
Adsorption characteristics of zinc ions on sodium dodecyl sulfate in process of micellar-enhanced ultrafiltration
HUANG Jin-hui(黄瑾辉), ZENG Guang-ming(曾光明), QU Yun-huan(曲云欢), ZHANG Zhen(张 振)
College of Environmental Science and Engineering, Hunan University, Changsha 410082, China
Received 13 October 2006; accepted 11 April 2007
Abstract: To separate zinc ions from aqueous solution efficiently, micellar-enhanced ultrafiltration(MEUF) of hollow ultrafiltration membrane was used with sodium dodecyl sulfate(SDS) as surfactant.The formation of micellar and the adsorption mechanism were investigated, including the influence of the ratio of SDS to zinc ions on the micelle quantity, the micelle ratio, the gross adsorptive capacity, the rejection of zinc ions and the adsorption isotherm law. The results show that the rejection rate of zinc ions reaches 97% and the adsorption of zinc ions on SDS conforms to the Langmuir adsorption isotherm and the adsorption is a chemical adsorption process.
Key words: micellar-enhanced ultrafiltration; zinc ion; surfactant; micelles
1 Introduction
Micellar-enhanced ultrafiltration(MEUF) was originated in 1968 when MICHAELS[1] proposed to use polymer or surfactant modified ultrafiltration. In 1979, LEUNG[2] first removed trace metal ion by using MEUF, and since then the technology has been studied. The method was easy to operate, what’s more, the experiment indicated that the rejection rates of Zn2+, Pb2+, Ni2+, Cu2+ and Ca2+ were higher than 99%, and the metal ions were also easily recovered from retentive solution through MEUF[3]. But restricted by the characteristic of the material of membrane and the frame of membrane module and due to the expensive cost, MEUF was still at the experimental stage.
Since 1980’s, with the fast development of membrane science, the kinds of membrane materials have been abundant and the resistance to contamination of membrane has become better and the cost has been gradually reduced. Meanwhile the contamination of toxic heavy metal ions grew seriously all over the world. The traditional techniques for the removal of heavy metal ions from aqueous effluents are incapable of reducing concentration to the levels required by law[4]. MEUF was considered as the most efficient material technique in removing heavy metal ions from aqueous streams, which demonstrates a huge prospect. The advantages of this method are the high removal efficiency, low energy consumption and easy operation in the whole process. In recent studies, almost all the metal ions can be separated via MEUF method, including Cd2+[5], Co2+[6], Ni2+[7], Cs+, Sr2+, Cr3+, Mn2+ [8], Pb2+[9], 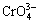 [10-11], Al3+[12], Zn2+[13-14], Cu2+[15-16], Cr3+[17],
[10-11], Al3+[12], Zn2+[13-14], Cu2+[15-16], Cr3+[17], 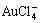 [18] and
[18] and 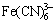 [19].
[19].
At present micellar-enhanced ultrafiltration is still at the laboratory stage. Researches on the application of MEUF are focused on surfactant species, surfactant concentration, trans-membrane pressure, operating time, pH and electrolyte concentration. Formation of surfactant micelle and mechanisms of micelle and metal ions in MEUF are ignored.
Zinc ions were separated from aqueous solution via micellar-enhanced ultrafiltration by using sodium dodecyl sulfate as surfactant in this research. Gross of the surfactant, amount of the surfactant micelle, ratio of S to M, ratio of S′ to M′, micelle ratio of S′ to S, adsorptive gross capacity, adsorptive capacity were used to describe the relationship among surfactant dosage, metal ions initial concentration, surfactant micelle formation process, and adsorption process. The adsorption isotherm rule was investigated and the adsorption type was detected by XPS. Mechanisms of MEUF were analyzed by surfactant micelle formation, adsorption rules, as well as microcosmic detection.
