
Dissolution behaviors of Ta2O5, Nb2O5 and their mixture in KOH and H2O system
WANG Xiao-hui(王晓辉)1, 2, ZHENG Shi-li(郑诗礼)1, XU Hong-bin(徐红彬)1, ZHANG Yi(张 懿)1
1. National Engineering Laboratory for Hydrometallurgical Cleaner Production Technology,
Key Laboratory of Green Process and Engineering, Institute of Process Engineering,
Chinese Academy of Sciences, Beijing 100190, China;
2. Graduate School of Chinese Academy of Sciences, Beijing 100039, China
Received 6 July 2009; accepted 21 January 2010
Abstract: The dissolution behaviors of Ta2O5, Nb2O5 and their mixture in KOH and H2O system were investigated. A L9(34) orthogonal design was used to study the effects of reaction temperature, mass ratio of KOH to Ta2O5, and reaction time on the dissolution rate of tantalum. It was found that the effect of reaction temperature on the dissolution rate of tantalum was much greater than that of the other factors. The results of factorial experiments showed that Ta2O5 was mainly transformed into insoluble potassium tantalate at low temperature (350 °C) and transformed into soluble potassium tantalate at high temperature (450 °C). The insoluble potassium tantalate was analyzed by XRD, which was proved to be KTaO3. Differently, almost all Nb2O5 was transformed into soluble potassium niobate at 350-450 °C. As for the mixture of Ta2O5 and Nb2O5, the dissolution rate of tantalum increased and the dissolution rate of niobium decreased as an interaction existed between niobium and tantalum. And increasing the mole ratio of Nb2O5 to Ta2O5 in the mixture was beneficial to the dissolution of both Ta2O5 and Nb2O5. In addition, the mechanism of the interaction between niobium and tantalum was also investigated through phase and chemical analysis.
Key words: Ta2O5; Nb2O5; KOH; dissolution behavior; mechanism; solid-solution
1 Introduction
Tantalum and niobium are important rare refractory metals and are widely used in steel, electronic and other high-tech industries[1-3]. The decomposition of the ore is the key step in extracting niobium, tantalum and their compounds from niobium-tantalum ore. At present, the hydrofluoric acid method is widely used in the tantalum-niobium hydrometallurgical industry for the decomposition of the ores[4-6]. However, due to the strong volatility, about 6%-7% of the hydrofluoric acid is lost during the decomposition process, which is harmful to human beings and equipments. As well, a large amount of wastewater containing fluoride is generated which needs to be treated[7-9]. More importantly, this method is only appropriate for high-grade niobium-tantalum ores[10]. Although the resources of niobium-tantalum ores are abundant in China, most of them are in low-grade and difficult to decompose by hydrofluoric acid[11]. Therefore, it is imperative to develop a new and clean production process, so as to achieve optimum resource utilization.
Recently, a new process for the leaching of low-grade niobium-tantalum ores using a KOH roast-water leach system was proposed by the Institute of Process Engineering, Chinese Academy of Sciences, China, with the objective to eliminate fluorine pollution at the source[12]. In this new process, low-grade refractory niobium-tantalum ore is decomposed using KOH molten salt instead of highly concentrated hydrofluoric acid and then is leached by H2O. The experimental results of the new process show that the decomposition rate for the low-grade refractory niobium-tantalum ore is almost 15% higher than that for the hydrofluoric acid process.
The new process of leaching niobium and tantalum from a low-grade niobium-tantalum ore is under development and there is a general lack of information. Although some studies have been performed on alkali fusion[13-14] and the phase equilibria of Nb2O5-K2CO3 system and Ta2O5-K2CO3 system have been given [15-16], no previous work has ever been reported on the fundamental dissolution behaviour of Nb2O5 and Ta2O5 in KOH molten salt and H2O system. The aim of this work is to investigate the dissolution behavior of Nb2O5, Ta2O5 and their mixture in KOH molten salt and H2O system. And the interaction between niobium and tantalum in the decomposition process was also investigated.
2 Experimental
2.1 Materials
All the chemical reagents employed were of analytical grade and deionized water was used in the corresponding procedures during the experiments. The Nb2O5 and Ta2O5 samples used for the present study were of reagent grade and were supplied by the Ningxia Orient Tantalum Industry Co., Ltd, China.
