
Nature of magnetic and electronic structure of double
perovskite A2FeMoO6
LIANG Pei(梁培), JIANG Jian-jun(江建军), MA Xin-guo(马新国), TIAN Bin(田斌)
Department of Electronic Science and Technology, Huazhong University of Science and Technology,
Wuhan 430074, China
Received 15 July 2007; accepted 10 September 2007
Abstract: The nature of magnetic and electronic structure in double perovskite structure A2FeMoO6 (A = Sr, a, Ca) was calculated using the local spin density approximation (LSDA) and the LSDA+U Coulomb interaction method of density functional theory. The result shows that Sr2FeMoO6 is magnetic metallic material, whereas Ba2FeMoO6 and Ca2FeMoO6 are half-metallic materials. Fe has great effect on the magnetic property of double perovskite structure A2FeMoO6 materials. Because of the orbit hybridization and polarization between the metal element and O element, the Mo element has magnetic properties. The static magnetic moment of double perovskite structure A2FeMoO6 materials, the value of the magnetic moment of these A2FeMoO6 for (A=Ca, Sr, Ba) are 3.626 43μB, 2.678 64μB, 3.706 17μB, respectively. The magnetic moment of Fe element in the crystal cell are, 3.626 43μB, 2.678 64 μB, 3.706 17μB. And the energy of crystal cells are -28 540.561 907Ry, -24 268.037 272Ry, -44 106.187 179Ry. These values are in agreement with the experiment values.
Key words: double perovskite A2FeMoO6; full-potential linearized augmented plane wave; half-metal; local density approximation
1 Introduction
The growing interest in the development of spintronics devices has stimulated the search for materials with large spin polarization and ferromagnetic order above room temperature[1]. In particular, much effort has been devoted in the past years to the study of ferromagnetic double perovskites of formula A2FeMoO6 (A = Ca, Sr, Ba) triggered by the work of KOBAYASHI et al[1]. The research has mostly been focused on the study of the compound Sr2FeMoO6 (SFMO), which presents a high Curie temperature (TC ≈400 K) and displays a strong magnetoresistance (magnetoresistance is defined as Rm = (R(H)-R(0))/R(0), where ρ(H) is the field dependent resistivity) up to room temperature[2].
In the 1960s, GALASSO et al[3] studied some compounds of the type AA′FeMoO6 and reported that at room temperature a cubic structure for AA′ is Ba2, a tetragonal one for AA′ is Sr2 and an orthorhombic one for AA′ is Ca2, as well as a maximum Curie temperature for AA′ is Sr2 (TC ≈ 420 K). Similar results were reported by NAKAGAWA[4], who also claimed that 8% oxygen vacancies hardly modify the lattice constants and the magnetic behavior. More recently, BORGES et al[5] reported a P21/n monoclinic structure for AA′=Ca2 and slightly different Curie temperatures. Research also proved that this series of materials to be half metallic ferromagnetic[6].
In despite of many research on this field, there are many open issues of fundamentals importance these concerning the electronic and magnetic structures of compounds. In this article we extended the first principle based on the density function theory to calculate the electronic and magnetic structure of the double perovskites A2FeMoO6 materials.
2 Methods
According to the results of BLAHA et al[7], we can obtain the structure of the double perovskites. In order to obtain the most stable structure of A2FeMoO6(A=Ca,Sr,Ba),we used Wien2k [8] to optimize the structure. And then the optimized crystal structure is listed in Table 1. As a preliminary study for the A2FeMoO6(A=Ca, Sr, Ba) film, we have investigated electronic and magnetic properties of bulk A2FeMoO6(A=Ca, Sr, Ba), using the full-potential linearized augmented plane wave (FLAPW) method[9] based on local spin density approximation (LSDA)[10] for exchange-correlation potential. The optimized lattice constants which we used in our calculation are listed in Table 1.
