Trans. Nonferrous Met. Soc. China 23(2013) 881-888
Phase equilibria in Mg-rich corner of Mg-Ca-RE (RE=Gd, Nd) systems at 400 °C
Hou-jun FEI1, Guang-long XU1,2, Li-bin LIU1,3, Hong BO1, Li-jun ZENG1, Cui-ping CHEN1
1. School of Materials Science and Engineering, Central South University, Changsha 410083, China;
2. IMDEA Materials Institute, Madrid 28040, Spain;
3. Key Laboratory of Non-ferrous Materials Science and Engineering of Ministry of Education, Central South University, Changsha 410083, China
Received 22 March 2012; accepted 2 July 2012
Abstract: The phase equilibria in Mg-rich corner of Mg-Ca-Gd and Mg-Ca-Nd ternary systems at 400 °C were determined through the equilibrated alloy method by using XRD, SEM, EPMA and DSC. Partial isothermal sections in Mg-rich corner of Mg-Ca-Gd and Mg-Ca-Nd ternary systems at 400 °C were constructed from 13 alloys. A three-phase region of α-Mg, Mg41RE5 and Mg2Ca was determined in both ternary systems. It is formed by a similar ternary eutectic reaction L→α-Mg+Mg2Ca+Mg41RE5 at 499.6 °C and 505.6 °C, respectively. It is found that the maximum solubility of Ca in Mg5Gd is 3.68% (molar fraction) and 3% of Gd can be dissolved in Mg2Ca in the Mg-Ca-Gd system at 400 °C. While in the Mg-Ca-Nd system,the maximum solubility of Ca in Mg41Nd5 is 3.57% and 1.24% of Nd can be dissolved in Mg2Ca at 400 °C. Other three-phase equilibria existing in Mg-rich corner of Mg-Ca-Gd system are α-Mg+Mg5Gd+Τ and Mg5Gd+Mg2Ca+Τ and the three-phase equilibrium in Mg-rich corner of Mg-Ca-Nd system is Mg3Nd+Mg2Ca+ Mg41Nd5.
Key words: Mg-Ca-Gd system; Mg-Ca-Nd system; Mg-rich corner; three-phase equilibrium; solubility
1 Introduction
Magnesium alloys, due to their low density and high specific strength, have attracted much attention in the fields of automotive and aerospace in the past decades [1,2]. However, the use of Mg-based light alloys is currently limited by their low yield strength and low creep resistance at elevated temperatures [3]. Alloying, especially the addition of Gd and Nd elements can effectively improve casting property and high temperature performance [4-6]. The formation of coherent Mg2Ca precipitated by adding Ca is suggested to be able to enhance the creep resistance and tensile strength due to its thermal stability [7,8]. Moreover, Ca could increase the ignition temperature of the molten Mg-based alloy and promote corrosion resistance because of the formation of the stable oxidization layer [9,10]. Therefore, Mg-Ca-Gd and Mg-Ca-Nd ternary systems are of great importance for design of Mg-based alloy with high creep resistance.
Phase diagram has always served as a roadmap for material research and materials processing design. This is particularly true in the development of new Mg-based materials with superior properties. However, the detailed information on phase equilibria in Mg-Ca-Gd and Mg-Ca-Nd systems is insufficient, together with the solubility in Mg-based solid solution (denoted as α-Mg) and binary compounds extending to ternary, which is a critical obstacle in alloy design and manufacture. Thus, the main purpose of this work is to determine the phase relationship in Mg-rich corner of Mg-Ca-RE (RE=Gd, Nd) systems at 400 °C.