2.2 Equipment
The dissolution process was carried out in a 500 mL SUS316 stainless batch reactor equipped with a thermometer, a mechanical stirrer and a reflux condenser. The reactor was heated by immersing in a furnace to reach and maintain the desired temperature within ±2 °C.
2.3 Procedure
All the experiments were conducted in batches. For each run, the required amounts of solid KOH were transferred to the reactor and then were heated to the desired temperature. When the temperature reached the pre-set value and kept stable, the mechanical stirrer was started and a certain amount of Nb2O5 (Ta2O5 or their mixture) was added to the reactor. The mixture was stirred at a constant speed under an atmospheric pressure. When the reaction time was reached, the products were cooled to room temperature quickly using cold airflow. Under the ambient conditions, the products were leached with a certain amount of water and filtered to obtain a solution and a solid residue. The resulting leaching solution and the residue were analyzed for Nb and Ta by ICP-OES. The dissolution rate (K) of the elements was calculated using the following expression:
K=[1-(mr/mo)]×100%
where mr and mo are the mass of the element calculated in the residue obtained in the leaching step and the mass of the element calculated in the Nb2O5(Ta2O5 or their mixture), respectively. The leaching residues were examined by X-ray diffraction analysis (XRD, using Phillips PW223/30).
3 Results and discussion
When Nb2O5 (Ta2O5) reacts with potash, K3NbO4 (K3TaO4) or KNbO3 (KTaO3) forms. When the mole ratio of K2O to Nb2O5(Ta2O5)≤1:1, the reaction product is mainly in the form of KNbO3 (KTaO3). And when the mole ratio of K2O to Nb2O5(Ta2O5)≥4:3, the reaction product is mainly in the form of K3NbO4 (K3TaO4). The KNbO3 (KTaO3) is insoluble and cannot be leached by water. By contrast, the K3NbO4 (K3TaO4) will hydrolyze to soluble K8Nb6O19 (K8Ta6O19) and then be leached in the water leaching process. The purpose of our research is to find the optium reaction conditions under which the highest dissolution rate of niobium and tantalum can be obtained. Therefore, the experiments below are all conducted under the condition of mole ratio of K2O to Nb2O5(Ta2O5)>4:3.
3.1 Dissolution behavior of Ta2O5
3.1.1 Effect of leaching parameters on dissolution of Ta2O5 using L9(34) orthogonal design
Through our preliminary experiments, we found that the reaction temperature, mass ratio of KOH to Ta2O5 and reaction time are the main parameters affecting the dissolution of Ta2O5. Thus, an L9(34) orthogonal design was used to investigate the effect of reaction temperature (Tr), mass ratio of KOH to Ta2O5 (Ra) and reaction time (t) on the dissolution rate of Ta2O5 in KOH and H2O system. The variable assignment and the level settings are listed in Table 1. The results of L9(34) orthogonal experiments are presented in Table 2.
Table 1 Experimental factors and levels

In Table 2, K1, K2 and K3 represent the sum of dissolution rate of Ta2O5 of level 1, level 2 and level 3 of a factor, respectively. K1/3, K2/3 and K3/3 represent the average of K1, K2 and K3, respectively. R represents the maximum difference value among K1, K2 and K3. The orthogonal experiment results of variance analysis are shown in Table 3.
Table 2 and Table 3 show that the most significant factor is reaction temperature, which is statistically significant at the 99% confidence level. In the selected range, the mass ratio of KOH to Ta2O5 and reaction time
Table 2 Results of L9(34) orthogonal experiments

Table 3 Variance analysis of orthogonal experiment results

have no significant influence on the dissolution of Ta2O5.
3.1.2 Effect of reaction temperature
According to the results of the orthogonal experiments, reaction temperature has much more significant influence on the dissolution of Ta2O5 than other factors do. Thus, the effect of reaction temperature was further investigated by factorial experiments under the conditions of reaction time of 1 h and mass ratio of KOH to Ta2O5 of 5:1. The results are shown in Fig. 1.