2 Experimental 2.1 Scheme
Firstly, the concentration of zinc ions was kept constant at 50 mg/L and the concentration of SDS was kept at its critical micelle concentration (CMC, 7.48 mmol/L). After mixing and stirring and setting, the solution passed through the hollow core fiber ultrafiltration membrane, then the permeate and retentive came into the feed permeate and retentive tank respectively. The experimental results were depicted at a constant operating pressure of 0.07 MPa. The operating time was 60 min.
2.2 Materials
The SDS used in this research is produced by Tianjin Kermel Chemical Reagents Development Center, China. Its molecular formula is C12H25NaSO4 and the relative molecular mass is 288.38 and the purity is 99%. Zinc ions were confected by zinc nitrate. Zinc nitrate is produced by Shanghai Tinxin Chemical Reagent Plant, China. Its molecular formula is Zn(NO3)2·6H2O and the relative molecular mass is 297.49 and the purity is 99%.
2.3 Procedure
SDS was added into zinc ions aqueous solution. After being fully mixed, the solution was fed into membrane module for linear continuous ultrafiltration by wriggle pump. The procedure is shown in Fig.1.
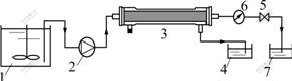
Fig.1 Schematic diagram of micellar-enhanced ultrafiltration process: 1 Feed solution; 2 Wriggle pump; 3 Ultrafiltration membrane; 4 Permeate tank; 5 Pressure control valve; 6 Manometer; 7 Retentive tank
2.4 Membrane module
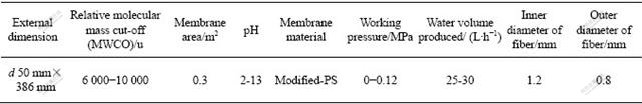
The hollow core fiber ultrafiltration membrane was produced by Tianjin Motianmo Co. (China). The characteristic of membrane is listed in Table 1.
Table 1 Membrane characteristics
2.5 Analysis
The concentration of SDS was determined by the methylene blue spectrophotometric method with Daojin UV-2550(P/N206-55501-93) spectrophotometer. The concentration of zinc ions was analyzed by atomic absorption spectrometry. The X-ray photoelectric spectra was measured by multi-function electron spectroscopy (Kratos XSAM800).
2.6 Performance parameter
1) Rejection R
To evaluate the efficiency of ultrafiltration in removing the metal ions from solution, the rejection rate R is expressed as
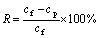 (1)
(1)
where cf is the concentration of the metal ions in the feeding solution (mg/L); cp is the concentration of the metal ions in the permeate (mg/L).
2) Gross of surfactant S
Gross of the surfactant added into solution in per unit time can be expressed as
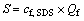 (2)
(2)
where cf, SDS is the concentration of surfactant SDS in the feed; Qf is the flux of feed.
3) Amount of surfactant micelle S′
The amount of the surfactant micelle that is formed in per unit time can be expressed as
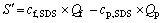 (3)
(3)
where cp, SDS is the concentration of surfactant SDS in the permeate; Qp is the flux of permeate.
4) Ratio of S/M
The molar ratio of the surfactant to the initial metal ions shows the added amount of surfactant in special solution and it can be written as
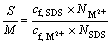 (4)
(4)
where 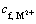 is the concentration of the heavy metal ions in the feed;
is the concentration of the heavy metal ions in the feed; 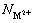 is the molar mass of heavy metal ions; NSDS is the molar mass of SDS.
is the molar mass of heavy metal ions; NSDS is the molar mass of SDS.
5) Ratio of S′/M′
The molar ratio of the surfactant micelle to the heavy metal ions adsorbed can demonstrate the adsorption of certain micelle to heavy metal ions and it can be expressed as
 (5)
(5)
where 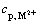 is the concentration of the heavy metal ions in the permeate.
is the concentration of the heavy metal ions in the permeate.
