一维碳纳米结构的有效合成及其 在锂离子电池负极材料中的应用
来源期刊:中国有色金属学报(英文版)2014年第4期
论文作者:唐晶晶 杨 娟 周向阳 陈光辉 黄 滨
文章页码:1079 - 1085
关键词:碳纳米纤维;碳纳米带;热解;KOH活化;锂离子电池
Key words:carbon nanofibers; carbon nanoribbons; pyrolysis; KOH activation; lithium ion batteries
摘 要:采用热解处理已合成的聚吡咯纳米线实现一维碳纳米纤维的有效合成。在KOH的活化作用下,原始的纤维结构发生变化,获得带状碳纳米结构。对所合成的碳纳米线及碳纳米带进行形貌及结构表征。测试这两种一维碳纳米材料应于于锂离子电池中负极材料的电化学性能。结果表明,一维碳纳米线及一维碳纳米带均表现出较优的循环性能及良好的倍率性能。碳纳米线材料在循环50次后仍保持530 mA·h/g的可逆容量。在前23次充放电循环中,碳纳米带的可逆容量均高于850 mA·h/g,充放电循环到第23次的容量保持率为86%。
Abstract: An efficient synthesis of carbon nanofibers by pyrolysis of as-prepared polypyrrole nanowires was reported. Under the subsequent KOH activation, a significant morphology variation was detected and the obtained sample took on a ribbon-like structure. The morphology and structure of the carbon nanofibers and carbon nanoribbons were characterized. When the as-prepared one-dimensional carbon nanostructures were used as anode materials in lithium ion batteries, both of them exhibited superior cyclical stability and good rate properties. After 50 cycles, the reversible capacity of carbon nanofibers electrode maintained 530 mA·h/g. Concerning carbon nanoribbons, the reversible capacity is always larger than 850 mA·h/g and the reversible capacity retention after 23 cycles is 86%.
Trans. Nonferrous Met. Soc. China 24(2014) 1079-1085
Jing-jing TANG, Juan YANG, Xiang-yang ZHOU, Guang-hui CHEN, Bin HUANG
School of Metallurgy and Environment, Central South University, Changsha 410083, China
Received 24 April 2013; accepted 18 September 2013
Abstract: An efficient synthesis of carbon nanofibers by pyrolysis of as-prepared polypyrrole nanowires was reported. Under the subsequent KOH activation, a significant morphology variation was detected and the obtained sample took on a ribbon-like structure. The morphology and structure of the carbon nanofibers and carbon nanoribbons were characterized. When the as-prepared one-dimensional carbon nanostructures were used as anode materials in lithium ion batteries, both of them exhibited superior cyclical stability and good rate properties. After 50 cycles, the reversible capacity of carbon nanofibers electrode maintained 530 mA·h/g. Concerning carbon nanoribbons, the reversible capacity is always larger than 850 mA·h/g and the reversible capacity retention after 23 cycles is 86%.
Key words: carbon nanofibers; carbon nanoribbons; pyrolysis; KOH activation; lithium ion batteries
1 Introduction
Lithium ion batteries have become the dominant power source since the first commercialization in 1991 by SONY [1]. Because of their high energy densities, lithium ion batteries have won wide application in mobile phones, portable electronic devices, large-scale energy storage and so on [2,3]. Up to now, most of the commercial anodes of lithium ion batteries are graphitic carbons, which have a limited theory capacity of 372 mA·h/g [4].