Table 1 Optimized lattice parameters of A2FeMoO6(A=Ca, Sr, Ba)
We have used 128 k-points in the first full Brillouin zone for the A2FeMoO6(A=Ca, Sr, Ba)double perovskite structures, respectively. About 100 augmented plane waves per atom were used as the variational basis set for all systems. Charge density and potential inside muffin-tin spheres with radii of 2.150 (a.u) for Ca, 1.960(a.u) for Fe, 1.890 (a.u) for Mo and 1.670(a.u) for O in Ca2FeMoO6 were expanded with l<8 lattice harmonics. For Ba2FeMoO6, the muffin-tin spheres radii are 2.500 (a.u) for Ba, 2.010 0 (a.u) for Fe, 1.930 0(a.u) for Mo and 1.7100 (a.u) for O in Sr2FeMoO6 cell. The muffin-tin spheres have radii of 2.500 (a.u) for Sr, 1.760 0 (a.u) for Fe, 1.880 0 (a.u) for Mo and 1.560 0 (a.u) for O. The core states are treated fully relativistically, and the valance states are treated semi-relativistically, including all relativistic term but spin-orbit coupling. Here, the 4f electrons of the Mo atom are treated as core ones, which means that we neglect the inter-atomic 4f interaction and the f-d hybridization, and are assumed to be fully spin polarized. Self-consistency is assumed when the RMts differences between the input and the output spin and charge densities are less than 10-4 e/a.u.3.
3 Results and discussion
3.1 Density of state
At first, electronic structure of the three different double perovskite compounds were calculated. In the following figures, the dash line stands for the Fermi surface. Fig.1 and Fig.2 show the spin down and spin up density of state (short for DOS) of Ba2FeMoO6, Fig.3 and Fig.4 show the spin down and spin up density of state of Ca2FeMoO6, Fig.5 and Fig.6 show the spin down and spin up density of state of Sr2FeMoO6.
Analyzing these results, conclusions can be obtained that the Ba2FeMoO6 and Ca2FeMoO6 are the half metallic materials[11] (we also note the half metallic nature of the ground state of this compound: the density of states for one direction spin band is present at Fermi level, whereas the other spin direction forms a gap at the Fermi level), while Sr2FeMoO6 is metallic material. Figs.1 and 2 show that there is none electron in the spin up direction, while there are some electrons in the spin down direction. The occupied part of the bands near the Fermi level in the minority spin channel is mainly composed of Fe d character which hybridizes with the oxygen p states and Mo d character. In the Figs.3 and 4 we can see in the spin up direction, there are several electrons, but in the spin down direction there is no electron, from this we also can infer that Ca2FeMoO6 is also a half metallic materials. The occupied part of the bands near the Fermi level in the minority spin channel is mainly composed of Fe d character which hybridizes with the oxygen p states and Mo d character. Figs.5 and 6 show the density of state of Sr2FeMoO6 for the majority and the minority spin channels; here the area of the DOS (density of state) in each figure is the weight of the indicated number of electrons in atoms. Both the minority down spin bands and majority up spin bands cross the Fermi level, we can infer that the Sr2FeMoO6 is metallic material. The spin down bands below 2 eV are predominantly of oxygen character, while bands crossing the Fermi level and ranging from -1 eV to about 2 eV have significant mixing between the Fed and Mod characters with some small admixture of oxygen p states. The presence of approximate cubic symmetry of the octahedral coordination of the oxygen atoms around the transition metal sites results in a splitting of the d levels into t2g and eg orbitals. For the minority spin channel, the Fe t2g and Mo t2g bands are partially filled, while Fe eg and Mo eg bands remain empty. The occupied part of the bands near the Fermi level in the majority spin channel is mainly composed of Fe d character which hybridizes with the oxygen p states.
