
Effect of modified starches on depression of diaspore
LI Hai-pu(李海普)1, ZHANG Sha-sha(张莎莎)1, JIANG Hao(蒋 昊)2, LI Bin(李 彬)1
1. School of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China;
2. School of Minerals Processing and Bioengineering, Central South University, Changsha 410083, China
Received 13 May 2010; accepted 4 July 2010
Abstract: Four modified starches with selected charge characteristics including cationic starch (CAS), carboxymethyl starch (CMS), amphoteric starch (AMS) and soluble starch (SS) were investigated as depressants for diaspore in reverse flotation test using cationic collector (dodecylamine). Adsorption examination, Zeta potential measurement and Fourier transform infrared (FTIR) spectroscopy were used to clarify the role of the surface charge characteristics of starches in determining the adsorption behavior and depression performance as well as the mineral-starch interaction. Results show that the positively charged starches (CAS and AMS) display higher adsorption amounts and also better depression performance compared with the non-ionic (SS) and anionic starch (CMS), benefiting from the favorable electrostatic attraction with diaspore and also electrostatic repulsion with collector. FTIR spectroscopy proves the presence of hydrogen bonds and chemical complexation between mineral and starches in an integrated manner.
Key words: starch; diaspore; reverse flotation; depressant
1 Introduction
Cationic reverse flotation desilication is a key process for the effective utilization of Chinese diasporic bauxite in terms of the improvement of aluminum/silicon ratio (A/S), by which the gangue minerals, such as kaolinite, illite and pyrophyllite, are purposely separated via floatation using cationic collectors, while the valuable component, diaspore, is depressed for enrichment. In this regard, depression of diaspore plays a significant role in promoting the separation efficiency in reverse flotation, and therefore has attracted a lot of interest from academia and industry. It is generally accepted that the depressing action occurs with the increased hydrophilicity of mineral, competitive adsorption and electrostatic repulsion between depressant and cationic collector, and strong interaction of depressant with the metallic ion of mineral[1]. Accordingly, selective depression could be obtained by introducing more hydrophilic groups, additional cationic substituents, and metal-affinity functionalities into the depressant molecules.
Among the currently available depressants, starch distinguishes itself primarily due to its high natural abundance, ready availability, renewability, low cost and environmental friendship[2-4]. Of particular to the researchers is the high number of hydroxyl groups therein which lend the polymeric product strong hydrophilicity as well as convenient modification. Natural starch and its modified derivatives have been widely employed as depressants of hematite in reverse flotation of iron ore[5-9], but only emerged as an area of academic study in the field of diasporic bauxite flotation in recent years[10-11]. In this work, a series of commercial starch products, which undergo specific chemical modification and exhibit certain characteristics that allow them to be categorized into cationic starch (CAS), anionic starch (carboxymethyl starch, CMS), amphoteric starch (AMS), and non-ionic starch (soluble starch, SS), were used as depressants for diaspore and their depression performances and features were investigated in a comparative manner for a better understanding of the interaction between mineral and polymeric depressants of the starch type.
2 Experimental
2.1 Minerals and reagents
All of the mineral samples were obtained from Xiaoyi, Shanxi Province of China. The mineral lumps were crushed, selected by hand and then ground to a diameter smaller than 0.076 mm. Soluble starch (Sinopharm Chemical Reagent Co. Ltd., Shanghai), carboxymethyl starch (Haixiang Biological Engineering Co. Ltd., Shandong), cationic starch (Jinshan Modified Starch Co. Ltd., Shandong), and amphoteric starch (Hongxing Modified Starch Co. Ltd., Jiangxi) were purchased and used as received. The starch solutions were freshly prepared by dispersing starch particles into a small amount of absolute ethanol, and then they were dissolved in hot distilled water. Dodecylamine (DDA) in analytical grade was used as the collector. The pH values of the respective suspension and solution were adjusted using 1 mol/L NaOH or 1 mol/L HCl solutions. The ionic strength of solutions was maintained at unity by suitable additions of a stock KCl solution.
2.2 Micro-flotation
The micro-flotation tests were carried out in an XFD-type laboratory flotation machine. Pure mineral particles (3 g) were placed in a plexiglass cell (40 mL) which was then filled with distilled water (30 mL). The suspension was agitated for 2 min and the pH was adjusted. After the desired amounts of depressant and collector being added, the suspension was agitated for 3 min, and then an 8 min flotation period was conducted. The suspensions and tailings were weighed separately after filtration and drying, and the recovery was calculated.