Many efforts have been taken to find an alternative negative electrode material with a higher capacity than commercial graphite to facilitate the fabrication of high power lithium ion batteries [5-7]. Among them, Li alloys are of great interest because of the high theoretical electrochemical capacity for alloying with Li (Si 4200 mA·h/g, Sn 994 mA·h/g) [8-11]. However, one critical issue of employing these Li alloys is the large volume changes accompanied with the incorporation of Li. Consequently, the large volume changes will cause the pulverization of electrode materials, resulting in a poor cycling behavior. Concerning amorphous carbon materials, they maintain a considerable structural stability compared with graphite. Meanwhile, amorphous carbon materials hold significant amount of lithium storage sites, which is responsible for their high lithium storage capacity [12,13]. Besides, studies on amorphous carbon materials indicate that the huge specific surface area and short ions transport route of carbon nanotubes, carbon nanofibers, and carbon nanospheres and so on, are ascribed to the excellent rate capacity [14-16]. Thus, nanostructured carbon materials have attracted increased attention as anode materials for high energy and high power applications. Carbon nanofibers (CNFs) and carbon nanoribbons (CNRs) are two of typical one-dimensional carbon nanostructures [17]. Recently, many approaches have been taken to synthesize CNFs and CNRs, such as template synthetic method [18,19], electrospinning technique [20,21] and chemical vapor deposition [22].
Herein, CNFs and CNRs were facile synthesized by the pyrolysis of polypyrrole nanowires (PNWs) and the subsequent KOH activation. The preparation of PPy products was accomplished via in situ chemical oxidation polymerization. In contrast with other syntheses such as hard template synthesis and electrochemical polymerization, chemical oxidation polymerization is simple, fast and cheap [23,24]. Therefore, CNFs and CNRs products are readily achievable based on the formation of fibrous PPy products. Besides, experimental results demonstrated that both of the as-prepared CNFs and CNRs satisfied electrochemical property.
2 Experimental
2.1 Synthesis of CNFs
The CNFs sample was prepared via decomposition of as-prepared PPy nanowires, which were synthesized via chemical oxidative polymerization of pyrrole (Py) in the presence of cetyltrimethylammonium bromide (CTAB). In a typical procedure, 0.03 mol CTAB was dissolved in 3.3 mol/L HCl (300 mL) aqueous solution at 5 °C. Under vigorous stirring, 3 mL Py was added to the above surfactant aqueous solution to form a homogeneous white viscous solution. Then a solution of ammonium persulfate (APS, 0.01 mol/L) in aqueous 3.3 mol/L HCl (300 mL) was poured into the above white solution all at once under stirring. The solution was allowed to react at 5 °C for a further 4 h. The solid black PPy samples were collected by filtering and washing with absolute ethanol and deionized water before drying at room temperature. The apparatus used for the preparation of CNFs consisted of a horizontal reactor tube with a conventional horizontal furnace. The PPy powder after manual grinding was placed in an alumina boat at the center of the reactor tube. Then the furnace was heated up to 900 °C with a heating rate of 5 °C/min and kept for 2 h under a nitrogen atmosphere to obtain CNFs products.
2.2 Synthesis of CNRs
For KOH activation, the as-prepared CNFs with addition of KOH were placed in a Ni-boat in a vertical type furnace. The mass ratio of CNFs and KOH was 1:5. Activation conditions were 800 °C for 1 h under a nitrogen atmosphere. The activated powder was immerged in 2 mol/L HCl solution to neutralize the excess KOH, and then washed with abundant deionized water. CNRs powder was obtained after being dried at 100 °C.
2.3 Materials characterization
The morphology and structure of as-prepared samples were evaluated by scanning electron microscopy (SEM, NOVA NANO SEM230, Japan) and transmission electron microscopy (TEM, JEM-2100F, Japan). Attenuated total reflection Fourier transform infrared spectra were collected from a FT-IR spectrometer (Nicolet 6700).
2.4 Electrochemical characterization
Electrochemical experiments were performed using 2025 coin-type half cells. To prepare CNFs and CNRs electrodes, a mixture of active materials, poly (vinylidene fluoride) and acetylene carbon black with a mass ratio of 8:1:1 was dissolved in N-methyl- 2-pyrrolidone to produce homogeneous slurry with mortar and pestle. Then the obtained slurries were pasted onto pure Cu foils and dried at 120 °C. The electrolyte was 1 mol/L LiPF6 in ethylene carbonate/dimethyl carbonate (1:1). Pure lithium metal foil was used as the counter electrode. Coin-type cells were assembled in a argon-filled glovebox. Cyclic voltammograms (CV) experiments were performed in the potential range of 0.01-3.2 V versus Li/Li+ at a scan rate of 0.5 mV/s. Galvanostatic charging/discharging was carried out on NEWARE-BTS-5V10MA in the same potential range.