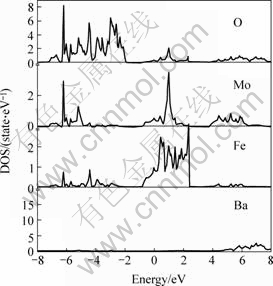
Fig.1 Spin down density of state of each atoms in Ba2FeMoO6 cell
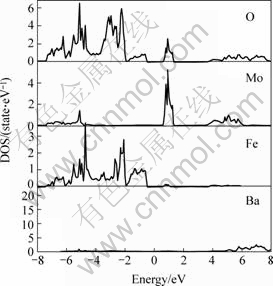
Fig.2 Spin up density of state of each atoms in Ba2FeMoO6 cell

Fig.3 Spin down density of state of each atoms in Ca2FeMoO6 cell
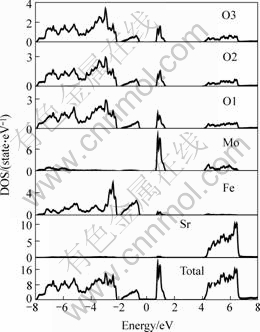
Fig.4 Spin up density of state of each atoms in Ca2FeMoO6 cell
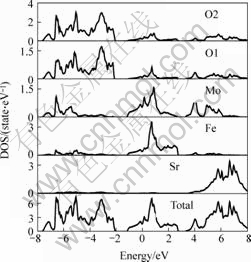
Fig.5 Spin down density of state of each atoms in Sr2FeMoO6 cell
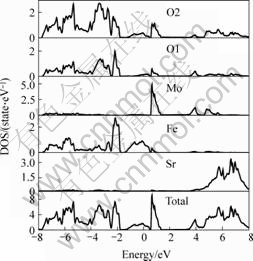
Fig.6 Spin up density of state of each atoms in Sr2FeMoO6 cell
3.2 Energy analysis
We also calculated the value of the energy of the A2FeMoO6 compounds, the results are listed in Table 2.
Table 2 Energy of A2FeMoO6 compounds (Ry)

3.3 Magnetic and magnetic moment
Next we will discuss spin distribution in FM state of A2FeMoO6 compounds. Spin populations on Fe ions are 3.626 43μB, 3.706 17μB and 2.6786 4μB, respectively in the Ca2FeMoO6, Ba2FeMoO6 and Sr2FeMoO6 cell(Table 3). Now spin distributions on the three kinds of ПA atoms are different. They are -0.000 05μB, -0.002 91μB and -0.001 19μB on atom Ca, Ba and Sr.
Table 3 Magnetic moment of atoms in compounds (μB)
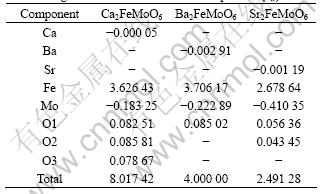
Obviously, the spin distributions of A2FeMoO6 compounds are changed simultaneously with corresponding Fe ions. This means that spin distribution on them are determined by the Fe ions that are directly bridged by them. Our calculation shows that spin populations on atom O1, O2 and O3 are 0.082 51μB, 0.085 81μB and 0.078 67μB in Ca2FeMoO6 cell, while that on atom O1 is 0.085 02μB, in the Ba2FeMoO6 cell and that on atom O1 and O2 are 0.056 36μB and 0.043 45μB, respectively.
From Table 3 we can obtain that the sum of the magnetic moment of the atoms is not equal to the total magnetic moment. The reason for this is that when we use WIEN2k to calculate the electronic and magnetic structure of the compounds, we use the muffin-tin spheres; some of the area is omitted, so the sum of the atom magnetic moment is less than the total magnetic moment.
4 Conclusions
1) Using an accurate full-potential density functional method, a systematical investigation were carried out on the electronic, magnetic, and structural properties of double perovskite structure A2FeMoO6(A is Ca or Sr, Ba).
2) Half-metallic ferromagnetism is observed in some of these double perovskite structure compounds, such as Ba2FeMoO6 and Ca2FeMoO6 are half-metallic materials, whereas Sr2FeMoO6 is magnetic metallic material.
3) Fe has great effect on the magnetic property of double perovskite structure A2FeMoO6 materials. Because of the orbit hybridization and polarization between the metal element and O element, Mo element has magnetic properties.