2.3 Adsorption test
0.4 g mineral sample was transferred to a 100 mL Erlenmeyer flask, and was suspended in a known concentration of starch solution. Then, the flask was placed in a mechanical shaker at 25 °C for 12 h which is long enough for a complete adsorption. At last, the mineral suspension was centrifuged for 10 min at 5 000 r/min and the supernatant was piped out for determination of starch concentration by spectrophotometric method[12]. The amount of starch absorbed on diaspore was calculated from the initial and residual concentrations of starch.
2.4 Electrokinetic measurement
Diaspore ground to a diameter smaller than 5 ?m was dispersed in a desired amount of 10-3 mol/L KCl solution and stirred for 2 min. The pH was adjusted and measured. The starch stock solution was added to this mixture, followed by stirring for 3 min. Then, the Zeta potential was determined by a Brookhaven ZetaPlus Zeta potential analyzer (USA).
2.5 FTIR spectroscopy
Fourier transform infrared (FTIR) spectroscopy was used to characterize the nature of the interaction between starch and diaspore. FTIR absorption spectra were obtained with KBr pellets using a Fourier transform infrared spectrometer Nicolet 6 700 (Thermo Fisher Scientific, USA).
3 Results and discussion
3.1 Micro-flotation test
The recoveries of diaspore by the collector DDA (6 mmol/L) in the absence and presence of the starch depressants (0.1 g/L) were depicted as a function of pH value in Fig.1(a). It is shown that diaspore is largely recovered (>90%) by DDA in the range of pH 6-10 without the addition of any depressant. In sharp contrast, the recoveries of diaspore can be noticeably depressed by starch depressants. Especially, in the case of AMS, the recovery of diaspore is highly decreased to as low as 12% at pH 9. Similarly, the depression performances of the other three starches can be established in the order of CAS > SS > CMS under the present conditions.
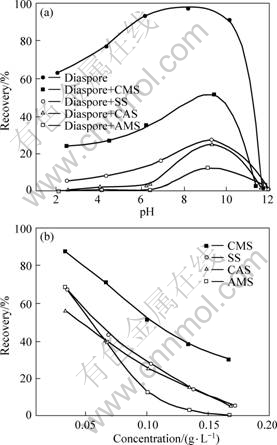
Fig.1 Recovery of diaspore by collector DDA (6 mmol/L) in the absence and presence of starches as depressant (0.1 g/L): (a) As function of pH; (b) At different concentrations of starches at pH 9
When the concentration of starch depressant was also taken into consideration to determine the appropriate dose at pH 9, it is found that the recovery of diaspore decreases almost linearly with the increase of the starch concentration in all cases (Fig.1(b)). By following this trend, AMS can even completely suppress the diaspore flotation with a low concentration of 0.17 g/L, while CAS and SS at the same concentration lead to the recoveries of diaspore not more than 5%. As for CMS, however, this concentration gives a moderate diaspore recovery (30%). Even so, the concentration of 0.10 g/L is still a suitable dose for all four starch depressants in view of the distinction in their performances.
The effect of starch depressants on the floatability of kaolinite was also investigated for a comparison. As seen from Fig.2, the recovery of kaolinite is more or less activated by the addition of starch reagents in a broad pH range, and nearly keeps a constant level with respect to the change of depressant concentration (data not shown). The activated flotation of kaolinite by the collector DDA in the presence of polymeric additive has been addressed elsewhere[13-14].
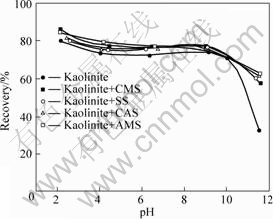
Fig.2 Recovery of kaolinite by collector DDA (6 mmol/L) in the absence and presence of starches as depressant (0.1 g/L) as function of pH
These observations that modified starches uniformly depress the diaspore and slightly activate kaolinite prove the selectivity required for the separation of diaspore and kaolinite by reverse flotation.
3.2 Adsorption test
Selective separation in reverse flotation is mainly achieved by the adsorption of the depressant onto the surface of valuable minerals. Therefore, the investigation of adsorption of starches on diaspore was carried out at different concentrations of depressant at pH 9 under the same ionic condition (10-3 mol/L KCl). As observed in Fig.3, approximately comparable adsorption amounts (ca. 2 mg/g) for different starches are obtained at the initial concentration of 20 mg/L. The subsequent increase of starch concentration results in the concomitant increase of adsorption amounts for all four depressants, among which CAS exhibits the fastest increasing speed in nearly linear growth while SS gives only a slow and reluctant growth. What’s more, it is noted that all starches except for CAS are approaching the adsorption equilibrium at a higher concentration around 0.15 g/L. For each starch, the adsorption amount and the depression performance can be reasonably well correlated; that is, the more adsorption, the more depression. However, there is no such one-to-one correspondence among the four depressants due to their difference in interaction with diaspore.