3 Result and discussion
3.1 Characterization of samples
Representative SEM and TEM images of PNWs, CNFs and CNRs synthesized by the above procedures are illustrated in Fig. 1. It is clear from Figs. 1(a) and (d) that in the system of Py/HCl/CTAB/APS, fibrous PPy generates and the diameter of CNFs ranges from 20 nm to 90 nm. It is difficult to detect individual PNW due to the cross-linked structure of nanowires. It is worth mentioning that there are no obvious structure changes after pyrolyzation process as shown in Figs. 1(b) and (e). The fibrous texture was preserved and the edges of CNFs took on “beads on a string” morphology as displayed in Fig. 1(e). However, after the KOH activation, obvious structural changes took place and ribbon-like products were obtained (Figs. 1(c) and (f)). The non-uniform and crimped edges were curved along the full length of CNRs.
According to the previous reports [25,26] and the experimental results, it is possible to speculate a formation mechanism of PNWs, CNFs and CNRs as exhibited in Fig. 2. With a small quantity of APS in the mixture, Py monomer was oxidatively polymerized by the persulfate anions in the lamellar (CTA)2S2O8 mesophase, which caused the generation of PPy sheets. However, with excess APS, PPy sheets tended to curve into wire-like morphology, and finally polymerized into PNWs [27]. The subsequent pyrolyzation process realized the carbonization of PNWs and the transformation into CNFs products, which maintained the original fiber shape. Chemical activation with alkali compounds is a well-known method to activate carbon materials with a high surface area. Due to the intercalation of K-ions into CNFs, which is ascribed to the curving of PPy sheets, the CNFs are prior to be opened along the axial direction to take on a ribbon-like morphology.

Fig. 1 SEM (a, b, c) and TEM (d, e, f) images of PNWs (a, d), CNFs (b, e), and CNRs (c, f)

Fig. 2 Schematic depiction of synthesis of PNWs, CNFs and CNRs
The surface functional groups of PNWs, CNFs and CNRs were analyzed by FT-IR spectrometer as shown in Fig. 3. Two very weak peaks around 2923 cm-1 and 2855 cm-1 were attributed to the stretching vibration mode of methylene, which indicated the complete eliminating of surfactants from the products. The intense peak at 1630 cm-1 confirmed the existence of C=C groups. The characteristic peaks of PNWs at about 1545 cm-1 and 1455 cm-1 are due to the fundamental stretching vibration of Py rings. Bands at 1306 cm-1 and 1035 cm-1 are assigned to the C—N stretching vibration and N—H deformation vibrations, respectively. The peaks near 1175 cm-1 and those between 680-515 cm-1 reflect the doping state of PNWs [28-30]. It is clear that after the pyrolysis of PNWs, the obtained CNFs and CNRs are much purer.
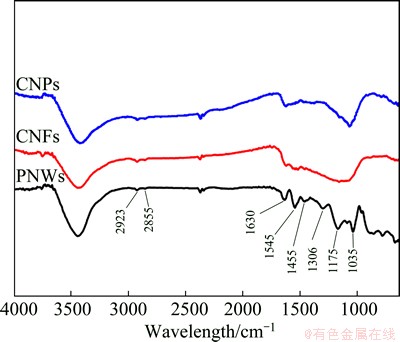
Fig. 3 FT-IR spectra of PNWs, CNFs and CNRs
3.2 Electrochemical performance
The electrochemical activities of one-dimensional carbon samples were evaluated using CV. Figures 4(a) and (b) present the CV profiles of CNFs and CNRs electrodes, respectively. The CV curves of CNFs and CNRs exhibit three apparent peaks at 0.8-1.0, 0.3-0.5 and 0.01 V in the first reduction process. The first peak can be attributed to the irreversible Li-insertion. The formation of solid electrolyte interphase (SEI) film and the decomposition of electrolyte are responsible to the second peak. The 0.01 V peak is ascribed to the reversible insertion of Li-insertion. Several broad peaks in the oxidation processes are attributed to Li-extraction from electrodes. For both CNFs and CNRs electrodes, the shape of the first cycling curve differs from that of the subsequent curves and no significant shift was observed after the 3rd cycle, which implied the stable SEI films and the good cycling performance of the obtained one-dimensional carbon materials.