4) The static magnetic moment of double perovskite structure A2FeMoO6 materials, the value of the magnetic moment of these A2FeMoO6 (A=Ca, Sr, Ba) are 3.626 43μB, 2.678 64μB, 3.706 17μB. The magnetic moments of Fe element in the crystal cell are 3.626 43 μB, 2.678 64 μB, and 3.70617 μB.
5) The energies of crystal cell are: -28 540.561 907 Ry, -24 268.037 272 Ry, and -44 106.187 179 Ry. These values are closed to experiment value.
References
[1] KOBAYASHI KI, KIMURA T, SAWADA H. Room temperature magnetoresistance in an oxide material with an ordered double perovskite structure[J]. Nature, 1998, 395: 677-680.
[2] BALCELLS LI, NAVARRO J, BIBES M, ROIG A, MARTINEZ B, FONTCUBERTA J. Cationic ordering control of magnetization in Sr2FeMoO6 double perovskite[J]. Applied Physic Letter, 2001, 78(6): 781-783.
[3] PHILIPP J B, MAJEWSKI P, ALFF L, ERB A, GROSS R, GRAF T, BRANDT M S, SIMON J, WHATHER T, MADER W, TOPWAL D, SARMA D D. Structural and doping effects in the half-metallic double perovskite A2CrWO6 (A=Sr, Ba, and Ca)[J]. Phys Rev B, 2003, 68: 144431
[4] BORGES R P, LHOSTIS S, BARI M A. Thin films of the double perovskite Sr2FeMoO6 deposited 2 6 by pulsed laser deposition[J]. Thin Solid Films, 2003, 429: 5–12.
[5] SARMA D D, MAHADEVAN P, SAHA-DASGUPTA T, RAY S, KUMAR A. Electronic Structure of Sr2FeMoO6[J]. Physical Review Letters, 2000, 85(12): 2549-2552
[6] SONG W H, DAI J M, YE S L, WANG K Y, DU J J. Preparation and magnetic properties of the double-perovskite A2FeMoO6 A=(Ca, Sr, Ba), polycrystals with nanometer-scale particles[J]. Journal of Applied Physics, 2001, 89(11): 7678-7680.
[7] BLAHA P, SCHWARZ K, MADSEN G K H, KVASNICKA D, LUITZ J. WIEN2k, Vienna University of Technology, 2002, improved and updated Unix version of the original copyrighted Wiencode, which was published by P.Blaha, K. Schwarz, P. Sorantin, S.B. Trickey, Comput. Phys. Commun, 1990, 59: 399.
[8] WIMMER E, KRAKAUER H, WEINERT M, Freeman A J. Full-potential self-consistent linearized-augmented-plane-wave method for calculating the electronic structure of molecules and surfaces: O2 molecule[J]. Phys Rev B, 1981, 24: 864.
[9] NAVARRO J, BALCELLS LI, SANDIUMENGE F, BIBES M, ROIG A, MART?INEZ B, FONTCUBERTAV J. Antisite defects and magnetoresistance in Sr2FeMoO6 double perovskite[J]. J Phys: Condens Matter, 2001, 13: 8481–8488.
[10] TOMIOKA Y, OKUDA T, OKIMOTO Y, KUMAI R, KOBAYASHI KI Magnetic and electronic properties of a single crystal of ordered double perovskite Sr2FeMoO6[J]. Physical Review B, 2000, 61(1): 442-448.
[11] MANAKO T, IZUMI M, KOKAYASHI KI. Epitaxial thin film of ordered double perovskite Sr2FeMoO6[J]. Appl Phys Lett, 1999, 74: 2215-2217.
Foundation item: Project(NCET-04-0702) supported by the New Century Excellent Talents in University of China; Project(50771047) supported by the National Natural Science Foundation of China
Corresponding author: LIANG Pei, Doctoral canditate; Tel: +86-27-87559279; E-mail: liangpei20002@yahoo.com.cn
(Edited by YANG Hua)