Fig.3 Adsorption of starch on diaspore as function of starch concentration (pH=9)
3.3 Zeta potential measurement
The effect of starches on the Zeta potential of diaspore is presented in Fig.4. The isoelectric point (IEP) of diaspore is located around pH 7.8 in the absence of starch, which is in good agreement with the previous report[15]. It is well known that the surface of mineral carries positive charges at pH lower than IEP and negative charges at pH higher than IEP, and generally, the adsorption of modified starches will affect the surface potential of mineral.
As shown in Fig.4, the adsorption of CMS strongly decreases the positive charges while increases the negative charges of diaspore so as to put the whole curve negatively in broad pH range, as expected for a negatively charged reagent. On the contrary, CAS causes the corresponding curve positively, resulting from the typical cationic character of starch. The IEP of diaspore is shifted to a higher pH (9.0) when being treated with AMS, which indicates that AMS is more or less positively charged. In comparison, SS keeps the IEP almost as the same and leads the zeta potential close to zero, exhibiting a nature of non-ionic polymer[16-17]. Based on these observations, the charges residing at the starch molecules could fundamentally account for the major difference in adsorption amounts of starches on diaspore at pH 9 (Fig.3), in which CAS and AMS that are positively charged result in the higher adsorption due to the extra electrostatic interaction between starch and diaspore compared with the non-ionic SS and anionic CMS. Nevertheless, the adsorption of CMS on diaspore still occurred in alkaline media, giving a hint for the existence of other types of interaction between CMS and diaspore, such as chemical complexation or hydrogen bonding, to overcome the electrostatic repulsion[18].
According to the Zeta potential measurement, it could be rationally deduced that the surface charge characteristics of starches exert a significant influence
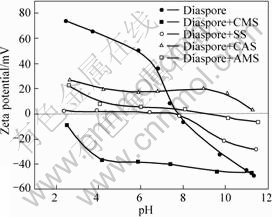
Fig.4 Zeta potential of diaspore as function of pH in the presence and absence of depressants
on their depression performances in inhibiting diaspore when working with the collector DDA. In the case of CMS, the negatively charged starch is prone to promote the adsorption of the cationic DDA collector by electrostatic attraction so as to decrease the hydrophilicity of diaspore surface and limit its depression, while CAS and AMS that are positively charged are able to exclude the adsorption of DDA to some degree by electrostatic repulsion and subsequently furnish a better depression performance, as verified by the flotation test (Fig.1(a)). The reason for the higher depression ability of AMS than CAS could be explained by the superior hydrophilicity of the former[19].
3.4 FTIR analysis
Infrared spectroscopy is a useful tool to study the interaction between mineral and reagent. In Fig.5, the FTIR spectra of diaspore samples conditioned with and without starches are presented. In the spectrum of diaspore, the three absorption bands are assigned to —OH stretching vibration at 2 117.76 and 1 985.84 cm-1, —OH bending vibration at 1 067.41 and 966.58 cm-1, and Al—O vibration at 704.27 and 579.91 cm-1. After adsorption, the bands due to —OH stretching of diaspore slightly shift in all cases, suggesting the starch-mineral interaction through their hydroxyl groups, most possibly by forming intermolecular hydrogen bonds. Likewise, the Al—O vibration is also found to be shifted to higher wavenumbers more or less, such as for
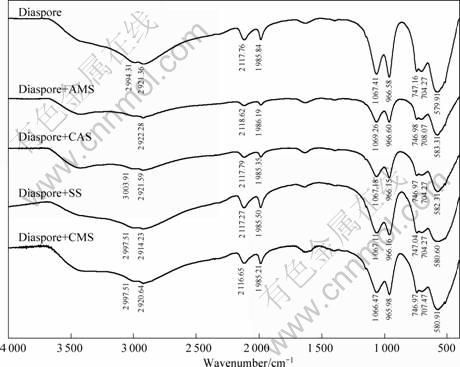
Fig.5 FTIR spectra of diaspore samples conditioned with and without starches
diaspore+CAS (704.27, 582.31 cm-1), diaspore+CMS (707.47, 580.91 cm-1), diaspore+AMS (708.07 and 583.31 cm-1), and diaspore+SS (704.27, 580.60 cm-1).