6) Micelle ratio S′/S
The ratio of surfactant micelle to the added amount of surfactant can be written as
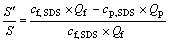 (6)
(6)
7) Adsorptive gross capacity M
Adsorptive gross capacity means the amount of heavy metal ions adsorbed of surfactant in per unit time. The reason may be that the heavy metal ions and micelle are completely rejected by ultrafiltration membrane and it can be expressed as
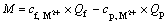 (7)
(7)
8) Adsorptive capacity Γ
The amount of heavy metal ions adsorbed per unit mass of surfactant can be expressed as
 (8)
(8)
3 Results and discussion 3.1 Effect of ratio of SDS to Zn2+ on adsorption in MEUF 3.1.1 Effect of S/M ratio on Zn2+ Rejection
The change of rejection of Zn2+ with S/M ratio is shown in Fig.2 at the initial Zn2+ concentration of 50 mg/L. The rejection of Zn2+ increases with the ratio of S/M increasing. When the S/M ratio is less than or equal to 5.8, Zn2+ rejection increases by 13.8% with doubled S/M ratio. As the S/M ratio is higher than 5.8, the rejection of Zn2+ increases flatly. Moreover, when S/M ratio is higher than 24.4, the rejection of Zn2+ reaches 97%.
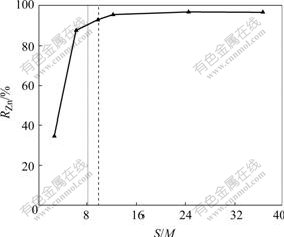
Fig.2 Effect of S/M ratio on rejection of Zn2+
When the rejection of Zn2+ reaches 90%, S/M ratio is 7.90, which is shown as the left imaginary line in Fig.2. The condition is controlled as follows: the added SDS 27.2 g/h, the formed amount of micelle 20.5 g/h, the initial amount of Zn2+ 0.79 g/h in solution, adsorbed Zn2+ 0.75 g/h, S′/M′ ratio of 6.20. This means every 6.2 SDS molecule adsorbing one zinc ions. Ratio of S/M added amount of surfactant at 1CMC is shown as the right imaginary line in Fig.2. And the condition controlled is as follows: the rejection of Zn2+ 92.54%, S/M ratio 9.80, amount of surfactant micelle 28 g/h, added gross amount of SDS 41.3 g/h, gross amount of Zn2+ 0.96 g/h in solution, adsorbed Zn2+ 0.91 g/h, S′/M′ of 7. This means every 7 SDS molecule adsorbing one zinc ions.
3.1.2 Effects of S/M ratio on micelle quantity
The effect of S/M ratio on gross of SDS micelle quantity, micelle quantity and the ratio of micelle are shown in Figs.3 and 4. For the aqueous solution, when the S/M ratio increases, a parallel increase in gross of SDS and micelle quantity could be obtained and the ratio of micelle increases. When S/M ratio is lower than 9.8 and the concentration of SDS is less than that of the CMC, micelles are still present in the solution, and the rejection of Zn2+ is observed because of the concentration polarization effect.
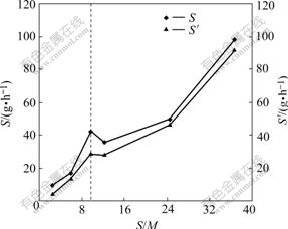
Fig.3 Effect of S/M ratio on gross of surfactant and amount of micelle
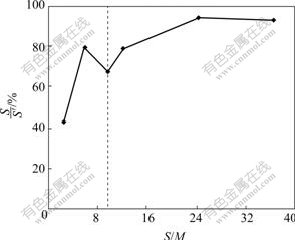
Fig.4 Effect of S/M ratio on S′/S ratio in solution containing Zn2+
3.1.3 Effect of S/M ratio on adsorption quantity of Zn2+
The effect of S/M ratio on gross adsorption quantity and adsorption quantity of Zn2+ is shown in Fig.5. When the concentration of SDS is lower than 1CMC, it is noted that as the S/M ratio increases, gross adsorption quantity of SDS to Zn2+ increases as well. It reaches the peak at the SDS concentration of 1CMC. However, from then the adsorption quantity of SDS to Zn2+ decreases with the increase in S/M ratio. It can be explained that adsorption of SDS micelle to Zn2+ reaches saturated state at 1CMC. When increasing the S/M ratio and the added SDS, adsorption of sodium cations at the micelle interface occurs there by competing with adsorption of Zn2+, so gross adsorption quantity of SDS micelle to Zn2+ is reduced. But per unit mass adsorption of SDS micelle to Zn2+ decreases with the S/M ratio increasing. Taking the rejection of 90% and the concentration of SDS at 1CMC for example, S/M ratios are 7.90, 9.80; gross adsorption quantities are 0.75 g/h, 0.91 g/h, and unit adsorption quantities are 0.039 g Zn2+/gSDS, 0.032 g Zn2+/gSDS, respectively.