The Mg-Gd [11-15], Mg-Nd [16,17] and Mg-Ca [18] binary systems have been investigated by experimental investigation and CALPHAD method. Recently, coupling the experimental thermochemical and phase diagram data with thermodynamic modeling, the thermodynamic assessments of all of these binary systems have been carried out by CACCIAMANI et al [12-14] and GUO et al [15] for the Mg-Gd system, MENG et al [16] and NIU et al [17] for the Mg-Nd system, and ALJARRAH and MEDRAJ [18] for the Mg-Ca system, respectively. The shapes of the phase diagrams of Mg-Gd and Mg-Nd systems are similar, except that an eutectic reaction L→α-Mg+Mg41Nd5 (544 °C and 93.5%Mg) is available in the Mg-Nd system in comparison with L→α-Mg+Mg5Gd, (548 °C and 91.2% Mg) [15] in the Mg-Gd system. In the Mg-Gd system, the solubility of Gd in α-Mg is about 3.8%, but only 0.2% of Nd can be dissolved into α-Mg [17]. Neither isothermal sections nor ternary compounds in the Mg-Ca-RE (RE=Gd, Nd) ternary systems have been reported before.
2 Experimental
A series of Mg-Ca-Gd and Mg-Ca-Nd alloy samples in the Mg-rich corner were designed in order to include the compounds in equilibrium with α-Mg. The samples were prepared mainly using Mg-30%Gd, Mg-30%Nd and Mg-45%Ca master alloys. Pure granular Mg (> 99.99%), Ca (> 99.9%), Nd (> 99.9%), Gd (>99.9%) were added in some samples in order to get the designed composition. All the raw materials were wrapped with tantalum foils and sealed in iron tubes filled with argon. The tubes were kept at 800 °C for 4 h, and then quenched in water. During the melting process, each tube was turned over every 45 min to promote homogenization in the alloys. All the samples were then kept at 400 °C for 1440 h and finally quenched in water.
The compositions of the alloys were determined by the chemical titration technique. The phase constitutions of the annealed alloys were examined by powder X-ray diffraction (XRD, Dmax-2500 VBX) with Cu Kα diffraction operated at a voltage of 40 kV and a current of 250 mA. The microstructure was observed using a FEI-Quanta-200 scanning electron microscope (SEM) with an acceleration voltage of 20 kV. The composition of equilibrium phases was analyzed using a JXA-8230 electron probe microanalyzer (EPMA) and also with a voltage of 20 kV. Some of the annealed samples were examined by heat flux differential scanning calorimeter (DSC) measurement, which was performed on a TA-SDTQ600 at a heating rate of 10 K/min. As the alloys volatilize seriously at high temperatures, only a single heating process was performed for each sample under a continuous flux of argon.
Table 1 Equilibrium phase constituents in Mg-rich corner of Mg-Ca-Gd system at 400 °C
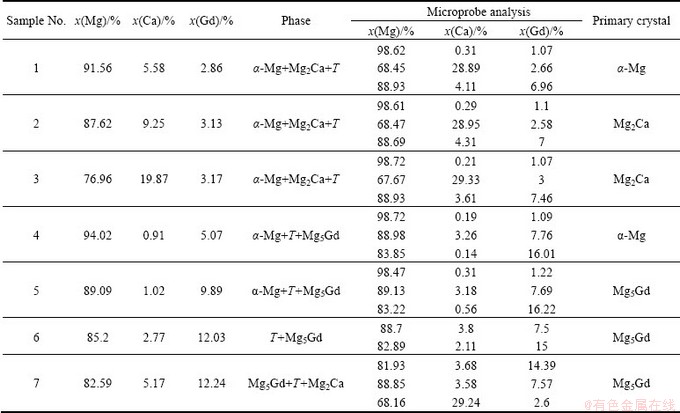
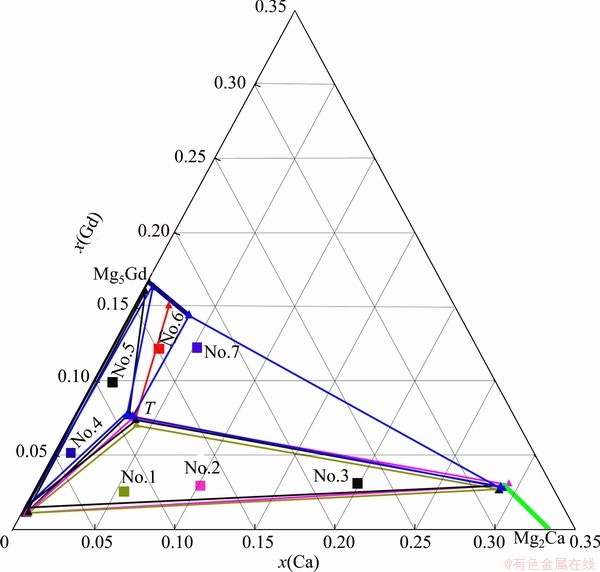
Fig. 1 Phase diagram of Mg-rich corner in Mg-Ca-Gd system at 400 °C
3 Results and discussion
3.1 Phase relation in Mg-rich corner of Mg-Ca-Gd system at 400 °C
The phases and their compositions in each annealed sample of Mg-Ca-Gd system obtained by XRD and EPMA are listed in Table 1 and the phase diagram of Mg-Ca-Gd system in Mg-rich corner at 400 °C is illustrated in Fig. 1.