The FTIR spectra of CAS, CMS, AMS, and SS are given in Fig.6. The adsorption bands in regions of 3 500–3 300 cm-1 and 3 000–2 800 cm-1 are assignable to —OH and —CH stretching vibration, respectively. The other bands correspond well to the vibrational modes of the chemical bonds reported previously[20-26]. Due to the intense infrared absorbance of diaspore, a difference spectroscopy was employed to highlight the starch adsorption. As shown in Fig.6, the —OH stretching band of CAS (3 420.65 cm-1), CMS (3 430.92 cm-1), and AMS (3 440.66 cm-1) are upshifted to 3448.35, 3 452.45, and 3 441.11 cm-1, respectively, evidencing the involvement of —OH groups of these starches in the adsorption on diaspore[17, 27]. What’s more, in the difference spectrum of CMS-adsorbed diaspore, two adsorption bands appearing at 1 598.62 and 1 416.13 cm-1 can be assignable to the subtle shifts of the original carboxyl groups of CMS (1 604.72, 1 418.91 cm-1), which is inductive of the association of carboxyl groups with the reactive Al sites on diaspore surface. These observations are well consistent with the findings in Zeta potential measurement and, consequently, suggest the presence of starch-diaspore chemical interaction by forming surface complex between polysaccharide oxygen atoms and surface aluminum atoms of diaspore. However, the adsorption of SS on diaspore was hardly observed even if by examining the difference spectroscopy (Fig.6). The possible reason for this could be the relatively small amount of SS absorbed on diaspore (as revealed in adsorption test) which is not sufficient to show adsorption bands in FTIR spectrum[28].
4 Conclusions
1) Starch derivatives were found to be applicable and selective as depressant for diaspore in reverse flotation using the cationic collector (dodecylamine, DDA), owing to their strong depression on diaspore and slight activation on kaolinite. The performances of four modified starches for the suppression of diaspore are determined in the order of amphoteric starch (AMS) > cationic starch (CAS) > soluble starch (SS) > carboxymethyl starch (CMS).
2) The surface charge of starches is essential to their adsorption amounts and depression performances with respect to diaspore. At pH 9, positively charged starches (CAS and AMS) display higher adsorption amounts due to the extra electrostatic interaction between starch and diaspore and also exhibit better depression performances by partly excluding the adsorption of the cationic collector DDA, compared with the non-ionic SS and anionic CMS. On the other hand, a higher hydrophilicity helps AMS to achieve a better depression performance than CAS.
3) The adsorption of positively charged starches (CAS and AMS) on diaspore in acidic media and anionic

Fig. 6 FTIR spectra of starches and corresponding difference spectra
starch (CMS) on diaspore in alkaline media solidly indicates the presence of starch-mineral interaction more than electrostatic force. FTIR spectroscopy is informative to support the existence of hydrogen bonds and chemical complexation (through hydroxyl or carboxyl oxygen with aluminum sites) between mineral and starches.
References
[1] HU Yue-hua, WANG Yu-hua. Flotation chemistry of aluminum and silicate and desilication of bauxite [M]. Beijing: Science Press, 2004. (in Chinese)
[2] RATH R K, SUBRAMANIAN S, PRADEEP T. Surface chemical studies on pyrite in the presence of polysaccharide-based flotation depressants [J]. Journal of Colloid and Interface Science, 2000, 229(1): 82-91.
[3] SHOGREN R L. Flocculation of kaolin by waxy maize starch phosphates [J]. Carbohydrate Polymers, 2009, 76(4): 639-644.
[4] L?PEZ VALDIVIESO A, CELED?N CERVANTES T, SONG S, ROBLEDO CABRERA A, LASKOWSKI J S. Dextrin as a non-toxic depressant for pyrite in flotation with xanthates as collector [J]. Minerals Engineering, 2004, 17(9/10): 1001-1006.
[5] PERES A E C, CORREA M I. Depression of iron oxides with corn starches [J]. Minerals Engineering, 1996, 9(12): 1227-1234.
[6] ARAUJO A C, VIANA P R M, PERES A E C. Reagents in iron ores flotation [J]. Minerals Engineering, 2005, 18(2): 219-224.
[7] MONTES-SOTOMAYOR S, HOUOT R, KONGOLO M. Flotation of silicated gangue iron ores: Mechanism and effect of starch [J]. Minerals Engineering, 1998, 11(1): 71-76.
[8] WEISSEBORN P K, WARREN L J, DUNN J G. Selective flocculation of ultrafine iron ore. 1. Mechanism of adsorption of starch onto hematite [J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 1995, 99(1): 11-27.
[9] MA X D. Role of solvation energy in starch adsorption on oxide surfaces [J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2008, 320(1/2/3): 36-42.
[10] HU Yue-hua, LI Hai-pu, JIANG Yu-ren. Effect of hydroxamic acid starch on reverse flotation desilicate from diasporic bauxite [J]. Transactions of Nonferrous Metals Society of China, 2002, 12(5): 974-978.