It can be seen from Fig. 1 that the dissolution rate of Ta2O5 increases significantly with the increasing reaction temperature. To investigate this phenomenon, the residues obtained after Ta2O5 is decomposed by KOH molten salt and dissolved by water were dried at 120 °C for 10 h and then analyzed by XRD. The results are shown in Fig.2. It can be seen from Fig.2 that all diffraction peaks of the insoluble residue are attributable to KTaO3. The XRD pattern is consistent with that reported in JCPDS No.01-077-0917. In order to prove that the KTaO3 is not formed in the water dissolution procedure, we used ethanol instead of water in the water dissolution procedure. There is also KTaO3 in the residue as shown in Fig.2. This indicates that besides converting into K3TaO4, a part of tantalum directly converts into insoluble KTaO3 in the KOH decomposition procedure. And this is the reason for the low dissolution rate of Ta2O5 under low reaction temperature. From Fig.1 we can also find that higher temperature results in lower conversion rate of insoluble KTaO3. Therefore, increasing the reaction temperature is beneficial to the dissolution of Ta2O5. But when the reaction temperature is higher than 540 °C, the dissolution rate of Ta2O5 does not change significantly.
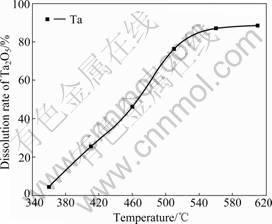
Fig.1 Effect of reaction temperature on dissolution rate of Ta2O5 (Reaction conditions: reaction time 1 h and mass ratio of KOH to Ta2O5 of 5:1)
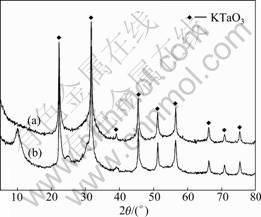
Fig.2 XRD pattern of residue obtained after Ta2O5 being decomposed by KOH molten salt and leached by water (a) and ethanol (b)
3.2 Dissolution behavior of Nb2O5
The effects of reaction temperature, mass ratio of KOH to Nb2O5 and reaction time on the dissolution rate of Nb2O5 were examined. The results are listed in Table 4.
Table 4 Effects of reaction temperature, alkaline-to-ore mass ratio and reaction time on dissolution rate of Nb2O5

Table 4 shows that the dissolution rate of Nb2O5 is almost 100% in the selected range of reaction conditions, which indicates that most Nb2O5 is converted into K3NbO4 in the KOH decomposition procedure and then is dissolved by water. This also indicates that the dissolution behavior of Nb2O5 in KOH and H2O system is different from that of Ta2O5.
3.3 Dissolution behavior of mixture of Ta2O5 and Nb2O5
According to the results of the above experiments, the dissolution behaviors of Ta2O5 and Nb2O5 in KOH and H2O system are different. Ta2O5 converts into K3TaO4 and KTaO3 while Nb2O5 converts only into K3NbO4. As we know, tantalum and niobium often coexist in minerals with the similar properties. Therefore, it is necessary to investigate the dissolution behavior of the mixture of Ta2O5 and Nb2O5. As the dissolution behaviors of Ta2O5 and Nb2O5 in KOH and H2O system are different, when they are mixed together, there may be interaction between them. Therefore, we emphatically investigated the effect of mass ratio of Nb2O5 to Ta2O5 on the dissolution behavior of the mixture of Nb2O5 and Ta2O5. The Nb2O5 and Ta2O5 mixture was obtained through ball-mill mixing of pure Nb2O5 and Ta2O5. We use mass fraction of Nb2O5 to represent the mass ratio of niobium to tantalum in the mixture. The results are presented in Fig.3.
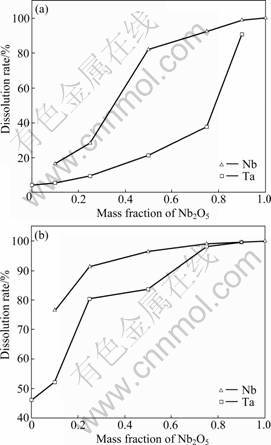
Fig.3 Dissolution behavior of mixture of Ta2O5 and Nb2O5 in KOH and water system at 350 °C (a) and 400 °C (b) (Reaction conditions: reaction time 1 h and KOH-to- Ta2O5(Nb2O5) mass ratio 5:1)
From Fig.3. we can see that when the Nb2O5 and Ta2O5 mixture reacts with KOH and water system, in case that the reaction is carried out at 350 °C and with increasing the mass fraction of Nb2O5, the dissolution rate of tantalum increases slowly at first and then rapidly, while the dissolution rate of niobium increases rapidly at first and then slowly. And in case that the reaction is carried out at 400 °C, the dissolution rates of tantalum increase significantly with increasing the mass fraction of Nb2O5 in the mixture while the increase of niobium is rather small. This indicates that an interaction exists between niobium and tantalum when Nb2O5 and Ta2O5 mixture reacts with KOH and H2O system. The existence of niobium can promote the dissolution of tantalum, while the existence of tantalum can inhibit the dissolution of niobium. To investigate the mechanism of the interaction between niobium and tantalum, phase analysis and component analysis of the residues obtained were made. The results are shown in Fig.4 and Table 5.