The three-phase fields of α-Mg+Mg2Ca+Τ, α-Mg+ Mg5Gd+Τ and Mg5Gd+Mg2Ca+Τ exist in Mg-rich corner of Mg-Ca-Gd system at 400 °C, and the ternary phase Τ can form in most of the alloys and can be in equilibrium with all the phases in the Mg-rich corner. It can provide some possibilities for the alloy design. The solubility of Gd in Mg2Ca and that of Ca in Mg5Gd compounds are estimated to be 3% and 3.68%, respectively.
The microstructures and XRD patterns of annealed alloys 1 and 3 are shown in Fig. 2. There are obviously three kinds of contrast in both of BSE images. The phase with a black contrast has been detected to be α-Mg with a composition of 98.6Mg-0.3Ca-1.1Gd and the charcoal gray phase is Mg2Ca with a composition of nearly 68Mg-29Ca-3Gd. The XRD pattern also demonstrates the existence of Mg and Mg2Ca as displayed in Figs. 2(b) and (d). The XRD result also indicates the existence of a phase with the same structure as Mg41Ce5, which is associated with the light phases in SEM image. It could be inferred that some Ca atoms dissolved and occupied the positions of Gd atoms, and improved the stability of the Mg41Gd5 phase which is unstable in the Mg-Gd binary system. This phase is denoted as compound Τ in this ternary system and no solubility range has been observed. The trace of bright white contrast in the BSE images is pure Gd which has not been melted.
Some information of the solidification process can be extracted from the annealed microstructure. It is obvious that the primary crystal is α-Mg in alloy 1 and Mg2Ca in alloy 3. Figures 2 (c) and (f) give the DSC curves of alloys 1 and 3 and there is an endothermic peak at about 499.6 °C on each heating curve. It can be inferred that these two similar curves were caused by the same eutectic reaction L→α-Mg+Mg2Ca+Τ, with a further consideration of the microstructure of the alloys. For Mg-based material design, the formation of Mg2Ca primary crystal should be avoided.
Figure 3 shows the equilibria of α-Mg+Mg5Gd+ Τ in sample 5. In Fig. 3(a), the dark phase is α-Mg and the charcoal gray phase is the compound Τ as described above, which can be proved by the EPMA and XRD results. There is still a light phase which has an mole ratio of Mg:Gd nearly 5:1. Considering the very small solubility of Ca, it can be confirmed that it is compound Mg5Gd which is the nearest stable phase to α-Mg in the Mg-Gd binary system. The diffraction peaks of the three phases above can all be indexed from the XRD patterns, as shown in Fig. 3(b).