Fig. 4 CV profiles of CNFs (a) and CNRs (b) electrodes
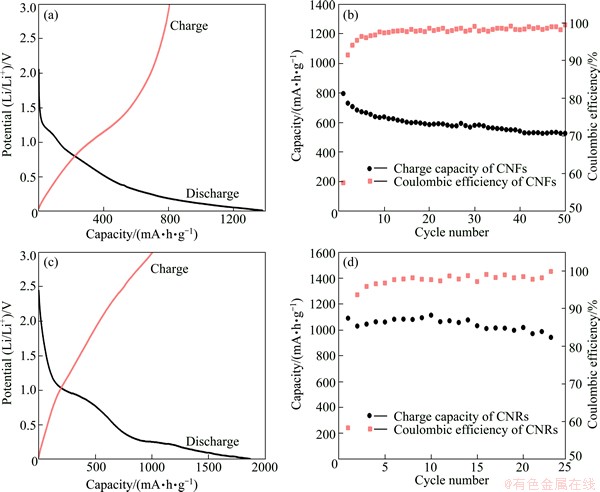
Fig. 5 Initial voltage profiles of CNFs (a) and CNRs (c) electrodes and cyclability of CNFs (b) and CNRs (d) electrodes tested at current density of 0.15C
Figure 5 shows the cycling performance of CNFs and CNRs electrodes tested by galvanostatic discharge-charge experiments at 0.15C (1C=372 mA/g). Figures 5(a) and (c) display the initial voltage profiles of CNFs and CNRs electrodes, showing the features of amorphous carbon materials. There are two sloping voltage ranges (3.2-1.2 V and 1.2-0.01 V) that can be detected, which are attributed to the decomposition of electrolyte and the formation of the SEI film, the insertion of Li-ions into the electrodes, respectively. At the first cycle, CNFs and CNRs electrodes show large Li-ion storages as high as 1382 mA·h/g and 1868 mA·h/g, respectively. However, the initial irreversible capacities of 808 mA·h/g and 1089 mA·h/g can also be seen at the first cycle as shown in Figs. 5(a) and (c), respectively, leading to relatively low initial coulombic efficiency of 58.5% and 58.3% for CNFs and CNRs, respectively. The irreversible capacity loss during the initial cycle is ascribed to the decomposition of electrolyte and the consumption of Li-ions to form SEI. Besides, during the initial discharging process of CNRs electrode, there are two sloping voltage ranges (3-0.9 V and 0.9-0.01 V) which is obviously different from that of CNFs electrode. The first slope is attributed to the decomposition of the electrolyte and the formation of the SEI film, while the other corresponds to the insertion of lithium ions into the porous of CNRs generated by KOH activation [31]. Figure 5(b) shows the cycling performance of CNFs electrodes. It is clear seen from Fig. 5(b) that at the second and the sequential cycles, the coulombic efficiency is above 90% and increases to nearly 100% with cycling. Besides, after 50 cycles, the reversible capacity of CNFs electrode maintains 530 mA·h/g, which indicates a capacity retention of 65.6% from the initial cycle. Concerning CNRs anodes, the reversible capacity is always larger than 850 mA·h/g and reversible capacity retention after 23 cycles is 86.4% (Fig. 5(d)). The better electrochemical performance of CNRs can be assigned to the activation effect of KOH, which is beneficial to generating a large surface area and a lot of active sites to accommodate more Li-ions. These results demonstrate that both CNFs and CNRs anodes deliver good cycle stability and large reversible capacity. Although the specific capacity of CNFs or CNRs is higher than the theoretical capacity of graphite (372 mA·h/g), the specific capacity is still fading with cycling, which is ascribed to the amorphous nature of hard carbon materials [32-34].