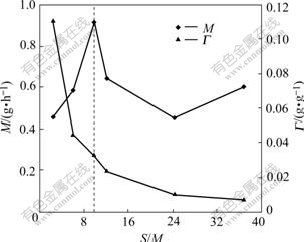
Fig.5 Effect of S/M ratio on adsorption quantity and gross adsorption quantity of Zn2+
Fig.6 shows the relation curve of S/M and S′/M′ in solution containing Zn2+. The straight line passes the zero point and the slope ratio of straight line is 0.924 1.
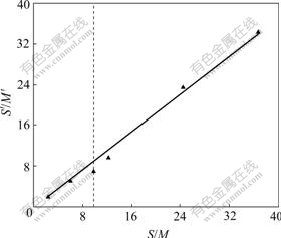
Fig.6 Effect of S/M ratio on S′/M′ ratio in solution containing Zn2+
3.2 Adsorption isotherms of Zn2+ onto SDS micelle in MEUF
The Langmuir adsorption isotherm is perhaps the best known equation among all isotherms describing adsorption, which is often expressed as
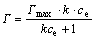 (9)
(9)
where Γmax is the maxinum amout of adsorbate adsorbed per unit mass of adsorbent; ce is the adsorbate concentration reaching equilibrium adsorption; k is the equilibrium adsorption constant that is related to energy of adsorption.
The above equation can be rearranged to the following liner form:
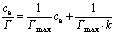 (10)
(10)
The linear form can be used for linearization of experimental data by plotting ce/Γ versus ce.
The adsorption equilibrium model used in adsorption of Zn2+ onto SDS micelle in MEUF is the Langmuir equation and the adsorption isotherms are shown in Figs.7 and 8, after fitting, which can be expressed as
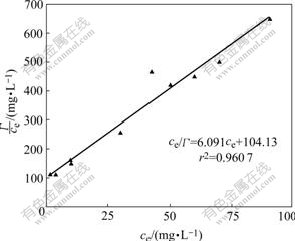
Fig.7 Langmuir fitting curve of Zn2+ onto SDS
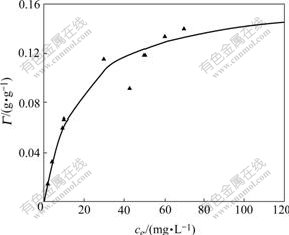
Fig.8 Langmuir adsorption isotherms of Zn2+ onto SDS
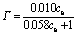 (11)
(11)
where Γ is the amount of Zn2+ adsorbed per unit mass of micelle; ce is the concentration of Zn2+ reaching equilibrium adsorption.
It can be seen that the adsorption process of Zn2+
3.3 Adsorption mechanism of interaction between Zn2+ and SDS in MEUF
To study the adsorption mechanism of Zn2+ onto SDS, X-ray photoelectric spectra of pure SDS, Zn(NO3)2 and the mixed material was measured. The sample preparation conditions such as analytical pure SDS, solid Zn(NO3)2 and feeding in MEUF (the concentration of SDS of 1CMC was taken as the concentration of Zn2+ was 50 mg/L, the pH value was not adjusted and the operating time was 60 min), were desiccated at 60-80 ℃. The binding energy data of proof sample C 1s, O 1s, S 2p and Zn 2p can be seen in Tables 2 and 3.