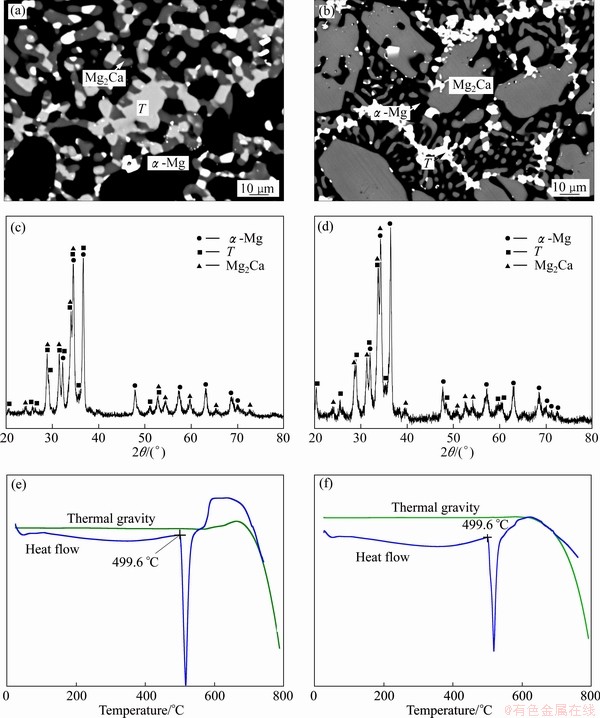
Fig. 2 BSE images (a, b), XRD patterns (c, d) and DSC curves (e, f) of sample 1 (a, c, e) and sample 3 (b, d, f) in Mg-Ca-Gd system after equilibrium treatment at 400 °C
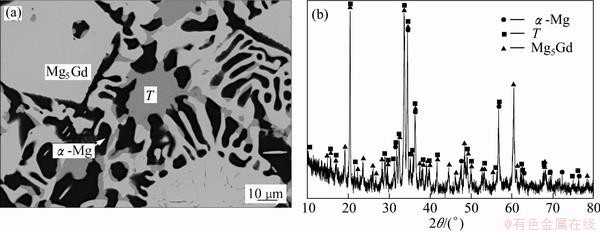
Fig. 3 BSE image (a) and XRD pattern (b) of sample 5 in Mg-Ca-Gd system after equilibrium treatment at 400 °C
Table 2 Equilibrium phase constituents in Mg-rich corner of Mg-Ca-Nd system at 400 °C
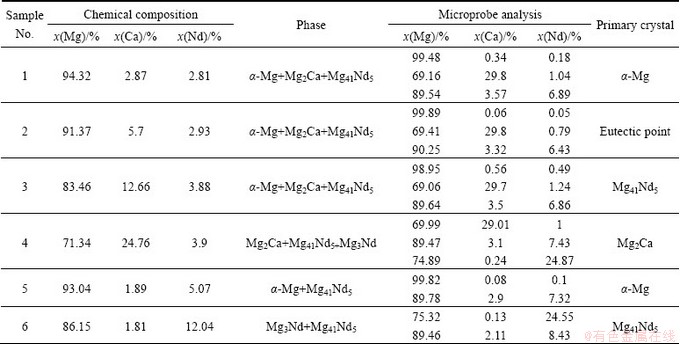
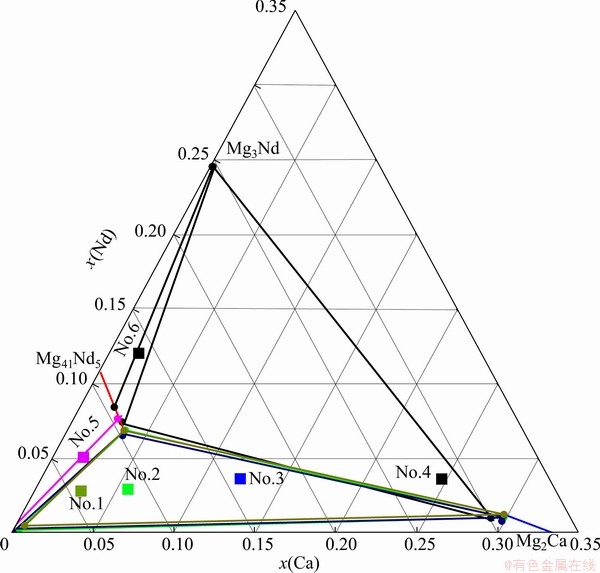
Fig. 4 Phase diagram of Mg-rich corner in Mg-Ca-Nd system at 400 °C
3.2 Phase relation in Mg-rich corner of Mg-Ca-Nd system at 400 °C
The phases and their compositions in each annealed sample obtained by XRD and EPMA in Mg-Ca-Nd system are listed in Table 2 and the phase diagram of Mg-Ca-Nd system in Mg-rich corner at 400 °C is illustrated in Fig. 4.
