressure and roasting time.
The recovery rate of antimony increases from 66.00 wt% to 73.81 wt% with temperature from 650 to 800 °C, and decreases with temperature increased further to 900 °C due to the reduction of Sb4O6 to the nonvolatile Sb. Similarly, the recovery rate of antimony increases substantially with the CO partial pressure range of 1.25 vol% to 7.5 vol%, and then decreases gradually when the CO partial pressure is over 7.5 vol%. Under optimized conditions of roasting temperature of 800 °C, CO partial pressure of 7.5 vol% and roasting time of 120 min, 98.40 wt% of arsenic removal rate and 80.40 wt% antimony recovery rate could be obtained. In addition, the products of “As2O3”can be used for preparing ferric arsenate which realized the harmless treatment of it, and the copper existed in the roasted residue in the form of Cu and Cu2O could be recycled into this two stage roasting processes after it is reoxidized.
References
[1] BINZ F, FRIEDRICH B. Recovery of antimony trioxide flame retardants from lead refining residues by slag conditioning and fuming [J]. Chemie Ingenieur Technik, 2015, 87(11): 1569–1579. DOI: 10.1002/cite.201500071.
[2] ANDERSON C G. The metallurgy of antimony [J]. Chemie der Erde-Geochemistry, 2012, 72: 3–8. DOI: 10.1016/ j.chemer.2012.04.001.
[3] WEN Bing, ZHOU Jian-wei, ZHOU An-guo, LIU Cun-fu, XIE Li-na. Sources, migration and transformation of antimony contamination in the water environment of Xikuangshan, China: Evidence from geochemical and stable isotope (S, Sr) signatures [J]. Science of the Total Environment, 2016, 569–570: 114–122. DOI: 10.1016/ j.scitotenv.2016.05.124.
[4] SHANGGUAN Yu-xian, ZHAO Long, QIN Yu-sheng, HOU Hong, ZHANG Nai-ming. Antimony release from contaminated mine soils and its migration in four typical soils using lysimeter experiments [J]. Ecotoxicology and Environmental Safety, 2016, 133: 1–9. DOI: 10.1016/ j.ecoenv.2016.06.030.
[5] HENCKENS M L C M, DRIESSEN P P J, WORRELL E. How can we adapt to geological scarcity of antimony? Investigation of antimony's substitutability and of other measures to achieve a sustainable use [J]. Resources, Conservation and Recycling, 2016, 108: 54–62. DOI: 10.1016/j.resconrec.2016.01.012.
[6] PADILLA P R, RAM REZ G, RUIZ M C. High-temperature volatilization mechanism of stibnitein nitrogen-oxygen atmospheres [J]. Metallurgical and Materials Transactions B, 2010, 40(6): 1284–1292. DOI: 10.1007/s11663-010-9429-6.
REZ G, RUIZ M C. High-temperature volatilization mechanism of stibnitein nitrogen-oxygen atmospheres [J]. Metallurgical and Materials Transactions B, 2010, 40(6): 1284–1292. DOI: 10.1007/s11663-010-9429-6.
[7] HENCKENS M L C M, DRIESSEN P P J, WORRELL E. Metal scarcity and sustainability, analyzing the necessity to reduce the extraction of scarce metals [J]. Resources, Conservation and Recycling, 2014, 93: 1–8. DOI: 10.1016/ j.resconrec.2014.09.012.
[8] TANG Shi-jia, GAO Guang-ming, PENG En-sheng, SUN Zhen-jia. Fractal feature of western fracture zone in Xikuangshan antimony mine and its geological significance [J]. Journal of Central South University of Technology, 2000, 7(4): 212–215. DOI: 10.1007/s11771-000-0056-2.
[9] SHAWABKEH R A. Hydrometallurgical extraction of zinc from Jordanian electric arc furnace dust [J]. Hydrometallurgy, 2010, 104(1): 61–65. DOI: 10.1016/j.hydromet.2010.04.014.
[10] LUNDGREN M, LEIMALM U, HYLLANDER G,  KVIST L S, BJ
KVIST L S, BJ RKMAN B. Off-gas dust in an experimental blast furnace, Part 2: Relation to furnace conditions [J]. ISIJ International, 2010, 50: 1570–1580. DOI: 10.2355/ isijinternational.50.1560. 10.1007/s11771-014-2168-0.
RKMAN B. Off-gas dust in an experimental blast furnace, Part 2: Relation to furnace conditions [J]. ISIJ International, 2010, 50: 1570–1580. DOI: 10.2355/ isijinternational.50.1560. 10.1007/s11771-014-2168-0.