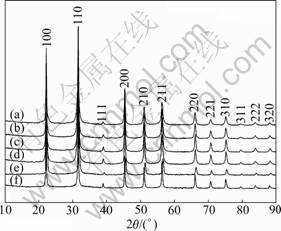
Fig.4 XRD patterns of residues obtained under different reaction conditions: (a) 350 °C, w(Nb2O5)=0.75; (b) 350 °C, w(Nb2O5)=0.5; (c) 350 °C, w(Nb2O5)=0.25; (d) 350 °C, w(Nb2O5)=0.1; (e) 400 °C, w(Nb2O5)=0.5; (f) 400 °C, w(Nb2O5)=0.25
Mole ratio of Ta to Nb in residue
Table 5 Composition of residues obtained under different reaction conditions
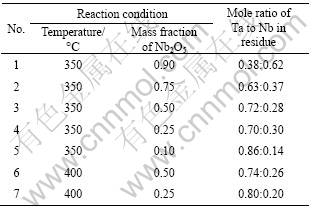
From Fig.4, we can find that the XRD patterns of the residues are very close to the XRD pattern of KTa0.77Nb0.23O3 (JCPDS No.70-2011), which is a solid solution of KTaO3 and KNbO3. But from Table 5, we can find that the mole ratio of Ta to Nb in the residues are all different from 0.77:0.23, which indicates that the residues are not KTa0.77Nb0.23O3. As we know, the Nb5+ and Ta5+ are quite similar in chemical properties and ionic radius and there may be isomorphism replacement between Nb5+ and Ta5+. KTaO3 and KNbO3 can form continuous solid solution by isomorphism replacement between Nb5+ and Ta5+. Thus, we conjecture that the residues of Nb2O5 and Ta2O5 mixture are KTaO3-KNbO3 solid solutions.
According to Vegard’s law[17], the lattice parameter of continuous solid solution has a linear relationship with the mole fraction of one component in it. Therefore, the line of lattice parameter to mole fraction of niobium in the residues is plotted. The results is shown in Fig.5. From Fig.5. we can see that, the relationship between the lattice parameter and mole fraction of niobium is accorded with Vegard’s law. This result indicates that the leaching residues of Nb2O5 and Ta2O5 mixture are really KTaO3 and KNbO3 solid solutions. This result also indicates that when Nb2O5 and Ta2O5 mixture reacts with KOH, by isomorphism replacement between Nb5+ and Ta5+, a part of Nb5+ ions enter the crystal lattice of KTaO3, forming KTaO3- KNbO3 solid solution. This is why the leaching rate of Nb2O5 decreases when it is mixed with Ta2O5. And when the mole fraction of Ta2O5 increases (the mole fraction of Nb2O5 decreases), more Nb5+ ions enter the KTaO3 lattice. Therefore, the leaching rate of Nb2O5 decreases with increasing the mole fraction of Ta2O5. Similarly, when Nb2O5 and Ta2O5 mixture reacts with KOH, there may be a part of Ta5+ ions entering the lattice of K3NbO4, forming K3NbO4-K3TaO4 solid solution and then may be leached. Therefore, the leaching rate of Ta2O5 increases with the increase of Nb2O5 mole fraction in the mixture. But as the K3NbO4 and K3TaO4 are difficult to obtain, further investigation is needed for an in-depth explanation.

Fig.5 Relation between lattice parameter and mole fraction of Nb
In short, increasing the niobium to tantalum ratio in Nb2O5 and Ta2O5 mixture is effective for increasing the dissolution rate of niobium and tantalum.
4 Conclusions
1) The dissolution behavior of Ta2O5, Nb2O5 and their mixture in KOH and H2O system was investigated. Under the different reaction temperatures, Ta2O5 will be partly converted into K3TaO4 and then be dissolved and partly converted into insoluble KTaO3. Increasing the reaction temperature is beneficial to the dissolution of Ta2O5.
2) Under the same reaction conditions, Nb2O5 will be almost 100% converted into K3NbO4 and then be dissolved.