The rate capability behaviors of CNFs and CNRs anodes at higher rates are presented in Fig. 6. When the C rate increases from 0.15C to 1.2C to 3C, both CNFs and CNRs electrodes exhibit the decreasing capacity retention. However, the reversible capacities of about 625 and 998 mA·h/g are detected for CNFs and CNRs electrodes after the rate returned to 0.15C, respectively. The excellent rate capability is ascribed to good electron conductivity and the unique one-dimensional structure where well-defined one-dimensional electronic conducting paths facilitate efficient electronic diffusion.
4 Conclusions
1) The fiber-like and ribbon-like carbon nanostructures were efficiently synthesized by pyrolyzation of easily prepared PNWs products and the subsequent activation of KOH. The structure, morphology and electrochemical properties of obtained samples were investigated by SEM, TEM, FI-IR and electrochemical measuremments.
2) The SEM and TEM results show that there are no obvious structure changes before and after the pyrolyzation process. The fibrous texture is preserved after the first heat treatment. However, after the KOH activation process, profound structural changes take place and ribbon-like products are created.
3) The FT-IR spectra reflect the N-doping state of PNWs, CNFs and CNRs.
4) When evaluating the electrochemical properties, both CNFs and CNRs electrodes satisfied capacity retention, stability and excellent rate performance.

Fig. 6 Rate capabilities of CNFs (a) and CNRs (b) electrodes
Acknowledgements
The authors would like to thank Mr. Hong-zhuan LIU for technical supports with experiments.
References
[1] BROUSSELY M, BIENSAN P, SIMON B. Lithium insertion into host materials: The key to success for Li ion batteries [J]. Electrochimica Acta, 1999, 45(1): 3-22.
[2] LI Xiu-wan, LI Dan, QIAO Li, WANG Xing-hui, SUN Xiao-lei, WANG Peng, HE De-yan. Interconnected porous MnO nanoflakes for high-performance lithium ion battery anodes [J]. Journal of Materials Chemistry, 2012, 22: 9189-9194.
[3] XIANG Xiao-xia, HUANG Zheng-zheng, LIU En-hui, SHEN Hai-jie, TIAN Ying-ying, XIE Hui, WU Yu-hu, WU Zhi-lian. Lithium storage performance of carbon nanotubes prepared from polyaniline for lithium-ion batteries [J]. Electrochimica Acta, 2011, 56(25): 9350-9356.
[4] WANG Da-wei, LI Feng, LIU Min, LU Gao-qing, CHENG Hui-ming. 3D aperiodic hierarchical porous graphitic carbon material for high-rate electrochemical capacitive energy storage [J]. Angewandte Chemie International Edition, 2007, 120(2): 379-382.
[5] WANG Chang-bin, YIN Long-wei, XIANG Dong, QI Yong-xin. Uniform carbon layer coated Mn3O4 nanorod anodes with improved reversible capacity and cyclic stability for lithium ion batteries [J]. ACS Applied Materials Interfaces, 2012, 4(3): 1636-1642.
[6] ZHOU Liang, ZHAO Dong-yuan, LOU Xiong-wen. Double-shelled CoMn2O4 hollow microcubes as high-capacity anodes for lithium-ion batteries [J]. Advanced Materials, 2012, 24(6): 745-748.
[7] LI X F, DHANABALAN A, BECHTOLD K, WANG C L. Binder-free porous core-shell structured Ni/NiO configuration for application of high performance lithium ion batteries [J]. Electrochemistry Communication, 2010, 12(9): 1222-1225.
[8] SONG T, LEE D H, KWON M S, CHOI J M, HAN H, DOO S G, CHANG H, PARK W I, SIQMUND W, KIM H, PAIK U. Silicon nanowires with a carbon nanofibers branch as lithium-ion anode material [J]. Journal of Materials Chemistry, 2011, 21: 12619-12621.
[9] ZHOU Xiang-yang, TANG Jing-jing, YANG Juan, ZOU You-lan, WANG Song-can, XIE Jing, MA Lu-lu. Effect of polypyrrole on improving electrochemical performance of silicon based anode materials [J]. Electrochimica Acta, 2012, 70: 296-303.