Table 2 Binding energies of C 1s, O 1s and S 2p (eV)
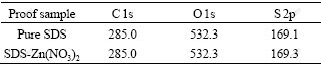
Table 3 Binding energy of Zn 2p (eV)

By comparing the X-ray photoelectric spectra of pure SDS and the mixed material from dried feed solution in MEUF, the binding energies of C 1s, O 1s don’t change after the interaction of SDS and Zn2+, and the peak doesn’t split. That is to say, C atom doesn’t react with O atom coordinately. After the micelle adsorbs Zn2+, S 2p peak doesn’t split and the binding energy increases. It can be considered that S atoms need to keep in a certain chemical condition around the adsorption. The change transfer from S atom to Zn2+, reduces the change density on S atom and increases the binding energy. Around the adsorption, the binding energy of Zn 2p decreases from 1 023.3 eV to 1 021.7 eV. It can be expressed that Zn2+ tends to gain the electron, namely it reacts with SDS micelle coordinately.
4 Conclusions
1) Micellar-enhanced ultrafiltration(MEUF) is a new technique combining surfactants and ultrafiltration membranes. The process is shown to be effective to remove Zn2+ from the aqueous solution.
2) The rejection of Zn2+ increases with the ratio of S/M increasing. When the S/M ratio is less than or equal to 5.8, Zn2+ rejection increases by 13.8% with doubling S/M ratio. As S/M ratio is higher than 5.8, the rejection of Zn2+ increases flatly. Moreover, when S/M ratio is higher than 24.4, the rejection of Zn2+ reaches 97%.
3) In MEUF, the change of ratio of SDS to Zn2+ can directly bring about the change of SDS concentration and gross micelle amount, which affects adsorption quantity of Zn2+ and the rejection in separation. For the aqueous solution, when the S/M ratio increases, a parallel increase in gross of SDS and micelle quantity can be got when S/M ratio is more than 9.8 and the concentration of SDS is more than 1CMC, and the ratio of micelle increases in the solution. When the concentration of SDS is less than 1CMC, the rejection of Zn2+ is observed because of the concentration polarization effect.
4) The adsorption process of Zn2+ onto SDS confirms to Langmuir adsorption isotherm model. The correlation coefficient r2 is 0.960 7, the maximum amount of adsorbed Zn2+ is equal to 0.166 g/g, and the adsorption equilibrium constant is equal to 0.058. Around the adsorption, the binding energy of Zn 2p decreases from 1 023.3 eV to 1 021.7 eV, while the binding energy of S 2p increases from 169.1 eV to 169.3 eV. It can be explained that Zn2+ can obtain electron. It means that Zn2+ tends to gain the electron, namely it reacts with SDS micelle coordinately. Chemical displacement is obvious in the adsorption of Zn2+ onto SDS.
References
[1] MICHAELS A S. Progress in separation and purification [M]. New York: Wiley-Interscience, 1968. 297-333.
[2] LEUNG P S. Ultrafiltration membranes and applications [M]. New York: Plenum Press, 1979. 415-421.
[3] HUANG Y, BATCHELOR B, KOSEOGLU S S. Crossflow surfactant-based ultrafiltration of heavy metals from waste streams [J]. Separation Science and Technology, 1994, 29(15): 1979-1998.