The three-phase fields of α-Mg+Mg2Ca+Mg41Nd5 and Mg3Nd+Mg2Ca+Mg41Nd5 exist in Mg-rich corner of Mg-Ca-Nd system at 400 °C, and the two-phase fields (α-Mg+Mg2Ca, α-Mg+Mg41Nd5 and Mg3Nd+ Mg41Nd5) are all quite broad. It can provide some possibilities for the strengthening phase design. The solubility of Nd in Mg2Ca and that of Ca in Mg41Nd5 compounds are estimated to be 1.24% and 3.57%, respectively.
It can be seen that alloys 1-3 in Mg-Ca-Nd system are in a three-phase equilibrium related to α-Mg and Mg2Ca phase. As shown in Figs. 5(a), (c) and (e), the dark phase is α-Mg and the charcoal gray phase is Mg2Ca dissolving about 1% Nd. While it is found that the light phase in Figs. 5(a), (c) and (e) has an mole ratio of Mg:Ca:Nd about 89.5:3.6:6.9 according to the EPMA results. It can be inferred that this is because Ca atoms dissolve into the crystal lattice of Mg41Nd5 and occupy the positions of some Nd atoms and cause a distortion of the lattice. In Figs. 5(b), (d) and (f), the correspondence of the diffraction peaks to the XRD patterns of Mg41Ce5 demonstrates that this phase has the same atomic site occupation as Mg41Ce5.
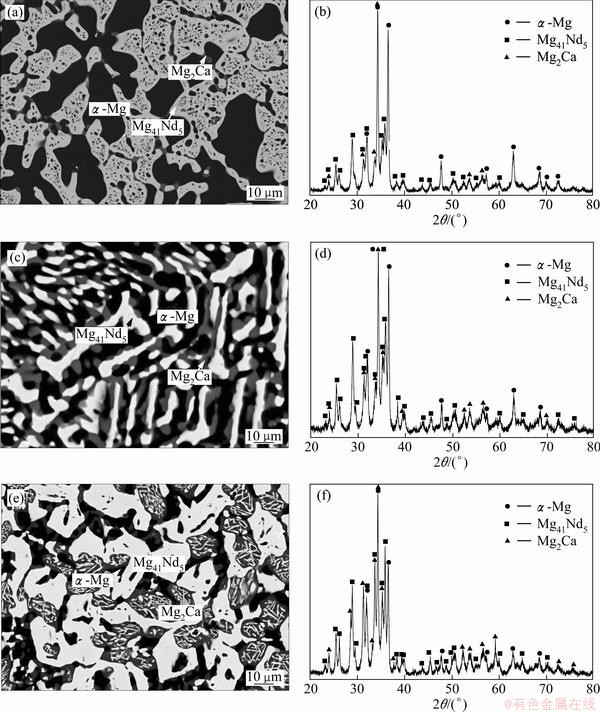
Fig. 5 BSE images (a, c, e) and XRD patterns (b, d, f) of samples 1(a,b), 2(c,d) and 3(e,f) in Mg-Ca-Nd system after equilibrium treatment at 400 °C
Similar to the Mg-Ca-Gd system, there is a eutectic reaction L→α-Mg+Mg2Ca+Mg41Nd5 in this three-phase field and the eutectic temperature is about 505.6 °C according to the DSC curve of alloy 2 given in Fig. 6. The primary crystal is α-Mg in alloy 1 (Fig. 5(a)) and Mg41Nd5 in alloy 3 (Fig. 5(c)), while alloy 2 is near the eutectic composition.
Further more, α-Mg is in two-phase equilibrium with Mg41Nd5 which dissolves 2.9% Ca in alloy 5 (Fig. 7). This proves that there is Mg41Nd5-based semi- continuous solid solution phase in this system but not a ternary compound as that in the Mg-Ca-Gd system.