[10] GUO Hua-jun, LI Xin-hai, WANG Zhi-xing, PENG Wen-jie, GUO Yong-xing. Si-doped composite carbon as anode of lithium ion batteries [J]. Transactions of Nonferrous Metals Society of China, 2003, 13(5): 1062-1065.
[11] LAI Jun, GUO Hua-jun, LI Xiang-qun, WANG Zhi-xing, LI Xin-hai, ZHANG Xiao-ping, HUANG Si-lin, LAN Gei. Silicon/flake graphite/carbon anode materials prepared with different dispersants by spray-drying method for lithium ion batteries [J]. Transactions of Nonferrous Metals Society of China, 2013, 23(5): 1413-1420.
[12] COURTEL F M, NIKETIC S, DUGUAY D, LEBDEH Y A, DAVIDSON I J. Water-soluble binders for MCMB carbon anodes for lithium-ion batteries [J]. Journal of Power Sources, 2011, 196(4): 2128-2134.
[13] QIE Long, CHEN Wei-min, WANG Zhao-hui, SHAO Qing-guo, LI Xiang, YUAN Li-xia, HU Xian-luo, ZHANG Wu-xing, HUANG Yun-hui. Nitrogen-doped porous carbon nanofibers webs as anodes for lithium ion batteries with a superhigh capacity and rate capability [J]. Advanced Materials, 2012, 24(15): 2047-2050.
[14] LANDI B J, GANTER M J, CRESS C D, DILEO R A, RAFFAELLE R P. Carbon nanotubes for lithium ion batteries [J]. Energy Environmental Science, 2009, 2: 638-654.
[15] KIM C, YANG K S, KOJIMA M, YOSHIDA K, KIM Y J, KIM Y A, ENDO M. Fabrication of electrospinning-derived carbon nanofibers webs for the anode material of lithium-ion secondary batteries [J]. Advanced Functional Materials, 2006, 16(18): 2393-2397.
[16] WANG Y, SU F B, WOOD C D, LEE J Y, ZHAO X S. Preparation and characterization of carbon nanospheres as anode materials in lithium-ion secondary batteries [J]. Industrial Engineering Chemistry Research, 2008, 47(7): 2294-2300.
[17] CHEN Ge, WANG Zhen-yao, XIA Ding-guo. One-pot synthesis of carbon nanotube@SnO2-Au coaxial nanocable for lithium-ion batteries with high rate capability [J]. Chemistry of Materials, 2008, 20(22): 6951-6956.
[18] WANG K X, BIRJUKOVS P, ERTS D, PHELAN R, MORRIS M A, ZHOU H S, HOLMES J D. Synthesis and characterization of ordered arrays of mesoporous [J]. Journal of Materials Chemistry, 2009, 19: 1331-1338.
[19] LIANG Hai-wei, GUAN Qing-fang, CHEN Li-feng, ZHU Zhu, ZHANG Wen-jun, YU Shu-hong. Macroscopic-scale template synthesis of robust carbonaceous nanofibers hydrogels and aerogels and their applications [J]. Angewandte Chemie International Edition, 2012, 51(21): 5101-5105.
[20] LAI C L, GUO Q H, WU X F, RENEKER D H, HOU H Q. Growth of carbon nanostructures on carbonized electrospun nanofibers with palladium nanoparticles [J]. Nanotechnology, 2008, 19: 195303-195309.
[21] WANG Ming-xi, HUANG Zheng-hong, KANG Fei-yu, LIANG Kai-ming. Porous carbon nanofibers with narrow pore size distribution from electrospun phenolic resins [J]. Materials Letters, 2011, 65(12): 1875-1877.
[22] SUBRAMANIAN V, ZHU H W, WEI B Q. High rate reversibility anode materials of lithium batteries from vapor-grown carbon nanofibers [J]. The Journal of Physical Chemistry B, 2006, 110(14): 7178-7183.
[23] CHARLES R M. Nanomaterials: A membrane-based synthetic approach [J]. Science, 1994, 266(5193): 1961-1966.