[4] QIU Yan-xing, CHENG Xian-xiong, HAO Zhi-wei, LUO Xian-ping. Present situation and development for wastewater containing cadmium treatment technology [J]. Sichuan Nonferrous Metals, 2002, 4: 38-41. (in Chinese)
[5] XU Zhen-liang, XU Hui-min, ZAI Xiao-dong. Treatment of water streams containing Pb2+ and Cd2+ by micellar-enhanced ultrafiltration [J]. Membrane Science and Technology, 2002, 22(3): 15-20. (in Chinese)
[6] AKITA S, CASTILLO L P, NII S, TAKAHASHI K, TAKEUCHI H. Separation of Co(II)/Ni(II) via micellar enhanced ultrafiltration using organophosphorus acid extractant solubilized by nonionic surfactant [J]. Journal of Membrane Science, 1999, 162: 111-117.
[7] YURLOVA L, KRYVORUCHKO A, KORNILOVICH B. Removal of Ni(II) irons from wastewater by micellar-enhanced ultraflitration [J]. Desalination, 2002, 144: 255-260.
[8] JUANG R S, XU Y Y, CHEN C L. Separation and removal of metal ions form dilute solutions using micellar-enhanced ultrafiltration [J]. Journal of Membrane Science, 2003, 218: 257-267.
[9] GZARA L. Removal of divalent lead cation from aqueous streams using micellar-enhanced ultrafitration [J]. Rev Sci Eau, 2000, 13: 289-304.
[10] GZARA L, DHAHBI M. Removal of chromate anions by micellar-enhanced ultrafiltration using cationic surfactants [J]. Desalination, 2001, 137: 241-250.
[11] BAEK K, YANG J W. Simultaneous removal of chlorinated aromatic hydrocarbons nitrate and chromate using micellar-enhanced ultrafiltration [J]. Chemosphere, 2004, 57(9): 1091-1097.
[12] HANKINS N, HILAL N, OGUNBIYI O O. Inverted polarity micellar enhanced ultrafiltration for the treatment of heavy metal polluted wastewater [J]. Desalination, 2005, 185: 185-202.
[13] HONG J J, YANG S M, LEE C H. Ultrafiltration of divalent metal cations from aqueous solution using polycarboxylic acid type biosurfactant [J]. Journal of Colloid and Interface Science, 1998, 202: 63-73.
[14] JUANG R S, XU Y Y, CHEN C L. Separation and removal of metal ions from dilute solutions using micellar-enhanced ultrafiltration [J]. Journal of Membrane Science, 2003, 218: 257-267.
[15] LIU C K, LI C W. Combined electrolysis and micellar enhanced ultrafiltration (MEUF) process for metal removal [J]. Separation and Purification Technology, 2005, 43(1): 25-31.
[16] TUNG C C, YANG Y M, CHANG C H, MAA J R. Removal of copper ions and dissolved phenol from water using micellar-enhanced ultrafiltration with mixed surfactants [J]. Waste Manage, 2002, 22: 695-701.
[17] AOUDIA M, ALLAL N, DJENNET A, TOUMI L. Dynamic micellar enhanced ultrafiltration: Use of anionic (SDS)–nonionic(NPE) system to remove Cr3+ at low surfactant concentration [J]. Journal of Membrane Science, 2003, 217: 181-192.
[18] AKITA S, YANG L, TAKEUCHI H. Micellar-enhanced ultrafiltration of gold(III) with nonionic surfactant [J]. Journal of Membrane Science, 1997, 133: 189-194.
[19] BAEK K, KIM B K, CHO H J, YANG J W. Removal characteristics of anionic metals by micellar-enhanced ultrafiltration [J]. Journal of Hazardous Materials, 2003, 99: 303-311.
Foundation item: Project(50608028) supported by the National Natural Science Foundation of China; Project(50225926) supported by the National Foundation for Distinguished Young Scholars; Project(20020532017) supported by the Doctoral Foundation of Ministry of Education of China; Project(2003AA644010) supported by the National High-Tech Research Program of China; Project([2005]100) supported by Scientific Research Fund of Hunan Provincial Education Department, China
Corresponding author: ZENG Guang-ming; Tel: +86-731-8822754; E-mail: zgming@hnu.cn
(Edited by YANG Bing)