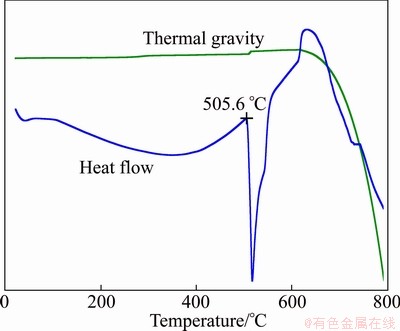
Fig. 6 DSC curve of sample 2 in Mg-Ca-Nd system after equilibrium treatment at 400 °C
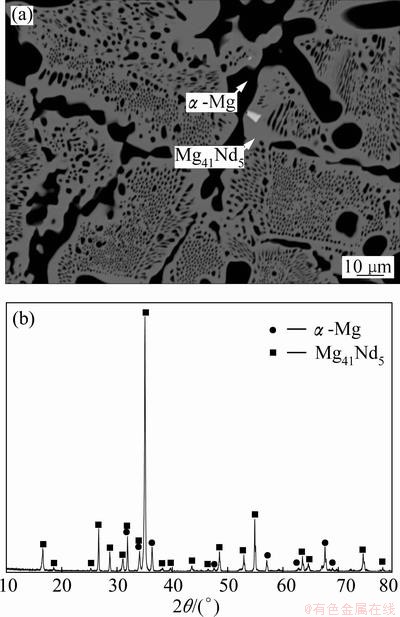
Fig. 7 BSE image (a) and XRD pattern (b) of sample 5 in Mg-Ca-Nd system after equilibrium treatment at 400 °C
4 Conclusions
1) A three-phase equilibrium involving α-Mg, Mg2Ca and Mg41RE5 phases exists both in Mg-Ca-Gd and Mg-Ca-Nd systems at 400 °C, and the Mg41RE5 compound has the same atomic site occupation as Mg41Ce5.
2) The Mg41RE5 is ternary compound Τ (89Mg- 4Ca-7Gd) in the Mg-Ca-Gd system due to the instability of the Mg41Gd5 binary compound while it is a Mg41Nd5-based solid solution in the Mg-Ca-Nd system because Mg41Nd5 is stable in the related binary system.
3) There is a ternary eutectic reaction L→α-Mg+ Mg2Ca+X(X=Τ, Mg41Nd5) at about 499.6 °C and 505.6 °C, respectively. The maximum solubilities of Gd and Nd in Mg2Ca are relatively large in both ternary systems at 400 °C.
References
[1] HSCHUMANN S H, HFRIEDRICH H H. Current and future use of magnesium in the automobile industry [J]. Materials Science Forum, 2003, 419: 51-56.
[2] MORDIKE B L, EBERT T. Magnesium properties-applications- potential [J]. Materials Science and Engineering A, 2001, 302: 37-45.
[3] LUO A, PEKGULERYUZ M O. Cast magnesium alloys for elevated temperature applications [J]. Journal of Materials Science, 1994, 29: 5259-5271.
[4] NIE J F, MUDDLE B C. Characterisation of strengthening precipitate phases in a Mg-Y-Nd alloy [J]. Acta Mater, 2000, 48(8): 1691-1703.
[5] NIE J F, OH-ISHI K, GAO X, HONO K. Solute segregation and precipitation in a creep-resistant Mg-Gd-Zn alloy [J]. Acta Mater, 2008, 56(20): 6061-6076.
[6] SHI Bin-qing, CHEN Rong-shi, KE Wei. Effect of element Gd on phase constituent and mechanical property of Mg-5Sn-1Ca alloy [J]. Transactions of Nonferrous Metals Society of China, 2010, 20(s): s341-s345.
[7] HLUOH A A, HPOWELLH B R, HBALOGHH M P. Creep and microstructure of magnesium-aluminum-calcium based alloys [J]. Metallurgical and Materials Transactions A, 2002, 33(3): 567-574.
[8] LI Y, HODGSON P D, WEN C. The effects of calcium and yttrium additions on the microstructure, mechanical properties and biocompatibility of biodegradable magnesium alloys [J]. Journal of Materials Science, 2011, 46: 365-371.
[9] YOU B S, PARK W W, CHUNG I S. The effect of calcium additions on the oxidation behavior in magnesium alloys [J]. Scripta Mater, 2000, 42: 1089-1094.