[11] HOU Xiao-chuan, YANG Yun-de, LI He, ZENG-li, XIAO Lian-sheng. Kinetics study of leaching arsenic from Ni-Mo ore roasting in dust mixture of hydrochloric and sulfuric acids [J]. Journal of Central South University, 2014, 21: 2176-2183.
[12] CHAI Li-yuan, SHI Mei-qing, LIANG Yan-jie, TANG Jing-wen, LI Qing-zhu. Behavior, distribution and environmental influence of arsenic in a typical lead smelter [J]. Journal of Central South University, 2015, 22: 1276– 1286. DOI: 10.1007/s11771-015-2644-1.
[13] YUAN Hai-bin, ZHU Yu-yan, ZHANG Ji-bin. Process of high-arsenic dust containing tin volatilization from DC submerged arc furnace [J]. Journal of Central South University: Science and Technology, 2013, 44(6): 2200–2205. (in Chinese)
[14] ARACENA A, JEREZ O, ANTONUCCI C. Senarmontite volatilization kinetics in nitrogen atmosphere at roasting/ melting [J]. Transactions of Nonferrous Metals Society of China, 2016, 26(1): 294–300. DOI: 10.1016/S1003- 6326(16)64117-1.
[15] PADILLA R P, RAM REZ G, RUIZ M C. High-temperature volatilization mechanism of stibnite in nitrogen-oxygen atmospheres [J]. Metallurgical and Materials Transactions B, 2010, 41(6): 1284–1292. DOI: 10.1007/s11663-010-9429-6.
REZ G, RUIZ M C. High-temperature volatilization mechanism of stibnite in nitrogen-oxygen atmospheres [J]. Metallurgical and Materials Transactions B, 2010, 41(6): 1284–1292. DOI: 10.1007/s11663-010-9429-6.
[16] LI Lei, ZHANG Ren-jie, LIAO Bin, XIE Xiao-feng. Separation of As from As and Sb contained smoke dust by selective oxidation [J]. The Chinese Journal of Process Engineering, 2014, 14(1): 71–77. (in Chinese)
[17] TANG Hai-bo, QIN Qing-wei, GUO Yong, ZHENG Xin, XUE Ping, LI Guang-qiang. Pretreatment of high arsenic and antimony smelting dust for arsenic removal using roasting process [J]. Conservation and Utilization of Mineral Resources, 2014(3): 35–38. (in Chinese)
[18] BROOKS G A, RANKIN W J, GRAY N B. Thermal separation of arsenic and antimony oxides [J]. Metallurgical and Materials Transactions B, 1994, 25(6): 873–884. DOI: 10.1007/bf02662769.
[19] MAUSER J E. Heteronuclear compounds of arsenic and antimony [J]. Metallurgical and Materials Transactions B, 1982, 13(3): 511–513. DOI: 10.1007/BF02667768.
[20] BROOKS G A, RANKIN W J. Solid-solution formation between arsenic and antimony oxides [J]. Metallurgical and Materials Transactions B, 1994, 25(6): 865–871. DOI: 10.1007/BF02662768.
[21] WANG Jiang-sheng. Reclaiming valuable metals from copper converter dust [J]. Copper Industrial Engineering, 2005(1): 27–28. (in Chinese)
[22] KASHIWAKURA S, OHNO H, MATSUBAE-YOKOYAMA K, KUMAGAI Y, KUBO H, NAGASAKA T. Removal of arsenic in coal fly ash by acid washing process using dilute H2SO4 solvent [J]. Journal of Hazardous Materials, 2010, 181(1–3): 419–25. DOI: 10.1016/j.jhazmat.2010.05.027.
[23] GUO Xue-yi, SHI Jing, YI Yu, TIAN Qing-hua, LI Dong. Separation and recovery of arsenic from arsenic-bearing dust [J]. Journal of Environmental Chemical Engineering, 2015, 3(3): 2236–2242. DOI: 10.1016/j.jece.2015.06.028.
[24] GUO Xue-yi, YI Yu, SHI Jing, TIAN Qing-hua. Leaching behavior of metals from high-arsenic dust by NaOH–Na2S alkaline leaching [J]. Transactions of Nonferrous Metals Society of China, 2016, 26(2): 575–80. DOI: 10.1016/ S1003-6326(16)64118-3.
[25] MONTENEGRO V, SANO H, FUJISAWA T. Recirculation of high arsenic content copper smelting dust to smelting and converting processes [J]. Minerals Engineering, 2013, 49: 184–89. DOI: 10.1016/j.mineng.2010.03.020.