[11] LI Hai-pu, HU Yue-hua, WANG Dian-zuo, XU Jing. Effect of hydroxamic acid polymers on reversed flotation of bauxite [J]. Journal of Central South University of Technology, 2004, 11(3): 291-294.
[12] DUBIOS M, GILLES K A, HAMILTON J K, REBERS P A, SMITH F. Colorimetric method for determination of sugars and related substances [J]. Analytical Chemistry, 1956, 28(3): 350-356.
[13] SUN Wei, WANG Yu-hua, LI Hai-pu, HU Yue-hua. Nature of (001) and 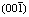 faces and flocculation flotation of kaolinite [J]. Transactions of Nonferrous Metals Society of China, 2003, 13(4): 968-971.
faces and flocculation flotation of kaolinite [J]. Transactions of Nonferrous Metals Society of China, 2003, 13(4): 968-971.
[14] HU Y H, SUN W, LI H P, XU Z H. Role of macromolecules in kaolinite flotation [J]. Minerals Engineering, 2004, 17(9/10): 1017-1022.
[15] LIU X W, HU Y H, XU Z H. Effect of chemical composition on electrokinetics of diaspore [J]. Journal of Colloid and Interface Science, 2003, 267(1): 211-216.
[16] LI Hai-pu. Structure-performance of modified polymers and the mechanism for flotation of aluminum and silicate minerals [D]. Changsha: Central South University, 2002. (in Chinese)
[17] XIA L Y, ZHONG H, LIU G Y, WANG S. Utilization of soluble starch as a depressant for the reverse flotation of diaspore from kaolinite [J]. Minerals Engineering, 2009, 22(6): 560-565.
[18] WANG J, SOMASUNDARAN P. Adsorption and conformation of carboxymethyl cellulose at solid-liquid interfaces using spectroscopic, AFM and allied techniques [J]. Journal of Colloid and Interface Science, 2005, 291(1): 75-83.
[19] WANG Dian-zuo. Molecular design of reagents for mineral and metallurgical processing [M]. Changsha: Central South University of Technology Press, 1996. (in Chinese)
[20] WANG Y B, XIE W L. Synthesis of cationic starch with a high degree of substitution in an ionic liquid [J]. Carbohydrate Polymers, 2010, 80(4): 1172-1177.
[21] WEI Y P, CHENG F, ZHENG H. Synthesis and flocculating properties of cationic starch derivatives [J]. Carbohydrate Polymers, 2008, 74(3): 673-679.
[22] CHEN Y X, LIU S Y, WANG G Y. Kinetics and adsorption behavior of carboxymethyl starch on alpha-alumina in aqueous medium [J]. Journal of Colloid and Interface Science, 2006, 303(2): 380-387.
[23] WANG Y L, GAO W Y, LI X. Carboxymethyl Chinese yam starch: Synthesis, characterization, and influence of reaction parameters [J]. Carbohydrate Research, 2009, 344(13): 1764-1769.
[24] BI Yan-li, ZHANG Hong-wei. Study on structural characteristics of phosphate amphoteric starch and its reinforcing effect on paper [J]. China Pulp & Paper, 2008, 27(12): 20-23. (in Chinese)
[25] ZHANG J P, LI A, WANG A Q. Study on superabsorbent composite. VI. Preparation, characterization and swelling behaviors of starch phosphate-graft-acrylamide/attapulgite superabsorbent composite [J]. Carbohydrate Polymers, 2006, 65(2): 150-158.
[26] PASSAUER L, BENDER H, FISCHER S. Synthesis and characterisation of starch phosphates [J/OL]. Carbohydrate Polymers, 2010: doi:10.1016/j.carbpol.2010.05.050.
[27] LI Hai-pu, HU Yue-hua, JIANG Yu-ren, WANG Dian-zuo. Interaction mechanism between modified starches and aluminum-silicate minerals [J]. The Chinese Journal of Nonferrous Metals, 2001, 11(4): 697-701. (in Chinese)
[28] PAVLOVIC S, BRANDAO P R G. Adsorption of starch, amylose, amylopectin and glucose monomer and their effect on the flotation of hematite and quartz [J]. Minerals Engineering, 2003, 16(11): 1117-1122.
(Edited by YANG Hua)
Foundation item: Projects(50804055, 50974134) supported by the National Natural Science Foundation of China; Project(09JJ3100) supported by Hunan Provincial Natural Science Foundation, China
Corresponding author: LI Hai-pu; Tel: +86-731-88830603; E-mail: lihaipu@mail.csu.edu.cn
DOI: 10.1016/S1003-6326(09)60327-7