[24] SHI Wei, GE Dong-tao, WANG Ji-xiao, JIANG Zhi-zhong, REN Lei, ZHANG Qi-qing. Heparin-controlled growth of polypyrrole nanowires [J]. Macromolecular Rapid Communications, 2006, 27(12): 926-930.
[25] ZHANG Xue-tong, ZHANG Jin, LIU Zhong-fan, ROBINSON Colin. Inorganic/organic mesostructure directed synthesis of wire/ ribbon-like polypyrrole nanostructures [J]. Chemical Communications, 2004: 1852-1853.
[26] WU A M, KOLLA H, MANOHAR S K. Chemical Synthesis of highly conducting polypyrrole nanofiber film [J]. Macromolecules, 2005, 38: 7873-7875.
[27] LI Ya-dong, LI Xiao-lin, DENG Zhao-xiang, ZHOU Bei-chuan, FAN Shou-shan, WANG Jun-wei, SUN Xiao-ming. From surfactant- inorganic mesostructures to tungsten nanowires [J]. Angewandte Chemie International Edition, 2002, 114(2): 343-345.
[28] SERRANO V G, VILLEGAS J P, FLORINDO A P, VALLE C D, CALAHORRO C V. FT-IR study of rockrose and of char and activated carobn [J]. Journal of Analytical and Applied Pyrolysis, 1996, 36(1): 71-80.
[29] CHO G, FUNG B M, GLATZHOFER D T, LEE J S, SHUL Y G. Preparation and characterization of polypyrrole-coated nanosized novel ceramics [J]. Langmuir, 2001, 17(2): 456-461.
[30] ZHANG Xue-tong, ZHANG Jin, SONG Wen-hui, LIU Zhong-fan. Controllable synthesis of conducting polypyrrole nanostructures [J]. The Journal of Physical Chemistry B, 2006, 110(3): 1158-1165.
[31] JI Li-wen, ZHANG Xiang-wu. Fabrication of porous carbon nanofibers and their application as anode materials for rechargeable lithium-ion batteries [J]. Nanotechnology, 2009, 20(15): 155705.
[32] FLANDROIS S, SIMON B. Carbon materials for lithium-ion rechargeable batteries [J]. Carbon, 1999, 37(2): 165-180.
[33] KIM C, YANG K S, KOJIMA M, YOSHIDA K, KIM Y J, KIM Y A, ENDO M. Fabrication of electrospinning-derived carbon nanofibers webs for the anode material of lithium ion secondary batteries [J]. Advanced Functional Materials, 2006, 16(18): 2393-2397.
[34] BUIEL E, DAHN J R. Li-insertion in hard carbon anode materials for Li-ion batteries [J]. Electrochimica acta, 2013, 45(1-2): 121-130.
唐晶晶,杨 娟,周向阳,陈光辉,黄 滨
中南大学 冶金与环境学院,长沙 410083
摘 要:采用热解处理已合成的聚吡咯纳米线实现一维碳纳米纤维的有效合成。在KOH的活化作用下,原始的纤维结构发生变化,获得带状碳纳米结构。对所合成的碳纳米线及碳纳米带进行形貌及结构表征。测试这两种一维碳纳米材料应于于锂离子电池中负极材料的电化学性能。结果表明,一维碳纳米线及一维碳纳米带均表现出较优的循环性能及良好的倍率性能。碳纳米线材料在循环50次后仍保持530 mA·h/g的可逆容量。在前23次充放电循环中,碳纳米带的可逆容量均高于850 mA·h/g,充放电循环到第23次的容量保持率为86%。
关键词:碳纳米纤维;碳纳米带;热解;KOH活化;锂离子电池
(Edited by Hua YANG)
Foundation item: Projects (51204209, 51274240) supported by the National Natural Science Foundation of China
Corresponding author: Xiang-yang ZHOU; Tel: +86-731-88836329; Fax: +86-731-88710171; E-mail: hncsyjy308@163.com
DOI: 10.1016/S1003-6326(14)63165-4