3) When the mixture of Ta2O5 and Nb2O5 reacts in KOH and H2O system, by the formation of KTaO3- KNbO3 and K3NbO4-K3TaO4 solid solutions, the dissolution rate of Ta2O5 increases while the dissolution rate of Nb2O5 decreases. And increasing the mole ratio of Nb2O5 to Ta2O5 in the mixture is beneficial to the dissolution of both Ta2O5 and Nb2O5.
References
[1] MILLER G L. Tantalum and niobium [M]. London: Butterworths Scientific Publications, 1959: 17-66.
[2] FENG Jing-su. The recent advance in applications of niobium [J]. Rare Metal Materials and Engineering, 1994, 23(3): 7-12. (in Chinese)
[3] QU Nai-qin. Properties and applications of Ta/Nb and their alloys [J]. Rare Metals and Hard Alloys, 1998, 133: 48-54. (in Chinese)
[4] EL-HUSSAINI O M. Extraction of niobium and tantalum from nitrate and sulfate media by using MIBK [J]. Mineral Processing and Extractive Metallurgy Review, 2001, 22: 633-650.
[5] HE Chang-yi, LIU Zhi-ming, ZHANG Hao-jun. Treatment of fluorine-containing waste gas from hydrometallurgy of tantalum and niobium ore [J]. Nonferrous Metals, 1998, 4: 141-142. (in Chinese)
[6] LIU Jian-zhong. Current status of China’s Ta and Nb initial materials and its countermeasures [J]. Rare Metals and Hard Alloys, 1997, 130: 39-41. (in Chinese)
[7] HE Ji-lin, ZHANG Zong-guo, XU Zhong-ting. Hydrometallurgical extraction of tantalum and niobium in China [J]. Tantalum-Niobium International Study Centre Bulletin, 1998, 93: 1-6. (in Chinese)
[8] XUE Mei. Environmental pollution and treatment of tantalum and niobium hydrometallurgy [J]. Rare Metals and Cemented Carbides, 2005, 33(4): 55-59. (in Chinese)
[9] HAN Jian-she, ZHAO Ming-zhi, WANG Cai-ming, ZHANG Jin, ZHOU Yong. Research on the “Three Wastes” disposal technology for Ta and Nb hydrometallurgy [J]. Rare Metals and Cemented Carbides, 2006, 34(4): 40-44. (in Chinese)
[10] GUPTA C K, SURI A K. Extractive metallurgy of niobium [M]. London: CRC Press, 1994: 98-127.
[11] LIU Jian-zhong, LI Mei-xian. Status and development advices on China’s tantalum and niobium resources [J]. Hunan Nonferrous Metals, 1999, 15(3): 60-62. (in Chinese)
[12] WANG Xiao-hui, ZHENG Shi-li, XU Hong-bin, ZHANG Yi. Leaching of niobium and tantalum from a low-grade ore using a KOH roast-water leach system [J]. Hydrometallurgy, 2009, 98: 219-223.
[13] OKA Y, MIYAMOTO M. Extraction of tantalum and niobium oxides from columbite (I): Decomposition of the ore [J]. J Electrochem Soc, 1949, 17: 63-69.
[14] BHATTACHARYA H. Investigations on the analysis of mixed oxides of the elements of Group Va and IVa (Part I): Preparation of pure tantalum and niobium pentoxide from India columbite and their analytical separation [J]. J Indian Chem Soc, 1952, 11(29): 871-875.
[15] ARNOLD R, FREDERIC H. Phase equilibria in the system K2CO3-Nb2O5 by the method of differential thermal analysis [J]. J Am Chem Soc, 1955, 77: 2115-2119.
[16] ARNOLD R, SOL T, FREDERIC H. Phase diagram of the system KNbO3-KTaO3 by the methods of differential thermal and resistance analysis [J]. Journal of American Chemical Science, 1955, 77: 4228-4230.
(Edited by LI Xiang-qun)
Foundation item: Project(2009AA06Z103) supported by the National High-tech Research and Development Program of China; Project(51004094) supported by the National Natural Science Foundation of China; Project(2007CB613501) supported by the National Basic Research Program of China
Corresponding author: WANG Xiao-hui; Tel/Fax: +86-10-82544856; E-mail: wangxh@home.ipe.ac.cn
DOI: 10.1016/S1003-6326(09)60409-X