[10] VOSTRY P, STULIKOVA I, SMOLA B, RIEHEMANN W, MORDIKE B L. Structure and stability of microcrystalline Mg-Ca alloy [J]. Materials Science and Engineering A, 1991, 137: 87-92.
[11] NAYEB-HASHEMI A A, CLARK J B. Phase diagrams of binary magnesium alloys [M]. Metals Park: ASM International, 1988.
[12] CACCIAMANI G, SACCONE A, BORZONE G, DELFINO S, FERRO R. Computer coupling of thermodynamics and phase diagrams: The gadolinium-magnesium system as an example [J]. Thermochim Acta, 1992, 199: 17-24.
[13] de NEGRI S, SACCONE A, CACCIAMANI G, FERRO R. The Al-R-Mg (R=Gd, Dy, Ho) systems. Part I: Experimental investigation [J]. Intermetallics, 2003, 11: 1125-1134.
[14] CACCIAMANI G, de NEGRI S, SACCONE A, FERRO R. The Al-R-Mg (R=Gd, Dy, Ho) systems. Part II: Thermodynamic modeling of the binary and ternary systems [J]. Intermetallics, 2003, 11: 1135-1151.
[15] GUO C, DU Z, LI C. A thermodynamic description of the Gd-Mg-Y system [J]. Calphad, 2007, 31: 75-88.
[16] MENG Fan-gui, LIU Hua-shan, LIU Li-bin, JIN Zhan-peng. Thermodynamic optimization of Mg-Nd system [J]. Transactions of Nonferrous Metals Society of China, 2007, 17(1): 77-81.
[17] NIU C J, LIU M, LI C R, DU Z M, GUO C P. Thermodynamic description on the miscibility gap of α-Mg in the Mg-Zn, Mg-Nd and Mg-Zn-Nd systems [J]. Calphad, 2010, 34: 428-433.
[18] ALJARRAH M, MEDRAJ M. Thermodynamic modeling of the Mg-Ca, Mg-Sr, Ca-Sr and Mg-Ca-Sr systems using the modified quasichemical model [J]. Calphad, 2008, 32: 240-251.
Mg-Ca-RE (RE=Gd, Nd)体系富镁角400 °C相平衡
费厚军1,徐广龙1, 2,刘立斌1,3,薄 宏1,曾丽君1,陈翠萍1
1. 中南大学 材料科学与工程学院,长沙 410083;
2. IMDEA Materials Institute, Madrid 28040, Spain;
3. 中南大学 有色金属材料科学与工程教育部重点实验室,长沙 410083
摘 要:采用平衡合金分析法, 利用X射线衍射分析、扫描电镜显微结构分析、电子探针成分分析以及差示扫描量热分析,对Mg-Ca-Gd 和Mg-Ca-Nd 2个体系富镁角400 °C的相平衡关系进行研究。通过对13个合金样品的分析构建2个三元系富镁角400 °C的等温截面。研究发现,在400 °C时2个体系中均存在α-Mg,Mg41RE5 和 Mg2Ca组成的三相区,它们是由一个相似的共晶反应L→α-Mg+Mg2Ca+Mg41RE5而形成的,共晶温度分别为499.6 °C和505.6 °C。结果表明,Mg-Ca-Gd体系在400 °C时Mg5Gd中Ca的溶解度为3.68%, 3%的Gd溶解到Mg2Ca中。而Mg-Ca-Nd体系在400 °C时Mg41Nd5中Ca的溶解度为3.57%,同时1.24%的Gd溶解到Mg2Ca中。此外这2个三元体系富镁角在400 °C的还分别存在由α-Mg+Mg5Gd+Τ 和 Mg5Gd+Mg2Ca+Τ组成以及由Mg3Nd+Mg2Ca+Mg41Nd5组成的三相区。
关键词:Mg-Ca-Gd体系;Mg-Ca-Nd体系;富镁角;三相平衡;溶解度
(Edited by Xiang-qun LI)
Foundation item: Projects (50731002, 50971136) supported by the National Natural Science Foundation of China
Corresponding author: Li-bin LIU; Tel: +86-731-88877732; E-mail: HPDC@mail.csu.edu.cn
DOI: 10.1016/S1003-6326(13)62543-1