[26] JIANG Xue-xian, HE Gui-xiang, LI Xu-guang, LU Jing. Experimental research on dearsenization of high arsenic fume [J]. Hydrometallurgy of China, 2010, 29(3): 199–202. (in Chinese)
[27] LI Yu-hu, LIU Zhi-hong, LI Qi-hou, LIU Fu-peng, LIU Zhi-yong. Alkaline oxidative pressure leaching of arsenic and antimony bearing dusts [J]. Hydrometallurgy, 2016, 166: 41–47. DOI: 10.1016/j.hydromet.2016.07.010.
[28] VIRCIKOVA E, HAVLIK M. Removing as from converter dust by a hydrometallurgical method [J]. JOM, 1999, 51(9): 20–23. DOI: 10.1007/s11837-999-0152-1.
[29] LI Yu-hu, LIU Zhi-hong, LI Qi-hou, ZHAO Zhong-wei, LIU Zhi-yong, ZENG Li. Removal of arsenic from Waelz zinc oxide using a mixed NaOH–Na2S leach [J]. Hydrometallurgy, 2011, 108(3, 4): 165-70. DOI: 10.1016/j.hydromet.2011. 04.002.
[30] SULLIVAN C, TYRER M, CHEESEMAN C R, GRAHAM N J. Disposal of water treatment wastes containing arsenic— A review [J]. Science of the Total Environment, 2010, 408(8): 1770–78. DOI: 10.1016/j.scitotenv.2010.01.010.
[31] LI Yu-hu, LIU Zhi-hong, LI Qi-hou, ZHAO Zhong-wei, LIU Zhi-yong, ZENG Li, LI Li. Removal of arsenic from arsenate complex contained in secondary zinc oxide [J]. Hydrometallurgy, 2011, 109(3, 4): 237-44. DOI: doi.org/ 10.1016/j.hydromet.2011.07.007.
[32] ZHONG Da-peng, LI Lei, TAN Cheng. Separation of arsenic from the antimony-bearing dust through selective oxidation using CuO [J]. Metallurgical and Materials Transactions B, 2017, 48(2): 1308–1314. DOI: 10.1007/s11663-016-0896-2.
[33] ZHANG Rong-liang, QIU Ke-qiang, XIE Yong-jin, HONG Yu-min, ZHENG Chun-dao. Treatment process of dust from flash smelting furnace at copper smelter by oxidative leaching and dearsenifying process from leaching solution [J]. Journal of Central South University: Science and Technology, 2006, 37(1): 73–78. (in Chinese)
[34] BERRE J F LE, GAUVIN R, DEMOPOULOS G P. Characterization of poorly-crystalline ferric arsenate precipitated from equimolar Fe(III)-As(V) solutions in the pH range 2 to 8 [J]. Metallurgical and Materials Transactions B, 2007, 38(5): 751–762. DOI: 10.1007/ s11663-007-9081-y.
(Edited by YANG Hua)
中文导读
含锑烟尘氧化焙烧处理后CO–CO2混合气体中锑资源的还原焙烧法回收
摘要:研究主要针对含锑烟尘经氧化焙烧处理后CO–CO2混合气体中锑资源的还原焙烧法回收进行了探讨。以CO–CO2混合气体作为还原剂,过程中锑物相还原为Sb4O6并进入气相,最后通过冷凝收尘方式实现其回收。温度由650 °C升高至800 °C时,锑回收率从66.00 wt%增至73.81 wt%,当温度进一步升高到900 °C时,Sb4O6被进一步还原为非挥发性金属锑,锑回收率降低。同样,CO分压对锑的挥发回收亦起双重作用。在最优焙烧条件下,即焙烧温度800 °C、CO分压7.5 vol%及焙烧时间120 min时,锑烟尘中砷挥发率可达98.40 wt%,同时锑回收率亦可达80.40 wt%。此外,挥发脱除的“As2O3”烟尘可用于制备砷酸铁,以实现其无害化处理。
关键词:含锑烟尘;砷锑分离;锑回收;还原焙烧;废物利用
Foundation item: Project(51564034) supported by the National Science Fund for Distinguished Regional Scholars, China
Received date: 2017-03-21; Accepted date: 2018-01-29
Corresponding author: LI Lei, PhD, Professor; Tel: +86–871–13987619187; E-mail: tianxiametal1008@163.com; ORCID: 0000-0003- 1205-3989

